Commentary - Interventional Cardiology (2015) Volume 7, Issue 5
Stent length as a potential indicator to select patients who may benefit from long-term dual antiplatelet therapy
- Corresponding Author:
- Gilles Lemesle
1Hôpital Cardiologique
Centre Hospitalier Régional et Universitaire de Lille
Lille, France
Tel: +33 3204 45301
Fax: +33 3204 44898
E-mail: gilles_lemesle@yahoo.fr
Abstract
“There is to date sufficient data to show us that a 1-year DAPT is not necessarily the best regimen for all CAD patients and that a tailored approach might be warranted.”
Keywords
stent length, percutaneous coronary intervention, dual antiplatelet therapy, thrombosis, myocardial infarction
6 to 12-month dual antiplatelet therapy (DAPT), combining aspirin and a P2Y12 receptor inhibitor, is currently the recommended treatment for patients with an acute coronary syndrome and those who underwent percutaneous coronary interventions (PCI) with drug-eluting stent (DES) implantation [1]. Thereafter, patients should be switched to single antiplatelet therapy (namely aspirin) and this treatment must be pursued lifelong in outpatients with stable coronary artery disease (CAD) [2].
However, the optimal duration of DAPT following coronary stenting is today a great matter of debate. Some authors have suggested individual adaptation of the duration of DAPT after PCI and that ‘one duration may not fit all’.
Risk stratification is then essential to easy select patients who could benefit the most from extended DAPT without increasing the risk of bleeding. The length of coronary artery lesions (and/or stents) is a simple parameter and a well-recognized predictor of PCI complexity and more importantly of long-term outcome, especially regarding the risk of stent thrombosis, restenosis and death [1,3–16]. In this manuscript, we discuss the role of lesion/stent length as a potential indicator for the selection of patients who may benefit from prolonged DAPT in clinical practice.
Definition of a long lesion/stent
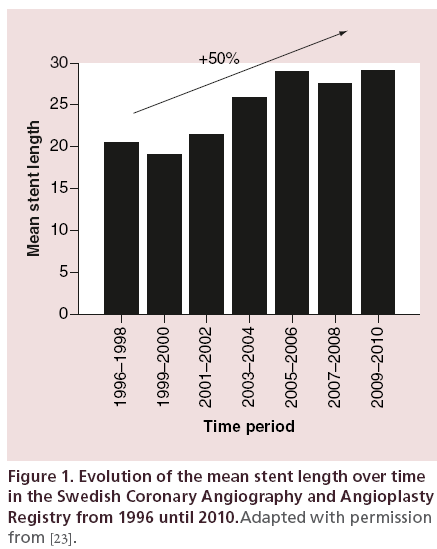
A homogeneous definition to what corresponds a long coronary artery lesion/stent is challenging; and for now, no consensual definition is admitted. In the past literature, this parameter has been evaluated using three main methods: as a continuous variable [3,6,14,17], by dichotomizing the study population by median (tertiles, quartiles, etc.) [7,15] or more frequently by using arbitrary cut-off values (15, 20, 30, 40, 50 mm) [6,13,18–22]. Of main importance, this definition has largely evolved over time, which adds more difficulties. Indeed, since the first stent implantation, there have been great progress and advances in PCI procedures and in devices, from the first bare metal stent (BMS) to the last generation of DES. Related to this and especially to the large decrease in the risk of restenosis, treated lesions are getting more complex and especially longer. Currently, long coronary artery lesion account for a large proportion of procedures, and full lesion coverage has become the preferred strategy. As a perfect illustration of this, Fokkema et al. published in 2013 the data of the Swedish Coronary Angiography and Angioplasty Registry and the evolution of PCI procedures in Sweden over the past 20 years [23]. Of note, 144,039 patients have been included in this registry and from the period 1996–1998 to the period 2009–2010, the mean stent length increased from 20.53 ± 8.47 mm to 29.09 ± 17.41 mm, which represents a 50% increase (Figure 1). This increase in stent length is very consistent in the different registries that have been published over time in the literature.
