Research Article - International Journal of Clinical Rheumatology (2021) Volume 16, Issue 1
Strontium ranelate as new modality in treatment of primary knee osteoarthritis
- Corresponding Author:
- Reham M. Shaat
Department of Rheumatology and Rehabilitation
Faculty of Medicine, Mansoura University, Egypt
E-mail: rehamshaat@mans.edu.eg
Abstract
Background: Osteoarthritis is a degenerative disease that has an effect on the whole joint structure which includes the synovial layer, cartilage, subchondral bone, ligaments and muscle groups across the joint. Objective: to evaluate effectiveness of strontium ranelate in treatment of patients with primary knee osteoarthritis (KOA). Methods: This randomized clinical trial was done on 30 patients with bilateral Knee osteoarthritis, they were divided into 2 groups. Group 1: 15 patients received strontium ranelate 2 gram and physiotherapy programme. Group 2:15 patients received physiotherapy programme only. All patients were assessed using visual analogue scale for pain (VAS), Western Ontario and McMaster Universities osteoarthritis index (WOMAC) and knee MRI. Results: This study included 45 patients with Knee osteoarthritis, 15 patients were excluded based on exclusion criteria, only 30 patients were randomized into 2 groups, 1 patient in group 1 and 2 patients in group 2 didn't complete follow up duration, and statistical data were done only on 27 patients. At the beginning of the study no significant difference was found between both groups in all parameters. A significant improvement in VAS and WOMAC score was found in group 1(p=0.034,p=0.004) in comparison to group 2 after 6 months follow up. There was statistically significant improvement in group 1 in synovitis/effusion detected by MRI in comparison to group 2 after 6 months of treatment. Conclusion: Strontium ranelate (SrR) has great role in reducing pain, synovitis, improving function in primary knee osteoarthritis and could be considered a disease modifying osteoarthritis drug.
Keywords
posteoarthritis â strontium ranelate â physiotherapy â knee â MRI
Key points
• Strontium ranelate improves pain and function in knee OA patients.
• Strontium ranelate could be effective disease modifying osteoarthritis drug.
Introduction
Osteoarthritis (OA) is the most common cause of functional disability in old patients. It is described by cartilage degradation and loss, subchondral bone damages, synovial inflammation, as well as other joint structural changes and mainly affects hand, knee, hip and spine [1]. Medications of osteoarthritis include acetaminophen, Non-Steroidal Anti- Inflammatory Drugs (NSAIDs), tramadol and opioids which improve symptoms but can't decrease the need for surgical operation as little evidence showing that they could slow down the progress of the disease. Otherwise, there is still no approved effective Disease- Modifying Osteoarthritis Drugs (DMOADs) for OA treatment [2]. Experimental studies revealed that the microstructural deterioration of subchondral bone could increase cartilage damage and improvement of this microstructure would slow the increase of cartilage impairment. A prospective study found that the progress of cartilage damage could be predicted by subchondral bone abnormalities [3]. A systematic review suggested that Magnetic Resonance Imaging (MRI) detected Bone Marrow Lesions (BMLs) were associated with structural progression and pain of OA [4]. Since cartilage is not innervated, the main symptom, joint pain, may be contributed to other structures such as subchondral bone. Thus, subchondral bone integrity should be considered in the treatment of OA [5]. Strontium ranelate (SrR), a bone-acting agent, has the capacity to dissociate the bone remodeling process and has effective role in improving symptoms [6]. The aim of our study is to evaluate effectiveness of strontium ranelate in treatment of patients with primary knee osteoarthritis.
Methods
This is a randomized clinical trial conducted on thirty patients with primary knee osteoarthritis. Patients were collected from Rheumatology and Rehabilitation Outpatient Clinic at Mansoura University Hospital. Patients were divided into two groups:
Group 1: included 15 bilateral Knee osteoarthritis patients who received oral strontium ranelate and physiotherapy program.
Group 2: included 15 bilateral Knee osteoarthritis patients who received physiotherapy program. Written consent was taken from each participant before intervention. This study was approved by the institution research board of faculty of medicine, Mansoura University, code: MS/15.09.44
Inclusion criteria
All patients in this study were diagnosed according to American College of Rheumatology clinical and radiological classification criteria for primary knee osteoarthritis [7]. Patients aged ≥ 40 years with symptoms of knee osteoarthritis according to American College of Rheumatology. Kellegren and Lawrence (KL) radiological classification 2 or 3 grade.
