Review Article - Interventional Cardiology (2011) Volume 3, Issue 3
Stroke outcomes in patients undergoing percutaneous coronary intervention in clinical practice today
- Corresponding Author:
- Uwe Zeymer
The Institut für Herzinfarktforschung Ludwigshafen an der Universität Heidelberg
Ludwigshafen, Germany
Tel: +49 621 503 4045
Fax: +49 621 503 4002
E-mail: uwe.zeymer@t-online.de
Abstract
Keywords
complication, outcome, PCI, stroke
Since cardiac catheterization and percutaneous coronary intervention (PCI) have long been established as a cornerstone of diagnosis and treatment for coronary artery disease, their use has increased dramatically over the last 30 years worldwide. For instance, over 2 million cardiac catheterization procedures are performed in the USA alone every year [1]. By pharmacological and technical improvement, cardiac catheterization and PCI are considered to be safe procedures with major adverse cardiac and cerebrovascular events (MACCE) appearing in less than 1% of all diagnostic cardiac catheterization procedures [2] and in approximately 2.5% of all PCIs to date [2–6]. Nevertheless, PCI is an invasive procedure causing mechanical stress to the arterial vascular system, which is thought to be one major cause for cerebral embolism and stroke in this setting [7–10]. Therefore, periprocedural complications are still a major concern in a continually aging population, carrying a higher risk for complications during PCI. Previously, high-risk patients with severe comorbidities had been excluded from cardiac catheterization and PCI, whereas now these patients are part of the routine population in clinical practice undergoing PCI today. In this context, periprocedural complications such as stroke affect thousands of patients worldwide every year.
Incidence
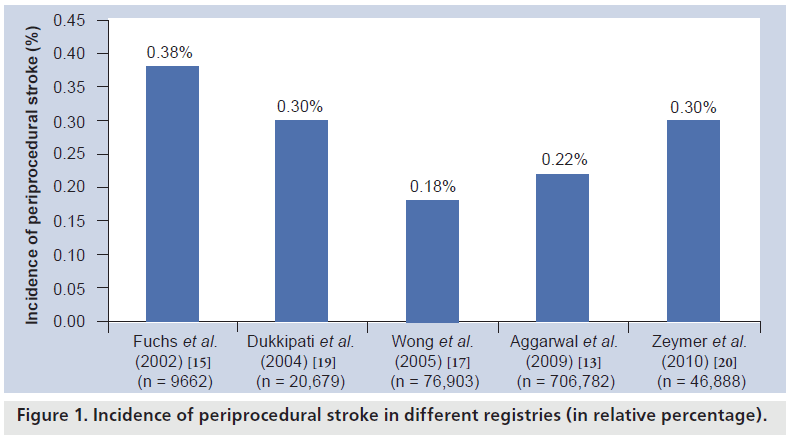
When cardiac catheterization began to be adopted in the 1970s and 1980s, the incidence of stroke after catheterization alone was reported to be in the range of 0.03–0.06% [11,12]. After the introduction of PCI, an increase in the incidence of stroke could be observed over the years, which is mostly due to an extended use of coronary angiography and PCI also in highrisk patients with severe vascular calcification and severe comorbidities, who had previously been excluded from heart catheterizations [13–17]. Today, stroke is still a rare complication after PCI. Large registries, reflecting a ‘real-world’ situation in clinical routine, have reported stroke to occur in 0.18–0.44% in an unselected population undergoing PCI today [15,17–19]. Our own group recently analyzed actual data from the PCI Registry of the Euro Heart Survey including 46,888 patients undergoing PCI at 176 centers in 33 ESC countries from 2005 until 2008 [20]. Today, the overall stroke rate of 0.3% in an unselected population undergoing PCI in Europe is in good accordance with previous reports by other registries. This reflects an almost constant rate of stroke after PCI over recent years in interventional cardiology, despite the improvement in pharmacological and technical issues, which is most likely due to the immutability of several risk factors for periprocedural stroke prior to PCI [14]. On the other hand, PCI is increasingly used to treat coronary artery disease even in octo- and nonagenerians today, carrying a higher risk for peri-interventional complication, in general (Figure 1).