Figure 1: Evolution of the mean stent length over time in the Swedish Coronary Angiography and Angioplasty Registry from 1996 until 2010.Adapted with permission from [23].
In 2005, the SYNTAX score was established as an angiographic tool assessing the complexity/severity of CAD [24]. This score has shown to accurately predict patient outcome after PCI [24–27]. The algorithm of this score is composed of 12 items including the number of lesions, chronic total occlusion, bi-/trifurcations, calcifications, tortuosity and so on – the ninth being the lesion length that become significant (+1) over 20 mm. This cut-off value is, however, empirical and to date, a stent length greater than 30 mm, which represents the mean of the stent length in all contemporary registries and trials [7,19,23,28–34] appears to be more correlated with daily practice.
Nevertheless, whatever the definition and/or cutoff value is used in the different studies, lesion/stent length has consistently been associated with poor outcome in the literature as illustrated in the following paragraphs.
Long lesion/stent as a marker of diffuse atherosclerosis & high-risk patients
As multivessel CAD or peripheral vascular disease, complex coronary artery lesions and notably lesion/ stent length emphasize a more diffuse atherosclerotic burden and are associated with worse outcomes (Figure 2). Indeed, it is today well recognized that CAD patients who present markers of diffuse atherosclerosis are at higher risk as compared with patients with focal disease [4–16,32,35,36]. As a perfect illustration of this, patients with high-SYNTAX score have shown to experience poor outcome after PCI [24–27].
Stent thrombosis
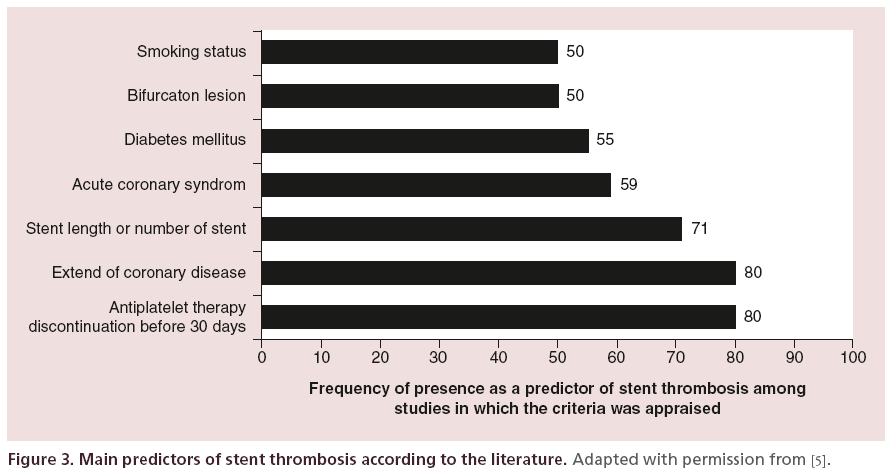
Many factors have been identified as predictors of stent thrombosis, and aside from factors related to the patient himself, lesion characteristics and factors related to the PCI procedure are also well-recognized parameters. Lesion/stent length has shown to be associated with the risk of stent thrombosis in various studies [4,5,37– 43]. In the observational study of Airoldi et al. [37], the mean stent length was 27.9 +/- 13.7mm and this parameter was strongly associated with the incidence of stent thrombosis (per 10 mm, HR: 2.75; 95% CI: 1.55–4.88; p = 0.001). These results were consistent with those of Iakovou et al [4]. In their prospective observational study, 2229 patients were included. The mean stent length was 27.89 +/- 13.32 mm and stent length was also an independent predictor of stent thrombosis (per 1 mm, HR: 1.03; 95% CI: 1.00–1.05; p = 0.01). Finally, in a cohort of 2954 patients who underwent PCI, total stent length was independently associated with an increased risk of stent thrombosis at 2 months (per 1 mm, HR: 1.04; 95% CI: 1.01–1.08; p = 0.009) [38]. Results of these studies were confirmed in a large meta-analysis of 30 studies, published in 2012, focusing on the risk of stent thrombosis after PCI with DES implantation, and including more than 200,000 patients [5]. In this meta-analysis, authors observed that best predictors of stent thrombosis appeared to be linked, aside from early DAPT discontinuation, to the extension of CAD and total stent length. According to this study, stent length was identified as a predictor of stent thrombosis in more than 70% of the studies that appraised this parameter and was in fine the third more frequent predictor of stent thrombosis identified in the literature (Figure 3).