Exclusion criteria
Patients having any systemic autoimmune disease like rheumatoid arthritis, spondyloarthropathy and gout, any trauma to the knee joint or orthopedic knee surgery. Recent intra-articular injection (especially glucocorticoids) less than three months before. Venous thromboembolism patients having chronic liver and kidney disease.
Data collection
Clinical data were collected from participants including demographic data, associated medical conditions such as diabetes mellitus, presence of any systemic autoimmune disease, gouty arthritis or pseudo gout, deep venous thrombosis, history of previous knee intraarticular steroid injection, previous trauma or surgery to knee joint.
Clinical examination
All patients were subjected to general systemic examination and musculoskeletal examination with stress on knee examination.
Radiological investigations
A) X ray:
Radiographs of the patients were obtained in both anteroposterior and lateral positions while patients were standing. Radiological findings were evaluated and graded by two clinicians according to the Kellgren– Lawrence (KL) radiological scale [8].
B) Magnetic Resonance Imaging:
Magnetic Resonance Imaging (MRI) was performed using 1.5 T units (Philips Ingenia). A standard knee coil (extremity coil) was used with the coil placed in the center of the magnet. The patient was lying supine with feet first. Routine examination of the knee was performed with a field of view 14 - 18 cm, slice thickness 3 - 4 mm, inter-slice gap about 1 mm in the axial, coronal and sagittal plane using the following parameter:
• For T1 WI, TR= 300-700 ms and TE= 10-30 ms.
• For T2 fast spin echo, TR= 250-3010 ms, TE= 100 and flip angle 120°.
• For Dual-DR-TSE, TR= 2500 ms, TE= 7 ms.
• For STIR, TR= 2000-2200, TE= 60, inversion time 150 ms.
WATSc was done for evaluation of the articular surface in sagittal and coronal planes using the following parameters, TR= 20 ms, TE= 7.9 ms, flip angle 2.5, slice thickness 2.5 mm and field of vision = 16 – 18 cm. Assessment of knee joint by using semi-quantitative MRI by (MOAKS) MRI Osteoarthritis knee score which is performed at 0, 6 months to assess changes during period of study [4].
In MOAKS the knee is partitioned into 14 articular sub regions for scoring articular cartilage and BMLs and what's more, the sub spinous area is included for BML scoring, Grading of size BML in each sub region develops according to the total volume of each sub region which is involved by BMLs.
• grade 0: absence of BML.
• grade 1: less than 33% of sub region volume.
• grade 2: between 33% and 66% of sub region volume.
• grade 3: more than 66% of sub region volume.
Hoffa synovitis is graded according to degree of hyperintensity in Hoffa’s fat pad into 3 grades: grade (0) normal, grade (1) mild, grade (2) moderate, grade (3) sever. Grading of synovitis/ effusion depends on measuring the amount of effusion. Grade 0: normal amount, grade 1: little amount (constant fluid in retro patellar space), grade 2: moderate amount (mild convexity of retro patellar space), grade 3: huge amount (distension of the capsule). Grading of size of articular cartilage loss in each sub region depends on the percentage of surface area of sub region has either partial or full thickness cartilage loss. All sub regions are included in this grading except sub spinous area.
• grade 0: no cartilage loss.
• grade 1: less than 10% of region of cartilage surface area.
• grade 2: ranged between 10% and 75% of region of cartilage surface area.
• grade 3: more than 75% of region of cartilage surface area .
