Commentary - Interventional Cardiology (2013) Volume 5, Issue 2
Stent retrievers: the future treatment of choice for endovascular recanalization in acute ischemic stroke
- Corresponding Author:
- Raul G Nogueira
Marcus Stroke and Neuroscience Center Grady Memorial Hospital
Emory University School of Medicine
Emory Faculty Office Building
80 Jesse Hill Drive SE Room 398
Atlanta, GA 30303, USA
Tel: +1 404 616 4013
Fax: +1 404 659 0849
E-mail: rnoguei@emory.edu
Abstract
Keywords
stent retriever, stroke, thrombectomy
Every 40 s, someone in the USA suffers a stroke. Stroke is the leading cause of serious long-term disability and the fourth leading cause of death in the USA. More than 140,000 people die each year from stroke in the USA [1]. At present, the goal in treating acute ischemic stroke (AIS) is to recanalize the occluded artery to assure regional reperfusion and to rescue the ‘ischemic penumbra’ (the term generally used to define ischemic but still viable cerebral tissue). The administration of intravenous recombinant tissue plasminogen activator effectively improves functional outcome after an acute stroke [2]. However, intravenous thrombolysis is safest and most effective within a period of fewer than 3–4.5 h [3], has multiple contraindications (such as recent bleed or surgery) and poor recanalization rates (may be as low as 6–10% in proximal major artery occlusion) [4]. In view of these limitations, researchers have been looking for better techniques to achieve recanalization of major cerebral arteries and, more importantly, reperfusion of salvageable ischemic brain tissue. This article brief ly summarizes the different advancements in the field of interventional stroke that have recently led to the development of the retrievable stents or ‘stentrievers’ for AIS therapy.
Intra-arterial thrombolysis
Direct infusions of a fibrinolytic agent within the thrombus allow a smaller dose of the drug to reach higher local concentrations. This, in theory, leads to a better rate of recanalization with a reduced risk of symptomatic intracranial hemorrhage (sICH) and systemic complications. Intra-arterial thrombolysis (IAT) has been validated based on the PROACT and PROACT II trials [5,6]. The PROACT II trial tested the safety and efficacy of intra-arterial prourokinase plus heparin versus heparin alone, in patients presenting within 6 h of middle cerebral artery occlusion. Even though sICH was higher in the IAT group, partial or complete recanalization and good functional outcome at 90 days were significantly higher in this group. The US FDA required a second confirmatory study in order to approve IAT as a standard therapy in AIS. However, for multiple reasons, including the development of the potentially safer and more effective clot retrieval devices, such a study never substantialized.
Mechanical thrombectomy as a stroke therapy
Early uncontrolled trials showed better recanalization rates with mechanical thrombectomy devices compared with IAT [7–9]. As a result, the FDA cleared the Merci retrieval device (Concentric Medical, CA, USA), a corkscrew-like Nitinol device, in 2004, followed by the Penumbra device (Penumbra Inc., CA, USA), a combination of a separator wire and an aspiration catheter, in 2008. Studies with the Merci device demonstrated a recanalization rate of up to 70%, a 32% rate of good functional outcome at 90 days, a 7.3% sICH rate and an overall 35.2% mortality rate [8]. Use of the Penumbra system demonstrated an 82% recanalization rate, 25% rate of good functional outcome at 90 days and a 10% rate of sICH. There are currently no published randomized clinical trials to assess the clinical efficacy of mechanical thrombectomy in comparison with systemic thrombolysis within 4.5 h or with the standard-of-care beyond a 4.5-h window.
Intracranial angioplasty & stenting for mechanical thrombectomy in AIS
Since 2006, multiple small case series have reported angioplasty, balloon-mounted stents and self-expanding stents as possible interventions for AIS [10]. A pooled analysis of these data shows that angioplasty with or without stenting can be achieved within a reasonable time and often yields high rates of recanalization. However, intracranial stenting requires dual antiplatelet therapy, which carries an additional risk of sICH and other hemorrhagic complications, particularly if administered in conjunction with thrombolytics. Furthermore, intracranial stents carry the long-term risk of in-stent restenosis. These limitations have led to the development of retrievable stents, stentrievers.