Figure 3: Main predictors of stent thrombosis according to the literature. Adapted with permission from [5].
Restenosis
Lesion/stent length has been identified as a strong predictor of restenosis after PCI as well [6,8,10–13,44]. In 2000, Kereiakes et al. reported in a meta-analysis of the Multi-link stent trials including 1091 patients after PCI with a BMS implantation (mean stent length 18.8 ± 6.2 mm) that the risk of restenosis was directly and linearly correlated to the stent length ranging from 12% in patients with a stent of 8 mm to 36% in patients with a stent of 35 mm [11]. Despite the fact that the risk of restenosis was overall decreased with the use of DES, lesion/stent length was still independently associated with the risk of restenosis with such stents [6,9,14–16]. As an example, Caputo et al. showed that the risk of target lesion revascularization increased from 3% in patients treated with a stent <15 mm to 5% in patients treated with a stent >24mm (with a 20% increased risk per additional 10 mm of length) [6].
Myocardial infarction not related to stent complication
If coronary events observed within the first months after PCI are essentially due to complications related to the stent and/or the PCI procedure, coronary events observed at distance (6–12 months after PCI) have been shown to be almost equally related to both complications of the stent and new atherosclerotic plaque progression and/or rupture [34,45]. Today, no study has truly evaluated the association between lesion/ stent length and the risk of myocardial infarction not related to stent complication. However, several studies have reported that lesion/stent length is a strong predictor of the occurrence of myocardial infarction (all-cause taken together) [6,7,14,15]. Of note, myocardial infarctions (all-cause taken together) were much more frequent than stent thrombosis alone in these studies suggesting that lesion/stent length can predict both types of myocardial infarctions: those related to stent thrombosis and those related to new plaque rupture. In the study published by Caputo et al., the incidence of stent thrombosis was below 1% when the incidence of myocardial infarction (all-cause taken together) was more than 6% [6]. The rate of myocardial infarction was 3.9% in patients treated with a stent <15 mm and 9.7% in patients treated with a stent >24 mm (p <0.01) and this difference was not only explained by the difference observed in terms of stent thrombosis. Thus, these data clearly suggest that lesion/stent length is also able to predict the risk of myocardial infarction not related to stent complications.
Vascular events not related to coronary arteries
For now, no study has directly focused on the relation between lesion/stent length and the risk of vascular events not related to coronary arteries (e.g., stroke, peripheral disease, aortic complications…). It is, however, clearly recognized that patients with multivessel CAD and patients with complex lesions, as evaluated by the SYNTAX score for example, have higher risk of such complications [46–48]. Head et al. recently published the results of the SYNTAX trial at 5 years and reported a 2.5-fold increased risk of stroke between patients with a SYNTAX score <22 and patients with a SYNTAX score ≥33 in the PCI group [49].
Mortality
As a logical consequence, lesion/stent length has been identified as a predictor of death in several studies [14,32]. Suh et al. recently reported in their study of 3145 patients that the better cut-off value to predict stent thrombosis at 3 years after PCI was 31.5 mm using a receiver operating characteristic (ROC) curve analysis [14]. More importantly, they reported that the 3-year all-cause mortality was almost twice higher in patients with a stent length ≥31.5 mm as compared with those with a stent length <31.5 mm: 5.2 versus 3% (p = 0.005). Choi et al. reported very consistent results using an arbitrary cut-off value of 32 mm [32]. In their study of 8445 patients with DES implantation, the 3-year rate of death was higher (even if not significant) in patients with a stent length ≥32 mm than in patients with a stent length <32 mm: 6.9 versus 5.2% (p = 0.08). It should, however, be emphasized that no large study has today identified lesion/stent length as an independent predictor of all-cause death after adjustment on potential confounders.