Randomization
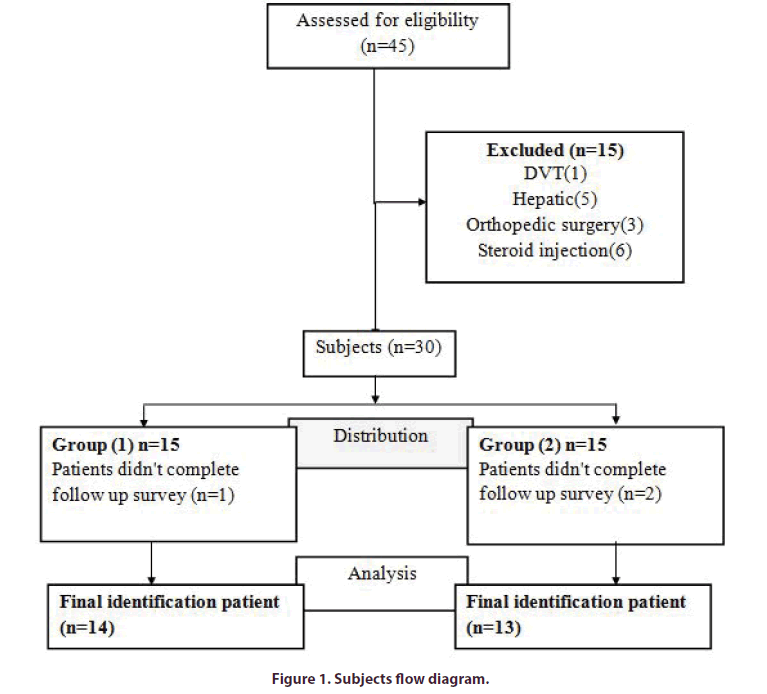
Patients were randomly allocated into two treatment groups, for assigning groups, pieces of paper were prepared in the same number of the patients. The name of treatment methods was written on the pieces of paper. Then patients were asked to take one paper and this is the treatment type for each person was specified (Figure 1).
Treatment protocol
The patients were randomly divided into 2 groups.
A) Group 1:
Patients took strontium ranelate 2gm (one sachet daily with 50 ml water at bedtime at least 2 h after food), the compliance of patient was assessed by counting the sachet number that patient returns at each visit and safety is determined by detecting side effects such as blood pressure and heart rate each visit and patients were subjected to physiotherapy program in the form of (US therapeutic acoustic radiation and exercise program) 2 times each week for a duration of 6 months.
Ultra sound (US): Pulsed ultrasonic waves were given at a 1 MHz frequency and at an intensity of 1 watt/ cm2 using a transducer with a diameter of 5 cm and an effective area of radiation of 3.5 to 5cm² for a period of 9.5 min (Medserve, England) [9].
Patients took a supine position with the treated knee flexed at 90° and the sound head was held stationary over the tibiofemoral joint medial to the patellar tendon to help to penetrate energy into the joint space [10].
Exercise program: Patients were subjected to a group of exercise program consisted of a range of motion of all lower limb and strengthening exercises for 45 min ,with 5 min stretching exercises of lower limb muscles, repeated 3 times each week [11].
B) Group 2:
Received the same physiotherapy program as group 1. For both groups, pain medication in the form of Paracetamol only is allowed for 6 months provided that patients stop it 48h before a visit to allow proper symptoms assessment. One investigator was responsible for assessing pain and function scores, another investigator experienced in radiology assess radiological score and other investigator was responsible for clinical assessment, all investigators were blind to type of treatment taken by patients.
Outcome measures
Primary outcome measures
Assessment of pain: Patients were assessed before treatment, at 3rd and 6th month after treatment using visual analogue scale (VAS). VAS pain is a unidimensional scale help to assess pain intensity. It is a 10 cm horizontal line marked every 1 cm. Pain intensity ranges from 0 (no pain, the left end of the line) to 10 (worst possible pain, the right end of the line) [12].
Assessment of pain, function and stiffness: Patients were assessed before treatment, at 3rd and 6th month after treatment using Western Ontario and McMaster Universities OA index (WOMAC). WOMAC is a self-administered composite questionnaire with three components to assess pain, knee stiffness and difficulty in the activity of daily living [13].
Secondary outcome measures
MRI assessment of Knee joint: Assessment of knee joint by using semi-quantitative MRI by (MOAKS) MRI Osteoarthritis knee score which is performed at 0, 6 months to assess changes during period of study [4].
Statistical analysis
Statistical analysis was done by using SPSS statistical package for social science version 16. The qualitative data was presented in the form of number and percentage.
Chi-square test was used as a test of significance for qualitative data. The quantitative data were presented in the form of mean and standard deviation. Student t test was used to compare between two groups. Parried t test was used to compare within the same group. Significance was considered when p value ≤ 0.05.