Figure 1: Incidence of periprocedural stroke in different registries (in relative percentage).
Risk factors
Several risk factors have been retrospectively associated with an increased risk for periprocedural stroke in large registries. The higher the patient’s atherosclerotic and vascular risk, the higher the patient’s risk for stroke during cardiac catheterization and PCI, in general. Consecutively, vascular risk factors such as arterial hypertension, diabetes mellitus and an advanced age have been clearly associated with a higher stroke risk during PCI [13,19,17]. The risk for stroke is also significantly increased in PCI, if performed under emergency conditions for an acute coronary syndrome, which usually accompanies longer fluoroscopy times and more use of contrast, compared with diagnostic cardiac catheterization procedures only [13–15,18,19]. This is most likely caused by a heavier vascular calcification and a possibly less careful guiding of catheters through the aorta, under the pressure of time in patients presenting with an acute coronary syndrome, which has been demonstrated to be associated with an increased risk of cerebral embolization by scraping at aortic atherosclerotic plaques [10]. In addition, the possibility for hemodynamic compromise is increased in patients presenting with an acute coronary syndrome, which is known to consecutively increase the risk for intracardial thrombus formation, as well as the risk for stroke. Furthermore, a history of stroke or TIA, renal failure, the use of an intraaortic balloon pump, congestive heart failure and interventions during bypass grafts, have also been shown to be associated with stroke complicating PCI today [13,15,17,19].
Pathophysiology
In the vast majority of all periprocedural cerebrovascular accidents, stroke occurs during or within the first 24 h after PCI [19,21] and cerebral microembolism is considered to be the major cause for periprocedural strokes. This is supported by transcranial Doppler and MRI studies evaluating the frequency of cerebral embolism during cardiac catheterization, which have shown cerebral microembolism to appear in 15–35% of all patients undergoing cardiac catheterization [8,21–23]. Fortunately, most of the patients undergoing cerebral embolism stay asymptomatic, which is most likely due to small emboli not involving essential cerebral territories. Interestingly, stroke after PCI seems to involve the anterior as well as the posterior circulation in equal proportions, whereas the majority of strokes usually affect the anterior circulation in the general stroke population [24]. Potential embolic sources are atherosclerotic plaques in the aortic arch during the advancement of catheters through the aorta, thrombus formation on catheter tips and an air embolism. Atherosclerotic plaques in the aortic arch are known to be an independent predictor for recurrent stroke [25]. Keeley et al. observed a dislodgement of atherosclerotic material in more than 50% of all patients undergoing PCI [10], which also supports the embolic genesis of many peri-interventional.
Embolic sources for stroke during PCI by far seem to outweigh other causes such as hemodynamic strokes due to high-grade carotid artery stenosis. Nevertheless, cerebral microembolism is not the only cause of stroke during PCI. Retrospective analysis demonstrated hemorrhagic strokes to occur in up to 46% of all periprocedural strokes [15], especially if PCI was performed for an acute coronary syndrome including an aggressive antithrombotic therapy [26]. Therefore, differentiation between ischemic and hemorrhagic strokes by CT or MRI scan prior to treatment is of paramount importance for the clinical outcome of the patient.
Clinical outcome
From a patient’s point of view, stroke is one of the most devastating complications that can occur after PCI. Several retrospective analyses of large PCI registries have demonstrated a strong association between periprocedural stroke and high mortality and morbidity rates [13,15,17–19]. Mortality rates range from 22 to 37% in large registries. An actual analysis of the PCI Registry of the Euro Heart Survey showed a mortality rate of 12.5% in patients with stroke undergoing an elective PCI, in contrast to 24.1% if PCI was performed for an acute coronary syndrome [20]. Despite its rare occurrence, stroke is still responsible for a notable proportion of PCI-related in-hospital death [15], consecutively having an enormous impact on the patient’s prognosis and on quality of life. Dukkipati et al. reported that 72% of all patients with a cerebrovascular accident after PCI had persistent neurological deficit at the time of hospital discharge [19]. A total of 26% of patients needed to be discharged with skilled home care, 22% to a rehabilitation program and 9% were dependent on a nursing home or assisted living [19]. Most of all patients suffering from stroke after PCI demonstrated motor or speech deficits, as well as mental status changes, followed by vision disorders, facial nerve paresis or unresponsiveness.