Impact of prolonged dual antiplatelet therapy in patients with long lesion/stent
The rationale behind long-term DAPT prescription after coronary interventions is based on the protection against the risk of stent thrombosis and new atherosclerotic plaque progression or rupture (in all artery territories). As mentioned above, the optimal duration of DAPT after PCI is currently highly controversial. In this context, the use of first generation DES has had a major impact on antithrombotic prescription in CAD patients. Indeed, since 2006, with the rising concern about late safety of first generation DES [50–52], the duration of DAPT has been progressively increased in clinical practice in order to better protect patients from the risk of late and very late coronary events, especially stent thrombosis [53]. Nevertheless, the benefit obtained on ischemic endpoints must be weighed against the risk of bleeding, since the use of more potent therapies leads to an inherent increase in bleeding complications. Of importance, bleeding has been shown to be independently associated with mortality after PCI [54–56].
Until recently, most registries that have focused on this specific question and included high-risk real-life patients, have suggested a potential benefit to pursue long-term DAPT at distance of the initial PCI with first generation DES, especially in regards to the risk of late and very late stent thrombosis [4,57,58]. These studies, however, suffer from the lack of randomization and inherent biases. By contrast, randomized trials that have included relatively few and low-risk patients did not report such a benefit [28–30]. These trials, however, suffer from the lack of power related to the low number of events observed. As a consequence, clear-cut conclusions were very difficult to draw from these data.
Within the last year, two large and powerful randomized trials tried to answer the specific question of the optimal duration of DAPT and have changed the landscape of antithrombotic management in stable CAD outpatients [34,59]. Conducted by Mauri et al. in 2014 [34], the DAPT trial has suggested that extended duration of dual antiplatelet could be beneficial in patients who have had no complication/event within the first year after PCI and who were at low risk of bleedings. In this study, 9961 event-free patients were randomized at 12 months after implantation of a DES to an additional 18 months of thienopyridine treatment (clopidogrel or prasugrel) or placebo (with continuation of aspirin in both groups). Both primary endpoints, stent thrombosis and major adverse cardiovascular and cerebral events were significantly reduced in the prolonged DAPT group. Rate of myocardial infarction was significantly reduced, and 45% of them were related to stent thrombosis. This remarkable benefit was, however, counterbalanced by a significant increase in bleedings. Similar results were observed in the PEGASUS-TIMI 54 trial [59], in which 21,162 patients with history of myocardial infarction were randomized to placebo or ticagrelor (60 mg or 90 mg bid). Either dose of ticagrelor as compared with placebo reduced significantly the primary endpoint of cardiovascular death, myocardial infarction or stroke by 15%. Significant increase in relevant bleeding was, however, also observed in this study. These two trials highlight the fragile balance between reducing ischemic events and increasing bleeding complications with extended antiplatelet therapies with hazardous effect on all-cause mortality.
Whatsoever, we have now strong evidences suggesting that prolonged DAPT will be beneficial in selected stable CAD patients at high risk of ischemic events and low risk of bleeding. As a consequence, we have a crucial need in current practice for useful and simple markers to better select these patients. In this context, procedural characteristics and especially lesion/stent length look crucial to help physicians for their therapeutic decisions. As mentioned above and according to the recent literature, lesion/stent length is strongly associated with patient’s outcomes. Indeed, a stent length greater than 30 mm appears to be highly associated with the risk of ischemic events. This subgroup of patients with diffuse atherosclerosis could then benefit from prolonged DAPT.
In the past, it has been shown that a stronger antithrombotic regimen (using cilostazol on top of DAPT as compared with DAPT alone) within the first year after PCI for a long lesion allows to improve patient’s outcome [21,60]. We recently analyzed in a retrospective study the determinants and prognosis of long-term DAPT in 460 event-free patients treated for a long coronary lesion (over 30 mm, mean 35.7 +/- 7.1 mm) [19]. Patients were divided in two groups, one group who stopped DAPT at one year (n = 168) and other group who prolonged antiplatelet therapy (n = 292). In this cohort, a high proportion (64%) of these selected patients were treated with prolonged DAPT over 1 year and this strategy significantly and independently reduced all-cause death (1.7 vs 12.5%, p = 0.0001) and cardiovascular death (1.7 vs 8.3%, p = 0.001). It is of note that inclusion and exclusion criteria led to a very specific population (with high risk of ischemic events and low risk of bleeding), so results presented must be taken with caution.