Results
At the beginning of this study we examined 45 patients with bilateral knee osteoarthritis, 15 patients were excluded and only 30 patients were included, and divided in two groups 15 in each group, during follow up period 1 patient was lost in group 1 and 2 patients in group 2 , only 27 patients completed follow up and their data were included in statistical analysis.
Table 1 showed age, gender, BMI and disease duration of studied groups. No significant differences were detected between group one and group two.
Table 1. Demographical and clinical characteristics in the studied groups.
| Group 1 n=14 | Group 2 n=13 | Test of significant | P value | |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD | 56 ± 4.88 | 57 ± 4.55 | t=0.77 | 0.44 |
| Range | (50-62) | (50-62) | ||
| Gender | ||||
| Female n, (%) | 10 (71.42%) | 10 (76.92%) | x2= zero | 0.99 |
| Male n, (%) | 4 (28.57%) | 3 (23.07%) | ||
| BMI | ||||
| Mean ± SD | 35.75 ± 5.8 | 38.35 ± 7.89 | t=1.39 | 0.17 |
| Range | (29.3-48) | (20.7-48) | ||
| Disease duration(months) | ||||
| Mean ± SD | 32.57 ± 13.3 | 32.2 ± 13.17 | t=0.18 | 0.85 |
| Range | (12-60) | (12-60) | ||
Data were displayed as mean, standard deviation, range and percentage
Student t test was used
X2, chi square test
BMI, Body mass index
Table 2 showed a non-significant difference in pain as measured by VAS between both groups before treatment and 3 months after treatment but showed the difference was significant at 6 months after treatment.
Table 2. VAS before and after treatment in the studied groups.
| VAS | Group 1 Mean ± SD | Group 2 Mean ± SD | Test of significance | P value |
|---|---|---|---|---|
| Before treatment | 6.28 ± 1.62 | 7 ± 1.57 | t=1.63 | 0.108 |
| 3 months after treatment | 6.14 ± 1.53 | 6.92 ± 1.57 | t=1.84 | 0.071 |
| 6 months after treatment | 5.39 ± 1.19 | 6.30 ± 1.84 | t=2.1 | 0.034* |
VAS: Visual analogue scale
Table 3 showed a non-significant difference in total WOMAC between both groups before and 3 months after treatment but showed the difference was significant at 6 months after treatment.
Table 3. Total WOMAC score before and after treatment in the studied groups.
| Total WOMAC | Group 1 mean ± SD | Group 2 mean ± SD | Test of significance | p value |
|---|---|---|---|---|
| Before treatment | 44.39 ± 12.4 | 50.15 ± 10.9 | t=1.87 | 0.077 |
| 3 months after treatment | 43.78 ± 11.65 | 49.8 ± 10.68 | t=1.98 | 0.052 |
| 6 months after treatment | 37.78 ± 7.7 | 45.73 ± 11.53 | t=2.96 | 0.004* |
Table 4 showed no significant difference in BML at patella between both groups before and after treatment. Regarding comparison in the same group, there was no significant difference in BML at patella before and after treatment in group 1and in group 2 (Supplementary Figure 1).
Table 4. MRI findings of BML at patella before and after treatment.
| Patella (BML) | Group 1 n=28 (%) | Group 2 n=26 (%) | ||
|---|---|---|---|---|
| Before treatment | score 0 | 20 (71.4) | 20 (76.9) | X2=7.31 |
| score 1 | 2 (7.1) | 6 (23.1) | P= 0.066 | |
| score 2 | 4 (14.3) | 0 (0) | X2=0.89 | |
| score 3 | 2 (7.1) | 0 (0) | p=0.61 | |
| After treatment | score 0 | 20 (71.4) | 22 (84.6) | X2= 5.34 |
| score 1 | 3(10.8) | 4 (15.4) | P= 0.148 | |
| score 2 | 4(14.3) | 0 (0) | X2=0.50 | |
| score 3 | 1 (3.5) | 0 (0) | p=0.48 | |
Data were presented as number and percentage
Chi-square with linear trends was used
BML: Bone Marrow Lesion
Table 5 showed no significant difference in BML score at femur between two groups before and after treatment. Regarding comparison in the same group, there was no significant difference in BML at femur before and after treatment in group 1 and in group 2 (Supplementary Figure 2).