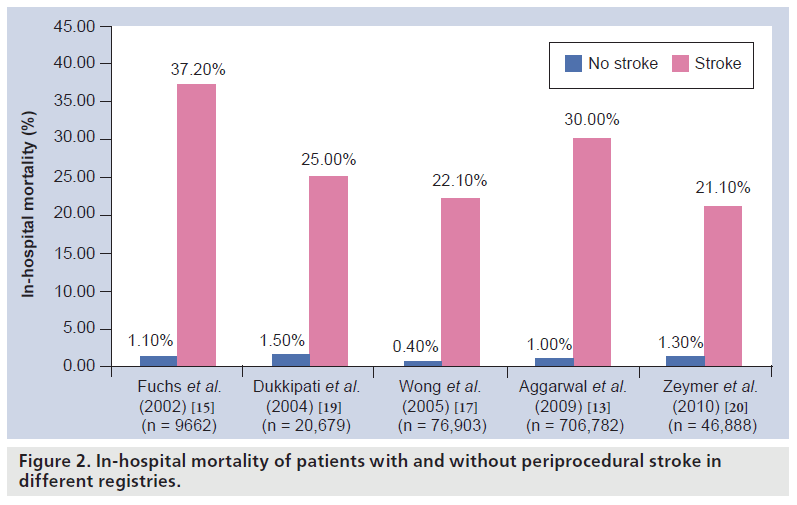
Besides its huge impact on a patient’s clinical outcome, periprocedural stroke has also been demonstrated to have a notable impact on economic issues by extending the patient’s postprocedural hospital stay by several days and expanding the cost (Figure 2) [15,16] .
Figure 2: In-hospital mortality of patients with and without periprocedural stroke in different registries.
Treatment options
Stroke is still a fatal complication after PCI, carrying a high morbidity and mortality rate. Unfortunately, most risk factors for periprocedural stroke are not modifiable prior to the intervention (for instance, advanced age, gender, history of stroke and acute coronary syndrome). Therefore, an improvement of the prognosis of patients developing this complication will mostly depend on an improvement in therapeutic options for cerebrovascular accidents. The low incidence of complications has so far precluded the development of an evidence- based treatment and therefore guidelines for a single therapeutic approach do not exist to date. No randomized trials exist regarding therapeutic options and the management of periprocedural stroke. Therefore, all available recommendations on this topic are generally based on expert opinions and small case series only [26–29].