Stentrievers
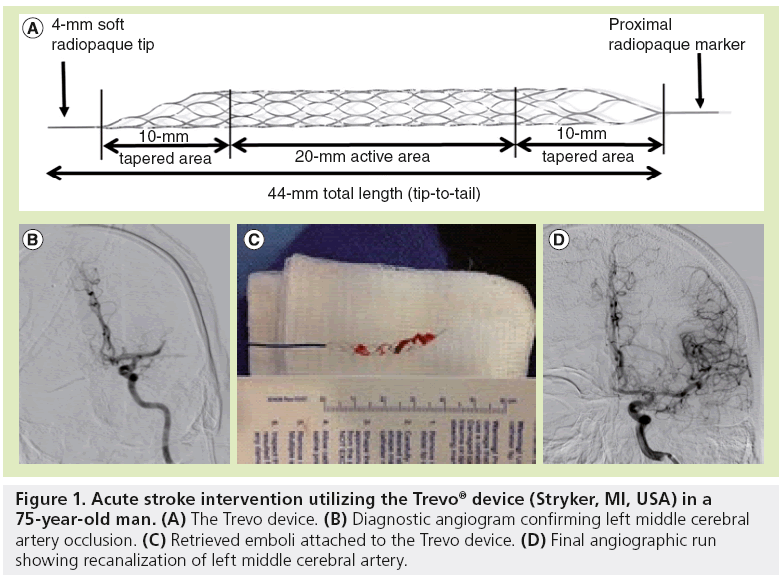
Stentrievers are Nitinol devices with a cylindrical form and cell-like struts. The device is placed across the thrombus, which upon deployment promotes a radial displacement of the clot, resulting in some degree of immediate reperfusion in 80–90% of cases. Flow restoration may rapidly re-establish oxygen supply in the ischemic brain region and enhance the delivery and efficacy of thrombolytic drugs. Stentrievers also allow thrombectomy to be performed by retracting the deployed stent into the guide catheter, whereby the struts of the stent engage the thrombotic material. The advantage of this system compared with the permanent intracranial stenting is that stentrievers require no anticoagulation or antiplatelet therapy as the stent is not deployed permanently. Moreover, the thrombotic material is removed as opposed to simply ‘trapped’ against the blood vessel wall. Also, stentrievers can be resheathed and used repeatedly to treat smaller distal branches (Figure 1). In short, stentrievers offer the advantage of immediate flow restoration while maintaining the ability of thrombus extraction along with eliminating the long-term risk of permanent intracranial stenting. Recently, the FDA granted clearance to both the Solitaire™ flow restoration stentriever device (ev3/Covidien Vascular Therapies, CA, USA) and the Trevo® Pro Retrieval System (Stryker, MI, USA) under 510(k) clearance, making these devices the first stentrievers to be cleared in the USA for recanalization of cerebral vessels in patients with AIS. The decision for clearance of these two devices was based on the results of two randomized trials demonstrating that the two new clot retrieval devices are more effective than their predecessor, the Merci retriever, in the setting of AIS [11,12]. In the SWIFT trial, the Solitaire flow restoration device was compared with Merci in 113 patients [11]; in TREVO 2, the Trevo device was compared with Merci in 178 patients [12]. Both trials enrolled patients within 8 h of symptom onset who had large intracranial artery occlusion and had a contraindication to, or had not responded to, intravenous tissue plasminogen activator. In the SWIFT trial, the primary end point of recanalization (defined as a thrombolysis in myocardial ischemia scale 2 or 3 flow without sICH) was more common with Solitaire (odds ratio: 4.87; 95% CI: 2.14–11.10). In TREVO 2, the primary end point of reperfusion (a thrombolysis in cerebral infarction grade of 2 or more) was more common with Trevo (odds ratio: 4.22; 95% CI: 2.01–08.86). The safety end points and the secondary clinical outcomes end points also supported a benefit of stentrievers as compared to the Merci retriever. Even with small sample sizes, the SWIFT and TREVO 2 trials, begin to answer the call for a high level of evidence about the safety and effectiveness of endovascular therapy in AIS. The stentriever technology is a major step forward in the successful treatment of AIS, and paves the way for new treatment strategies.
Figure 1: Acute stroke intervention utilizing the Trevo® device (Stryker, MI, USA) in a 75-year-old man. (A) The Trevo device. (B) Diagnostic angiogram confirming left middle cerebral artery occlusion. (C) Retrieved emboli attached to the Trevo device. (D) Final angiographic run showing recanalization of left middle cerebral artery.
Conclusion
Remarkable advances in the endovascular therapy of AIS lend great optimism to ongoing research efforts. Although much work is needed to prospectively validate the results of stentrievers for revascularization of acute cerebrovascular occlusion, the ongoing momentum in this field promises the possibility of continued progress.
Financial and competing interests disclosure
RG Nogueira and R Gupta are consultants for Stryker Neurovascular and Covidien, which are manufactures of stent-retriever devices. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Roger VL, Go AS, Lloyd-Jones DM et al. Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. Circulation 123(4), e18–e209 (2011).
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 333, 1581–1588 (1995).
- Hacke W, Kaste M, Bluhmki E et al.; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 359, 1317–1329 (2008).
- Saqqur M, Uchino K, Demchuk AM et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke 38, 948–954 (2007).
- del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a Phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke 29, 4–11 (1998).
- Furlan A, Higashida R, Wechsler L et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA 282, 2003–2011 (1999).
- Smith WS, Sung G, Starkman S et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 36(7), 1432–1438 (2005).
- Smith WS, Sung G, Saver J et al. Mechanical thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Stroke 39(4), 1205–1212 (2008).
- Penumbra Pivotal Stroke Trial Investigators. The Penumbra Pivotal Stroke Trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 40, 2761–2768 (2009).
- Gupta R, Tayal AH, Levy EI et al. Intra-arterial thrombolysis or stent placement during endovascular treatment for acute ischemic stroke leads to the highest recanalization rate: results of a multicenter retrospective study. Neurosurgery 68, 1618–1623 (2011).
- Saver JL, Jahan R, Levy EI et al.; SWIFT Trialists. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 380(9849), 1241–1249 (2012).
- Nogueira RG, Lutsep HL, Gupta R et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 380(9849), 1231–1240 (2012).