Table 5. MRI finding of BML at femur before and after treatment.
| Femur(BML) | Group 1 n=28 (%) | Group 2 n=26 (%) | ||
|---|---|---|---|---|
| Before treatment | score 0 | 18 (64.3) | 18 (69.2) | X2= 5.30 |
| score 1 | 4 (14.3) | 2 (7.7) | P= 0.151 | |
| score 2 | 4 (14.3) | 6 (23.1) | X2=4.92 | |
| score 3 | 2 (7.1) | 0 (0) | p=0.17 | |
| After treatment | score 0 | 21 (70.4) | 18 (75) | X2= 1.16 |
| score 1 | 6(25.9) | 6 (25) | P= 0.56 | |
| score 2 | 1(3.5) | 0 (0) | X2=5.6 | |
| score 3 | 0 (0) | 2 (7.6) | p=.093 | |
Data were presented as number and percentage
Chi-square with linear trends was used
Table 6 showed no significant difference in BML score at tibia between two groups before and after treatment. Regarding comparison in the same group, there was no significant difference in BML at tibia before and after treatment in group 1 and in group 2 (Supplementary Figure 3).
Table 6. MRI finding of BML at tibia before and after treatment.
| Tibia (BML) | Group 1 n=28 (%) | Group 2 n=26 (%) | ||
|---|---|---|---|---|
| Before treatment | score 0 | 21 (75) | 10 (38.5) | X2= 6 |
| score 1 | 1 (3.6) | 4 (15.4) | P= .073 | |
| score 2 | 5(17.9) | 8(30.8) | X2=6.07 | |
| score 3 | 1 (3.6) | 4 (15.4) | p=0.064 | |
| After treatment | score 0 | 21 (75) | 10 (38.5) | X2= 6.11 |
| score 1 | 7(25) | 14(53.8) | P= .069 | |
| score 2 | 0(0) | 1(3.8) | X2= 6.8 | |
| score 3 | 0 (0) | 1 (3.8) | p= 0.055 | |
Data were presented as number and percentage
Chi-square with linear trends was used
No significant difference of synovitis/ effusion between both groups before treatment (P= .77) but showed a significant difference between both groups after treatment (P=0.036*). Regarding comparison in the same group, there was a significant difference of synovitis/ effusion between before and after treatment in group 1(p=0.044*) (Supplementary Figure 4) but there was no significant difference between before and after treatment in group 2 (p=0.70).
No significant differences between both groups in articular cartilage loss at patella before treatment , after treatment in both groups (p=.081). Articular cartilage loss at femur before treatment and after treatment in both groups showed no change (P=.081). Articular cartilage loss at tibia before treatment and after treatment in both groups showed no change (P=.096).
Side effects of strontium
No recorded side effects.
Discussion
The guidelines for OA treatments include nonpharmacological, pharmacological and surgical approaches. The most recommended treatment for early OA is non-pharmacological approaches, such as weight loss, aerobic exercise, and physical therapy and knee braces [14].
Physical therapy is aimed to maintain joint mobility and improve muscle strength. Water or land-based exercise, aerobic walking, quadriceps strengthening, resistance exercise, and tai chi exercise decrease pain and disability from knee OA. Studies showed that physiotherapy can reduce pain and improve function as a short-term treatment of OA of the knee joint [15].
For a long time, treatment of OA concentrated on decreasing the pain and stiffness and on the improvement of functional abilities. None of the currently marketed medications is collectively perceived as a symptom and structure-adjusting drug in OA. Compounds with a possibility to impact the cartilage–subchondral bone unit, in view of their mechanical or biologic properties, may constitute a breakthrough in a therapeutic treatment of OA [16].
SrR able to disrupt the bone remodeling process and to disturb the balance between bone resorption and bone formation. Its effect on the subchondral bone makes it a strong Disease Modifying Osteoarthritis Drug (DMOAD) in the management of OA [17].
In our study, there was a significant improvement in pain as measured by VAS in group1. These outcomes were supported by Reginster et al. [18] who reported that there was a reduction in knee pain as assessed by VAS (p=0.065) in a group treated with SrR 2gm daily.