The situation in a catheterization laboratory is unique, because arterial access to the patient is already present and a local thrombolytic therapy can be initiated urgently, if an ischemic stroke occurs during PCI. Nevertheless, thrombolytic therapy is only temporarily used in a minority of patients in clinical practice [29], due to a huge uncertainty regarding the efficacy and safety of this treatment in the setting of PCI. In fact, a systemic thrombolytic therapy for stroke using a plasminogen activator is formally contraindicated in the majority of PCIs. This is due to the use of standard heparin and antithrombotic therapy during most PCIs today, which are known to increase the risk for intracranial bleeding during thrombolysis for stroke [29]. Initial concerns about limited effectiveness of thrombolysis in this special setting, owing to a predominant embolization of solid and heavily calcified particles from atherosclerotic plaques, which cannot be dissolved by thrombolytic therapies, were not affirmed by previously published case reports and two retrospective analyses. These two studies found most of the patients with peri-interventional stroke treated with intra-arterial thrombolysis to have better outcomes (using the NIH Stroke Scale [NIHSS]) compared with patients treated with an established and conservative treatment for stroke, with relatively low rates of intracranial bleeding [30,31]. This is also supported by small case reports using immediate cerebral angiography, intra-arterial thrombolysis and mechanical re-canalization of the occluded vessel in case of an ischemic stroke [27,28,32]. In these case reports, recanalization rates averaged 50%, carrying an additional risk of intracranial bleeding between 14 and 25% and a mortality rate of 8–19% [26]. Although results from randomized trials are missing, there is hope that intra-arterial thrombolysis could be relatively safe in the special setting of PCI. The PROACT II and FAST study, using either heparin or the glycoprotein IIb/IIIa antagonist abciximab during intra-arterial thrombolysis, implicate safety if intra-arterial thrombolytic therapy is performed in low-dose heparinized or glycoprotein IIb/IIIa antagonist-treated patients [33,34]. Nevertheless, a strong association between a higher dosage of heparin and an increase in intracranial bleeding rate was reported in PROACT I, which was the first randomized trial evaluating the safety and clinical efficacy of thrombolysis in patients with symptomatic MCA occlusion [35]. In this trial, the reduction in heparin dosage from 100 IU/kg bolus + 1000 IU/h every 4 h initially to 2000 IU bolus + 500 IU/h every 4 h demonstrated a decrease in the rate of symptomatic intracranial bleeding from 27.3 to 6.7% during thrombolysis. Consequently, the heparin dosage in PROACT II did not exceed an amount of 4000 IU, which led to a more acceptable intracranial bleeding rate of 10% [34].
Nevertheless, no randomized clinical trials exist to date evaluating the rate of intracranial hemorrhage during thrombolysis in patients treated with the combination of heparin, aspirin and P2Y12- ADP-receptor inhibitors (e.g., clopidogrel), as is common during PCI today.
In this special setting, it will also be of great interest to define safety of thrombolysis under new antihrombotic drugs such as prasugel and ticagrelor, which have been demonstrated to increase the risk for bleeding complications during PCI compared with clopidogrel.
For the ability to perform in heparinized patients and for superior recanalization rates, intra-arterial thrombolysis is considered to be the preferred thrombolytic approach compared with systemic thrombolysis if performed in this special setting [36]. Nevertheless, for high morbidity and mortality rates associated with stroke during PCI, systemic thrombolytic therapy can also be taken into account in patients who are not amenable for an immediate local thrombolytic approach after an individual risk assessment.
Data are available for the safety of mechanical embolectomy in case of large cerebral vessel occlusion in patients with abnormal hemostasis. Nogueira et al. reported that patients with significant abnormal hemostasis (INR >1.7, PTT >45 s or platelet count <100,000/μl) undergoing mechanical embolectomy were not at higher risk for intracranial hemorrhage, with equal mortality rates compared with patients with normal hemostasis [37]. They also observed a strong association between successful recanalization of the occluded vessel and a good outcome of a patient with abnormal hemostasis undergoing mechanical embolectomy, which emphasizes the need for aggressive local reperfusion therapy in patients who are not amenable to intravenous thrombolysis [37]. This data suggests mechanical recanalization of an occluded vessel to be another important therapeutic option and possibly relatively safe in the special setting of PCI. However, no data regarding mechanical recanalization in the setting of PCI are available as yet.
Hamon et al. proposed an algorithm for peri- and postprocedural stroke in 2008 from the available and limited data [26]. In their algorithm, immediate cerebral angiography should be performed if stroke appears during the catherization procedure and an experienced angiographer is available. In case of an acute arterial occlusion of a large vessel in the cerebral angiography, Hamon et al. proposed intra-arterial thrombolysis even without a prior cerebral CT scan to save time [26]. This topic is still under debate. Owing to relatively high rates of intracranial hemorrhage in the special setting of stroke complicating PCI (even if stroke is primarily caused by an embolic vessel occlusion), it seems to be a more safe and reasonable approach to exclude intracranial bleeding by CT scan prior to intra-arterial thrombolysis in our opinion [31].