Conclusion
There is to date sufficient data to show us that a 1-year DAPT is not necessarily the best regimen for all CAD patients and that a tailored approach might be warranted. Some patients at high risk of ischemic events and/or low risk of bleeding may clearly benefit from extended DAPT. Patients with long coronary lesion/ stent have been individualized as a very specific and high-risk population and might be a perfect target population for such a strategy regarding their diffuse atherosclerosis and their high risk of complications related or not related to the stent.
Financial & competing interests disclosure
G Lemesle has received fees and honoraria from Astra Zeneca, Lilly and Daichi Sankyo as a speaker and advisory board member. G Schurtz has received fees and honoraria from Astra Zenaca as a speaker. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Windecker S, Kolh P, Alfonso F et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 35, 2541–2619 (2014).
- Montalescot G, Sechtem U, Achenbach S et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 34, 2949–3003 (2013).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369, 667–678 (2007).
- Iakovou I, Schmidt T, Bonizzoni E, Colombo A et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293, 2126–2130 (2005).
- D’Ascenzo F, Bollati M, Clementi F et al. Incidence and predictors of coronary stent thrombosis: evidence from an international collaborative meta-analysis including 30 studies, 221,066 patients, and 4276 thromboses. Int. J. Cardiol. 167, 575–584 (2013).
- Caputo RP, Goel A, Pencina M et al. Impact of drug eluting stent length on outcomes of percutaneous coronary intervention (from the EVENT registry). Am. J. Cardiol. 110, 350–355 (2012).
- Claessen BE, Smits PC, Kereiakes DJ et al. Impact of lesion length and vessel size on clinical outcomes after percutaneous coronary intervention with everolimus- versus paclitaxel-eluting stents pooled analysis from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice) Randomized Trials. JACC Cardiovasc. Interv. 4, 1209–1215 (2011).
- Dietz U, Holz N, Dauer C, Lambertz H. Shortening the stent length reduces restenosis with bare metal stents: matched pair comparison of short stenting and conventional stenting. Heart 92, 80–84 (2006).
- Fearon WF. Impact of drug-eluting stent length on outcomes less is more..more or less. JACC Cardiovasc. Interv. 3, 189–190 (2010).
- Foley DP, Pieper M, Wijns W et al. The influence of stent length on clinical and angiographic outcome in patients undergoing elective stenting for native coronary artery lesions; final results of the Magic 5L Study. Eur. Heart J. 22, 1585–1593 (2001).
- Kereiakes D, Linnemeier TJ, Baim DS et al. Usefulness of stent length in predicting in-stent restenosis (the MULTI-LINK stent trials). Am. J. Cardiol. 86, 336–341 (2000).
- Mauri L, O’Malley AJ, Cutlip DE et al. Effects of stent length and lesion length on coronary restenosis. Am. J. Cardiol. 93, 1340–1346, A5 (2004).
- Mauri L, O’Malley AJ, Popma JJ et al. Comparison of thrombosis and restenosis risk from stent length of sirolimus-eluting stents versus bare metal stents. Am. J. Cardiol. 95, 1140–1145 (2005).
- Suh J, Park DW, Lee JY et al. The relationship and threshold of stent length with regard to risk of stent thrombosis after drug-eluting stent implantation. JACC Cardiovasc. Interv. 3, 383–389 (2010).
- Wong SC, Hong MK, Ellis SG et al. Influence of stent length to lesion length ratio on angiographic and clinical outcomes after implantation of bare metal and drug-eluting stents (the TAXUS-IV Study). Am. J. Cardiol. 95, 1043–1048 (2005).
- Shirai S, Kimura T, Nobuyoshi M et al. Impact of multiple and long sirolimus-eluting stent implantation on 3-year clinical outcomes in the j-Cypher Registry. JACC Cardiovasc. Interv. 3, 180–188 (2010).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 52, 1134–1140 (2008).
