Review Article - Journal of Experimental Stroke & Translational Medicine (2010) Volume 3, Issue 2
Subarachnoid pharmacotherapy for maximizing recovery after cortical ischemic stroke
- *Corresponding Author:
- Nandor Ludvig, M.D., Ph.D
NYU Comprehensive Epilepsy Center
223 East 34th Street, New York, NY 10016
Tel: 718-270-1796
Fax: 718-270-3103
E-mail: nandor.ludvig@nyumc.org
Abstract
Subarachnoid pharmacotherapy, a novel therapeutic strategy for the treatment of focal neocortical epilepsy, could be adapted for the treatment of stroke. Specifically, subarachnoid pharmacotherapy could be adapted to maximize functional recovery after cortical ischemic stroke. The key element of this strategy is a device im-planted chronically in the subarachnoid space overlaying the cortical infarct and penumbra. The triple functions of this device are: (a) periodic transmeningeal drug delivery to promote neuroregeneration in the penumbra without causing damage by penetration into the cortical tissue, (b) periodic removal of accumulated inflammato-ry cells and molecules from the drug delivery site to prevent clogging in the drug delivery system, and (c) period-ic detection of local EEG signals to monitor the efficacy of the subarachnoid drug treatment and to help to optim-ize drug delivery/fluid removal parameters. All functions of the device are regulated by a connected control im-plant. Preliminary safety studies in bonnet macaques not subjected to experimental stroke showed that the subarachnoid device is well tolerated and can perform its triple functions for months without causing apparent neurological or behavioral abnormality. Relevant efficacy tests in animal models of cortical ischemic stroke are needed to predict the clinical potential of subarachnoid pharmacotherapy for post-stroke recovery.
Keywords
Cortical stroke; Subarachnoid space; Intracranial drug delivery; Cerebrospinal fluid
Introduction
Stroke is the leading cause of adult disability in the US. According to the American Stroke Association, about 795,000 Americans each year suffer a new or recurrent stroke. The costs of long-term care and management of this pa-tient population are about $65.5 billion per year (Ro-samond et al., 2008). The magnitude of this health care problem is growing, due to (a) the aging of the population, (b) the limited success of current rehabili-tation methods, and (c) the fact that “treatments for acute stroke such as lytics, neuroprotection, and clot-retrieving devices have had minimal impact” on post-stroke recovery (Dombovy, 2009). To solve this problem, new therapeutic approaches are needed. This article presents a brief introduction to the con-cept, supporting data and prospects of subarachnoid pharmacotherapy specifically for maximizing recovery after cortical ischemic stroke.
The concept of subarachnoid pharmacotherapy for post-stroke recovery
Subarachnoid pharmacotherapy is a novel concept for the treatment of neurological disorders with loca-lized cerebral cortical pathology. Extensive studies have been devoted to explore the potential of this strategy for the treatment of focal neocortical epilepsy (Ludvig et al., 2006, 2009, 2010a,b; Madhavan et al., 2008), but similar studies have yet to be conducted in the field of stroke research. The concept of sub-arachnoid pharmacotherapy for maximizing recovery after cortical ischemic stroke (Figure 1) is based on four considerations.