If stroke occurs after the catheterization procedure and in all other cases (no experienced cerebral angiographer available, or in doubt of an arterial occlusion in cerebral angiography), brain imaging should be performed urgently to exclude intracranial hemorrhage according to the algorithm of Hamon et al. In case of a significant mismatch in perfusion/diffusion-weighted imaging or in CT perfusion, intra-arterial thrombolysis should also be considered [26].
Since this algorithm is based only on the available data, consisting of case reports and small retrospective analyses, it should be interpreted with caution. Updates are necessary, if further results from randomized clinical trials or registries regarding this topic are available. In the meantime, a practical approach to manage periprocedural stroke is required. Further investigation is necessary to evaluate the efficacy and safety of systemic or intra-arterial thrombolysis and mechanical embolectomy in the special setting of stroke after PCI.
Conclusion
Despite its rare occurrence, stroke is still one major complication after PCI having an enormous impact on a patient’s prognosis and quality of life. Most risk factors identified for periprocedural stroke are not modifiable prior to PCI. Therefore an improvement of the prognosis of patients developing this complication, will mainly be possible by improving therapeutical options. Intra-arterial thrombolysis seems to be a treatment option for some patients in this special setting after an individual risk assessment has been performed. Further studies and randomized clinical trials are necessary to affirm safety and efficacy of this treatment.
Future perspective
In our opinion, in the coming years, a patient’s prognosis after stroke complicating PCI will mainly depend on an improvement in therapeutic options such as intra-arterial thrombolysis or mechanical recanalization of the occluded vessel. To reduce morbidity and mortality of this important complication of PCI, it will be of paramount importance to develop therapeutic guidelines on how to manage periprocedural stroke in clinical practice. To date, no evidencebased data on therapeutic management in this special setting exists and therefore all decisions in treatment of this complication are left to the attending physician alone.
Owing to its rare occurrence, it will be challenging to perform randomized clinical trials on this topic in the future.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
▪ Although cardiac catheterization and percutaneous coronary intervention (PCI) are considered to be safe procedures today, periprocedural complications are still a major concern in a continually aging population, carrying a higher risk for complications during PCI.
Incidence
▪ Stroke is a rare but serious complication of PCI, which is reported to appear in 0.18–0.44% of all PCIs performed in an unselected population in clinical practice today. Despite pharmacological and technical improvements, the incidence of periprocedural stroke has remained constant over recent years in interventional cardiology.
Risk factors
▪ The higher the patient’s atherosclerotic and vascular risk, the higher the patient’s risk for stroke during cardiac catheterization and PCI.
▪ Risk factors for stroke complicating PCI include: arterial hypertension, diabetes mellitus, advanced age, PCI performed under emergency conditions, history of stroke or TIA, renal failure, use of an intra-aortic balloon pump, congestive heart failure and interventions at bypass grafts.
Pathophysiology
▪ Cerebral microembolism and intracranial hemorrhage are considered to be the major causes for periprocedural strokes. Studies have demonstrated cerebral microembolism to occur in 15 –35% of all patients undergoing cardiac catheterization, who mostly remain asymptomatic.
Clinical outcome
▪ From a patient’s point of view, stroke is one of the most devastating complications during PCI, which is still responsible for a notable proportion of PCI-related in-hospital death.
▪ Large registries demonstrate its association with high mortality rates ranging from 22 to 37% in clinical practice today.
▪ 72% of all patients with a cerebrovascular accident after PCI had persistent neurological deficit at the time of hospital discharge.
Treatment options
▪ Owing to its low incidence, no randomized trials exist on this topic, which has so far precluded the development of an evidence‑based treatment.
▪ Intra-arterial thrombolysis and mechanical embolectomy appear to be promising and relatively safe approaches in the treatment of periprocedural ischemic stroke, but further research is needed to validate their efficacy and safety in the special setting of PCI.
Future perspective
▪ Most risk factors identified for periprocedural stroke are not modifiable prior to PCI, and an improvement of the prognosis of patients developing this complication will mainly be possible by improving therapeutic options in the future.