- Kim YH, Park SW, Lee SW et al. Sirolimus-eluting stent versus paclitaxel-eluting stent for patients with long coronary artery disease. Circulation 114, 2148–2153 (2006).
- Manchuelle A, Delhaye C, Schurtz G et al. Dual antiplatelet therapy in patients with a long coronary artery lesion over 30 mm: determinants and impact on prognosis. Arch. Cardiovasc. Dis. 108, 235–243 (2015).
- Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N. Engl. J. Med. 356, 1009–1019 (2007).
- Lee SW, Lee JY, Ahn JM et al. Comparison of dual versus triple antiplatelet therapy after drug-eluting stent according to stent length (from the pooled analysis of DECLARE trials). Am. J. Cardiol. 112, 1738–1744 (2013).
- Park DW, Kim YH, Song HG et al. Comparison of everolimus- and sirolimus-eluting stents in patients with long coronary artery lesions: a randomized LONG-DES-III (Percutaneous Treatment of LONG Native Coronary Lesions With Drug-Eluting Stent-III) Trial. JACC Cardiovasc. Interv. 4, 1096–1103 (2011).
- Fokkema ML, James SK, Albertsson P et al. Population trends in percutaneous coronary intervention: 20-year results from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry). J. Am. Coll. Cardiol. 61, 1222–1230 (2013).
- Sianos G, Morel MA, Kappetein AP et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 1, 219–227 (2005).
- Valgimigli M, Serruys PW, Tsuchida K et al. Cyphering the complexity of coronary artery disease using the syntax score to predict clinical outcome in patients with three-vessel lumen obstruction undergoing percutaneous coronary intervention. Am. J. Cardiol. 99, 1072–1081 (2007).
- Su MI, Tsai CT, Yeh HI, Chen CY. The impact of SYNTAX score of non-infarct-related artery on long-term outcome among patients with acute ST segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. PLoS ONE 9, e109828 (2014).
- Yadav M, Palmerini T, Caixeta A et al. Prediction of coronary risk by SYNTAX and derived scores: synergy between percutaneous coronary intervention with Taxus and cardiac surgery. J. Am. Coll. Cardiol. 62, 1219–1230 (2013).
- Gwon HC, Hahn JY, Park KW et al. Six-month versus 12–month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 125, 505–513 (2012).
- Kim BK, Hong MK, Shin DH et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J. Am. Coll. Cardiol. 60, 1340–1348 (2012).
- Valgimigli M, Campo G, Monti M et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 125, 2015–2026 (2012).
- Lee SW, Park SW, Kim YH et al. A randomized, double-blind, multicenter comparison study of triple antiplatelet therapy with dual antiplatelet therapy to reduce restenosis after drug-eluting stent implantation in long coronary lesions: results from the DECLARE-LONG II (Drug-Eluting Stenting Followed by Cilostazol Treatment Reduces Late Restenosis in Patients with Long Coronary Lesions) trial. J. Am. Coll. Cardiol. 57, 1264–1270 (2011).
- Choi IJ, Koh YS, Lim S et al. Impact of the stent length on long-term clinical outcomes following newer-generation drug-eluting stent implantation. Am. J. Cardiol. 113, 457–464 (2014).
- Dilmanian H, Aronow WS, Mundia M et al. The average stent length is longer and the average stent diameter is shorter in patients with drug-eluting stents versus bare-metal stents during percutaneous coronary intervention. Am. J. Ther. 14, 277–279 (2007).
- Mauri L, Kereiakes DJ, Yeh RW et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 371, 2155–2166 (2014).
- Delsart P, Lemesle G, Lamblin N et al. Secondary medical prevention and clinical outcome in coronary artery disease patients with a history of non-coronary vascular intervention: a report from the CORONOR investigators. Eur. J. Prev. Cardiol. 22, 864–871 (2014).
- Gebel JM Jr. Secondary stroke prevention with antiplatelet therapy with emphasis on the cardiac patient: a neurologist’s view. J. Am. Coll. Cardiol. 46, 752–755 (2005).