Pain in OA is in all probability connected to inflammation and a parallel effect on bone. A study of patients with osteoporosis and radiological spinal OA announced a significant lessening in back pain after 3 years of treatment with strontium ranelate 2 g/ day [16]. Our study showed that there were significant improvements in total WOMAC score in (group 1) at 6 months of treatment.
Study conducted by Reginster et al. [18] reported that treatment with SrR 2g/day was associated with significantly lower total WOMAC score (p=0.045) and pain subscore (p=0.028), and a trend toward lower physical function subscore (p=0.099).
Bruyere et al. [19] showed differences between SrR 2gm/d group and placebo group reached significance after 6-24 months, there were significant differences in WOMAC pain (P=0.024) at 6 months, function at 12 months and stiffness at 24 months.
In the present study, there were no significant differences in BML scores between both groups at patella, femur and tibia before and after treatment and there was an improvement in BML score but not significant in group 1at 6 months after treatment.
Pelletier et al. [20] reported that the BML scores were significantly reduced in the medial compartment with daily 2 g SrR group at 36 months compared with placebo. The change in BML has been detected at 1 year of daily 2 g SrR in a study conducted by McIure et al. [21]. One study which evaluated biochemical markers of cartilage metabolism showed that some knees had changes in BML volumes over 3 months of daily 2 g SrR using semi quantitative MRI [22].
The correct mechanism by which SrR produces this impact on BML stays to be resolved. Nonetheless, in human OA subchondral bone osteoblasts, SrR appeared to diminish the production of several factors involved in bone remodeling such as MMPs and RANKL corresponding with the upregulation of osteoprotegerin [23].
In our study, there were significant improvements in grades of synovitis/ effusion in group 1(p=.044) and significant difference between two groups (P=0.036). The synovial membrane can produce chemokines and metalloproteinases which degrade cartilage, simultaneously, the products of cartilage breakdown stimulate the release of collagenase and other hydrolytic enzymes from synovial cells and lead to vascular hyperplasia of the synovial membrane in the osteoarthritis [24].
A therapeutic concentration of SrR significantly reduced the progression of cartilage lesions, which was associated with a decrease in the synthesis of catabolic factors such as MMPs by chondrocytes and IL-1β by the synovial membrane [25]. Synovial cells are capable of secretion of proteolytic enzymes and proinflammatory cytokines (IL-1β, IL-6, TNF-α) which mediate the development of pain associated with this disease [26].
As there is a link between the occurrence of cartilage degeneration and synovitis [24], and SrR was proven to reduce cartilage progression [25], this means that products of cartilage breakdown decrease and this explains why group1 showed a significant improvement in synovitis/effusion in our study.
In the acquired study, there were no significant differences in cartilage volume loss between both groups at patella, femur and tibia before and after treatment and there was no change in cartilage volume loss in group 1 at 6 months after treatment. This outcome is clarified by Hunter et al. [27] who uncovered that the change of cartilage morphology over 6 months using quantitative MRI is not noticeable. Measurements of cartilage morphology quantitatively more accurate than semi-quantitative measurements [28].
Pelletier et al. [20] evaluated the disease-modifying effect of strontium ranelate by conducting "Strontium ranelate Efficacy in Knee Osteoarthritis (SEKOIA) trial" which assessed Cartilage Volume Loss (CVL)and bone marrow lesions (BMLs) using MRI. They showed that there was a significantly decreased CVL on the plateaus at 12 (P=0.002) and 36 (P=0.003) months in the SrR 2 g/day group compared with placebo.
Zaim et al. [29] found that over 3 years, using WORMS score, the number of patients with a deterioration of the cartilage morphology was significantly lower in the SrR 2g group than in the placebo group in the lateral compartment (p=0.001). No difference was observed in the medial compartment.
Pelletier et al. [20] demonstrated that SR 2gm/d was shown to significantly reduce CVL by 35% and BML score change in medial central condyle at 36 months, suggested an association between the decrease in BML score and the reduction of CVL.