▪ It will be of paramount importance to develop therapeutic guidelines on how to manage periprocedural stroke in clinical practice to reduce morbidity and mortality of this important complication of PCI in the coming years.
References
- Khatri P, Kasner SE: Ischemic strokes after cardiac catheterization: opportune thrombolysis candidates? Arch. Neurol. 63, 817–821 (2006).
- Zeymer U, Zahn R, Hochadel M et al.: Incications and complications of invasive diagnostic procedures and percutaneous coronary interventions in the year 2003. Results of the quality control registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Z. Kardiol. 94, 392–398 (2005).
- Applegate RJ, Sacrinty MT, Kutcher MA et al.: Trends in vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention via the femoral artery, 1998 to 2007. JACC Cardiovasc. Interv. 1, 317–326 (2008).
- BQS Institut für Qualität und Patientensicherheit. Qualitätsreport: Koronarangiographie und perkutane Koronarintervention (PCI) (2008).
- Stathopoulos I, Jimenez M, Panagopoulos G et al.: The decline in PCI complication rate: 2003–2006 versus 1999–2002. Hellenic. J. Cardiol. 50, 379–387 (2009).
- Williams DO, Holubkov R, Yeh W et al.: Percutaneous coronary intervention in the current era compared with 1985–1986: the National Heart, Lung, and Blood Institute Registries. Circulation 102, 2945–2951 (2000).
- Bladin CF, Bingham L, Grigg L et al.: Transcranial Doppler detection of microemboli during percutaneous transluminal coronary angioplasty. Stroke 29, 2367–2370 (1998).
- Hamon M, Gomes S, Oppenheim C et al.: Cerebral microembolism during cardiac catheterization and risk of acute brain injury: a prospective diffusion-weighted magnetic resonance imaging study. Stroke 37, 2035–2038 (2006).
- Karalis DG, Quinn V, Victor MF et al.: Risk of catheter-related emboli in patients with atherosclerotic debris in the thoracic aorta. Am. Heart J. 131, 1149–1155 (1996).
- Keeley EC, Grines CL. Scraping of aortic debris by coronary guiding catheters: a prospective evaluation of 1,000 cases. J. Am. Coll. Cardiol. 32, 1861–1865 (1998).
- Bredlau CE, Roubin GS, Leimgruber PP et al.: In-hospital morbidity and mortality in patients undergoing elective coronary angioplasty. Circulation 72, 1044–1052 (1985).
- Dorros G, Cowley MJ, Simpson J et al.: Percutaneous transluminal coronary angioplasty: report of complications from the National Heart, Lung, and Blood Institute PTCA Registry. Circulation 67, 723–730 (1983).
- Aggarwal A, Dai D, Rumsfeld JS et al.: Incidence and predictors of stroke associated with percutaneous coronary intervention. Am. J. Cardiol. 104, 349–353 (2009).
- Brown DL, Topol EJ: Stroke complicating percutaneous coronary revascularization. Am. J. Cardiol. 72, 1207–1209 (1993).
- Fuchs S, Stabile E, Kinnaird TD et al.: Stroke complicating percutaneous coronary interventions: incidence, predictors, and prognostic implications. Circulation 106, 86–91 (2002).
- Weintraub WS, Mahoney EM, Ghazzal ZM et al.: Trends in outcome and costs of coronary intervention in the 1990s. Am. J. Cardiol. 88, 497–503 (2001).
- Wong SC, Minutello R, Hong MK: Neurological complications following percutaneous coronary interventions (a report from the 2000–2001 New York State Angioplasty Registry). Am. J. Cardiol. 96, 1248–1250 (2005).
- Cronin L, Mehta SR, Zhao F et al.: Stroke in relation to cardiac procedures in patients with non-ST-elevation acute coronary syndrome: a study involving >18 000 patients. Circulation 104, 269–274 (2001).