- Airoldi F, Colombo A, Morici N et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 116, 745–754 (2007).
- Lemesle G, Delhaye C, Sudre A et al. Impact of high loading and maintenance dose of clopidogrel within the first 15 days after percutaneous coronary intervention on patient outcome. Am. Heart J. 157, 375–382 (2009).
- Cutlip DE, Baim DS, Ho KK et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation 103, 1967–1971 (2001).
- Serruys PW, Daemen J, Morice MC et al. Three-year follow-up of the ARTS-II – sirolimus-eluting stents for the treatment of patients with multivessel coronary artery disease. EuroIntervention 3, 450–459 (2008).
- Chieffo A, Bonizzoni E, Orlic D et al. Intraprocedural stent thrombosis during implantation of sirolimus-eluting stents. Circulation 109, 2732–2736 (2004).
- Cheneau E, Leborgne L, Mintz GS et al. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation 108, 43–47 (2003).
- Lemesle G, Delhaye C, Bonello L, de Labriolle A, Waksman R, Pichard A. Stent thrombosis in 2008: definition, predictors, prognosis and treatment. Arch. Cardiovasc. Dis. 101, 769–777 (2008).
- Honda Y, Muramatsu T, Ito Y et al. Impact of ultra-long second-generation drug-eluting stent implantation. Catheter Cardiovasc. Interv. doi: 10.1002/ccd.26010. (2015)(Epubahead of print).
- Stone GW, Maehara A, Lansky AJ et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 364, 226–235 (2011).
- Kim EK, Song PS, Yang JH et al. Peripheral artery disease in korean patients undergoing percutaneous coronary intervention: prevalence and association with coronary artery disease severity. J. Korean Med. Sci. 28, 87–92 (2013).
- Moussa ID, Jaff MR, Mehran R et al. Prevalence and prediction of previously unrecognized peripheral arterial disease in patients with coronary artery disease: the Peripheral Arterial Disease in Interventional Patients Study. Catheter Cardiovasc. Interv. 73, 719–724 (2009).
- Khoury Z, Schwartz R, Gottlieb S, Chenzbraun A, Stern S, Keren A. Relation of coronary artery disease to atherosclerotic disease in the aorta, carotid, and femoral arteries evaluated by ultrasound. Am. J. Cardiol. 80, 1429–1433 (1997).
- Head SJ, Davierwala PM, Serruys PW et al. Coronary artery bypass grafting vs percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur. Heart J. 35, 2821–2830 (2014).
- Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation 115, 1440–1455; discussion 1455 (2007).
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48, 193–202 (2006).
- Lemesle G, Maluenda G, Collins SD, Waksman R. Drug-eluting stents: issues of late stent thrombosis. Cardiol. Clin. 28, 97–105 (2010).
- Lemesle G, Lamblin N, Meurice T et al. Dual antiplatelet therapy in patients with stable coronary artery disease in modern practice: prevalence, correlates, and impact on prognosis (from the Suivi d’une cohorte de patients COROnariens stables en region NORd-Pas-de-Calais study). Am. Heart J. 168, 479–486 (2014).
- Koskinas KC, Raber L, Zanchin T et al. Clinical impact of gastrointestinal bleeding in patients undergoing percutaneous coronary interventions. Circ Cardiovasc. Interv. 8(5), pii: e002053 (2015) (Epub ahead of print).
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 114, 774–782 (2006).
- Mehran R, Pocock S, Nikolsky E et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc. Interv. 4, 654–664 (2011).
- Eisenstein EL, Anstrom KJ, Kong DF et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA 297, 159–168 (2007).
- Lemesle G, Paparoni F, Delhaye C, Bonello L, Lablanche JM. Duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stent implantation: a review of the current guidelines and literature. Hosp. Pract. (1995) 39, 32–40 (2011).
- Bonaca MP, Bhatt DL, Cohen M et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 372, 1791–1800 (2015).
- Lee SW, Park SW, Kim YH et al. Comparison of triple versus dual antiplatelet therapy after drug-eluting stent implantation (from the DECLARE-Long trial). Am. J. Cardiol. 100, 1103–1108 (2007).