SrR may produce its effect as structure modifying drug in OA through its effect on chondrocyte and bone cell function. Its action on cartilage via chondrocytes was supported by decreased level urinary CTX-II which suggests lower cartilage metabolism and possibly reduced osteophyte formation. In vitro, SrR stimulates cartilage matrix formation, this action can restore the balance between chondrocyte formation and resorption through its direct effect on proteoglycan synthesis and its indirect effect on insulin growth factor-1 [30].
The presence of BMLs seen in MRI had been related to development and progression of cartilage loss, which includes areas adjacent to the bone marrow lesions [31]. SrR can act directly on the cartilage and regulate local biochemical mediators and macromolecules which are responsible for increasing the cartilage damage and indirectly, by affecting the BML, in this manner interfacing with the degradative cross-talk pathways between the subchondral bone and the cartilage [32].
Our obtained study found that no adverse events of SrR in group one were detected at 3months and 6 months after treatment. These results occurred due to a short duration of treatment. Our discoveries are bolstered by Halil et al. [33] who found that strontium ranelate treatment was not observed to be related to venous thromboembolism in post marketing studies and short-term treatment with the drug generally had no significant effect on hemostatic parameters in elderly women with osteoporosis in a small study.
Strontium ranelate was well tolerated. Register et al. [18] announced that the rate of venous thromboembolic occasions was <1% in SrR 2gm, and there were no cases of drug reaction with eosinophilia and systemic symptoms. There were no relevant differences in clinical or laboratory parameters, with the possible exception of creatine phosphokinase, which increased from baseline with treatment.
Study limitations
First, small number of patients was participated in this study. Second, short follow up duration. Finally, the quantitative cartilage volume assessment is more sensitive than the semi-quantitative scoring in the detection of treatment effect on OA cartilage changes.
Conclusion
Strontium ranelate could be effective in reducing pain and improving function in KOA patients.
Recommendation
Further studies enrolling larger number of patients are needed to confirm the effect of strontium ranelate on knee OA. Long duration is needed to show the effect of strontium ranelate on cartilage volume loss using semiquantitative MRI.
Author contributions
All authors have contributed to the concept and design of the study, interpretation of the data and revising the manuscript, and have approved the final draft.
Conflict of Interest
All authors declare that they have no conflict of interest.
Funding
This study was totally funded by all authors.
Acknowledgements
All authors wish to express deep appreciation and gratitude to all cooperative patients participated in this study.
Conflict of interest
Authors declare no conflict of interest.
Trial Registration
Clinical Trials.gov Identifier: NCT03937518
References
- Poole A. Osteoarthritis as a whole joint disease. HSS. J. 8(1), 4–6 (2012).
- Smelter E, Hochberg M. New treatments for osteoarthritis. Curr. Opin. Rheumatol. 25(3), 310–316 (2013).
- Bellido M, Lugo L, Roman-Blas J et al. Improving subchondral bone integrity reduces progression of cartilage damage in experimental osteoarthritis preceded by osteoporosis. Osteoarthr. Cartil. 19(10), 1228–1236 (2011).
- Hunter D, Guermazi A, Lo G et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr. Cartil. 19(8), 990–1002 (2011).
- Barr A, Campbell T, Hopkinson D et al. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis. Res. 17, 228 (2015).
- Tenti S, Cheleschi S, Guidelli G et al. What about strontium ranelate in osteoarthritis? Doubts and securities. Mod. Rheumatol. 24(6), 881–884 (2014).
- Wu C, Morrell M, Heinze E et al. Validation of American College of Rheumatology classification criteria for knee osteoarthritis using arthroscopically defined cartilage damage scores. Semin. Arthritis. Rheum. 35, 197–201 (2005).
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 16(4), 494–502 (1957).
- Huang M, Chen T, Weng M et al. Effects of pulse sonication on functional status of patients with knee osteoarthritis. International Society of Physical and Rehabilitation Medicine. Pp, 297–300 (2001).
- White D, Evans J, Truscott J et al. Can ultrasound propagate in the joint space of a human knee? Ultrasound. Med. Biol. 33, 1104–1111 (2007).
- Fitzgerald G, Oatis C. Role of physical therapy in management of knee osteoarthritis. Curr. Opin. Rheumatol. 16, 143–147 (2004).
- McCormack H, Horne D, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol. Med. 18, 1007–1019 (1988).