- Dukkipati S, O’Neill WW, Harjai KJ et al.: Characteristics of cerebrovascular accidents after percutaneous coronary interventions. J. Am. Coll. Cardiol. 43, 1161–1167 (2004).
- Zeymer U, Bauer T, Zahn R et al.: Stroke as complication of PCI is associated with a high in-hospital mortality. Results of the Euro Heart Survey PCI Registry. Presented at: ESC Congress 2010. Stockholm, Sweden, 28 August–1 September (2010)
- Busing KA, Schulte-Sasse C, Fluchter S et al.: Cerebral infarction: incidence and risk factors after diagnostic and interventional cardiac catheterization –prospective evaluation at diffusion-weighted MR imaging. Radiology 235, 177–183 (2005).
- Lund C, Nes RB, Ugelstad TP et al.: Cerebral emboli during left heart catheterization may cause acute brain injury. Eur. Heart J. 26, 1269–1275 (2005).
- Murai M, Hazui H, Sugie A et al.: Asymptomatic acute ischemic stroke after primary percutaneous coronary intervention in patients with acute coronary syndrome might be caused mainly by manipulating catheters or devices in the ascending aorta, regardless of the approach to the coronary artery. Circ. J. 72, 51–55 (2008).
- Segal AZ, Abernethy WB, Palacios IF et al.: Stroke as a complication of cardiac catheterization: risk factors and clinical features. Neurology 56, 975–977 (2001).
- Kronzon I, Tunick PA: Aortic atherosclerotic disease and stroke. Circulation 114, 63–75 (2006).
- Hamon M, Baron JC, Viader F et al.: Periprocedural stroke and cardiac catheterization. Circulation 118, 678–683 (2008).
- De Marco F, Fernandez-Diaz JA, Lefevre T et al.: Management of cerebrovascular accidents during cardiac catheterization: immediate cerebral angiography versus early neuroimaging strategy. Catheter Cardiovasc. Interv. 70, 560–568 (2007).
- Oezbek C, Heisel A, Voelk M et al.: Management of stroke complicating cardiac catheterization with recombinant tissue-type plasminogen activator. Am. J. Cardiol. 76, 733–735 (1995).
- Rother J, Laufs U, Bohm M et al.: Consensus paper on peri-interventional and postinterventional stroke during cardiac catheter procedures. Nervenarzt 80, 1205–1215 (2009).
- Arnold M, Fischer U, Schroth G et al.: Intra-arterial thrombolysis of acute iatrogenic intracranial arterial occlusion attributable to neuroendovascular procedures or coronary angiography. Stroke 39, 1491–1495 (2008).
- Khatri P, Taylor RA, Palumbo V et al.: The safety and efficacy of thrombolysis for strokes after cardiac catheterization. J. Am. Coll. Cardiol. 51, 906–911 (2008).
- Al-Mubarak N, Vitek JJ, Mousa I et al.: Immediate catheter-based neurovascular rescue for acute stroke complicating coronary procedures. Am. J. Cardiol. 90, 173–176 (2002).
- Eckert B, Koch C, Thomalla G et al.: Aggressive therapy with intravenous abciximab and intra-arterial rtPA and additional PTA/stenting improves clinical outcome in acute vertebrobasilar occlusion: combined local fibrinolysis and intravenous abciximab in acute vertebrobasilar stroke treatment (FAST): results of a multicenter study. Stroke 36, 1160–1165 (2005).
- Furlan A, Higashida R, Wechsler L et al.: Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 282, 2003–2011 (1999).
- del Zoppo GJ, Higashida RT, Furlan AJ et al.: PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke 29, 4–11 (1998).
- Mattle HP, Arnold M, Georgiadis D et al.: Comparison of intraarterial and intravenous thrombolysis for ischemic stroke with hyperdense middle cerebral artery sign. Stroke 39, 379–383 (2008).
- Nogueira RG, Smith WS: Safety and efficacy of endovascular thrombectomy in patients with abnormal hemostasis: pooled analysis of the MERCI and multi MERCI trials. Stroke 40, 516–522 (2009).