- Bellamy N, Campbell J, Hill J et al. A comparative study of telephone versus onsite completion of the WOMAC 3.0 Osteoarthritis Index. J. Rheumatol. 29, 783–786 (2002).
- Hochberg M, Altman R, April K et al. American College of Rheumatology 2012 recommendations for the use of non-pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis. Care. Res. 64(4), 465–474 (2012)
- Page C, Hinman R, Bennell K. Short term beneficial effects of exercise on pain and function. Int. J. Rheum. Dis. 14(2), 145–151 (2011).
- Bruyère O, Burlet N, Delmas D et al. Evaluation of symptomatic slow-acting drugs in osteoarthritis using the GRADE system. BMC. Musculoskelet. Disord. 9(1), 165 (2008).
- Han W, Fan S, Bai X et al. Strontium ranelate, a promising disease modifying osteoarthritis drug. Expert. Opin. Investig. Drugs. 26(3), 375–380 (2017).
- Reginster J, Badurski J, Bellamy N et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of adouble-blind, randomised placebo-controlled trial. Ann. Rheum. Dis. 72(2), 179–186 (2013).
- Bruyere O, Reginster J, Bellamy N et al. Clinically meaningful effect of strontium ranelate on symptoms in knee osteoarthritis: a responder analysis. Rheumatology (Oxford). 53(8), 1457–1464 (2014).
- Pelletier J, Roubille C, Raynauld J et al. Disease-modifying effect of strontium ranelate in a subset of patients from the Phase III knee osteoarthritis study SEKOIA using quantitative MRI: reduction in bone marrow lesions protects against cartilage loss. Ann. Rheum. Dis. 74(2), 422–429 (2015).
- McIure S, Bowes M, Wolstenholme C et al. The natural history of OA associated BMLs in the OAI progressor cohort. Osteoarthr. Cartil. 18, S184–S185 (2010).
- Garnero p, Peterfy C, Zaim S et al. Bone marrow abnormalities on magnetic resonance imaging are associated with type II collagen degradation in knee osteoarthritis – a three-month longitudinal study. Arthritis. Rheum. 52(9), 2822–2829 (2005).
- Tat S, Pelletier J, Mineau F et al. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone. 49, 559–567 (2011).
- Ashraf S, Walsh D. Angiogenesis in osteoarthritis. Curr. Opin. Rheumatol. 20(5), 573–580 (2008).
- Pelletier J, Kapoor M, Fahmi H et al. Strontium ranelate reduces the progression of experimental dog osteoarthritis by inhibiting the expression of key proteases in cartilage and of IL-1beta in the synovium. Ann. Rheum. Dis. 72(2), 250–257 (2013).
- Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6(11), 625–635 (2010).
- Hunter D, Bowes M, Eaton C et al. Can cartilage loss be detected in knee osteoarthritis (OA) patients with 3–6 months' observation using advanced image analysis of 3T MRI? Osteoarthr. Cartil. 18(5). 677–683 (2010).
- Wilidi L, Martel-Pelletier J, Abram F et al. Assessment of Cartilage Changes Over Time in Knee Osteoarthritis Disease-Modifying Osteoarthritis Drug Trials Using Semi-quantitative and Quantitative Methods: Pros and Cons. Arthritis. Care. Res. 65(5), 686–694 (2013).
- Zaim S, Guermazi A, Roemer F et al. Strontium ranelate effect on knee osteoarthritis progression. Oseoarthr. Cartil. 21, S28 (2013).
- Tat S, Lajeunesse D, Pelletier J et al. Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best. Pract. Res. Clin. Rheumatol. 24, 51–70 (2010).
- Neogi T. Clinical significance of bone changes in osteoarthritis. Ther. Adv. Musculoskeletal. Dis. 4(4), 259–267 (2012).
- Lafeber F, van Laar J. Strontium ranelate: ready for clinical use as disease-modifying osteoarthritis drug? Ann. Rheum. Dis. 72, 157–161 (2013).
- Halil M, Cankurtaran M, Yavuz B et al. Short-term hemostatic safety of strontium ranelate treatment in elderly women with osteoporosis. Ann. Pharmaco. Ther. 41(1), 41–45 (2007).