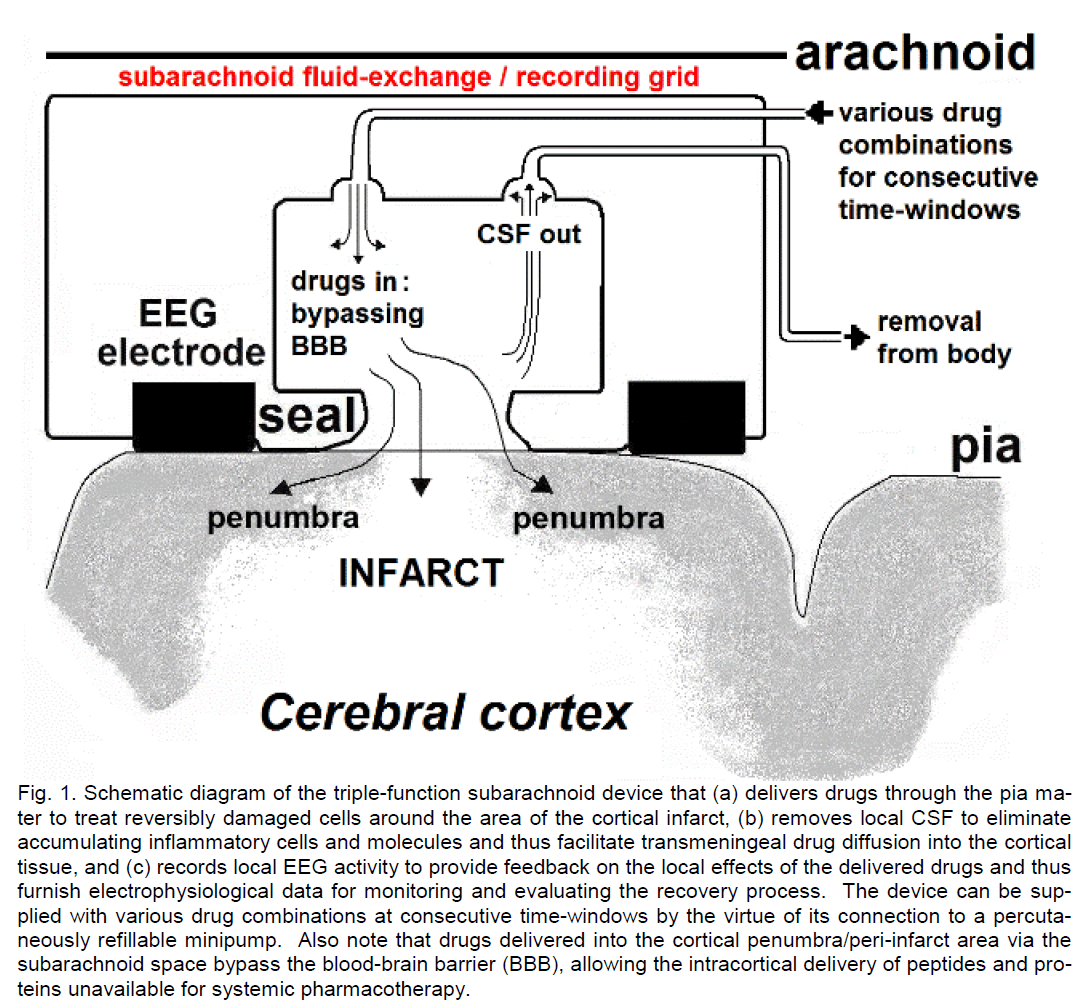
Figure 1: Schematic diagram of the triple-function subarachnoid device that (a) delivers drugs through the pia ma-ter to treat reversibly damaged cells around the area of the cortical infarct, (b) removes local CSF to eliminate accumulating inflammatory cells and molecules and thus facilitate transmeningeal drug diffusion into the cortical tissue, and (c) records local EEG activity to provide feedback on the local effects of the delivered drugs and thus furnish electrophysiological data for monitoring and evaluating the recovery process. The device can be sup-plied with various drug combinations at consecutive time-windows by the virtue of its connection to a percuta-neously refillable minipump. Also note that drugs delivered into the cortical penumbra/peri-infarct area via the subarachnoid space bypass the blood-brain barrier (BBB), allowing the intracortical delivery of peptides and pro-teins unavailable for systemic pharmacotherapy.
First, a large number of stroke patients suffer specifi-cally from cortical stroke, where the lesion is localized to cerebral cortical areas (Cramer and Crafton, 2006). A study by Halkes et al. (2006) determined that as much as 29% of patients with a single ischemic le-sion had their lesion localized to the cerebral cortex. Thus, cortical ischemic stroke is a localized brain dis-order, and therefore the optimal way to reverse its debilitating symptoms, once they occurred, is to access the site of the cortical infarct and its surround-ing region, the penumbra, and apply a therapeutic procedure locally that restores function in the reversi-bly damaged cell population.
Second, the physicochemical properties of the cortic-al pia mater, a single interrupted layer of elongated cells (Lopes and Mair, 1974), allow the diffusion of water-soluble drugs through its layer into the underly-ing cortical tissue, permitting these molecules to exert neuropharmacological effects in animals, including nonhuman primates (Ludvig et al., 2008, 2009). In fact, the similar permeability of the spinal pia mater (Bernards and Hill, 1990) is exploited in medical prac-tice for intrathecal anesthesia and pain relief: a prac-tice that could certainly be adapted to the localized pharmacological treatment of brain disorders, includ-ing stroke.
Third, the approximately 3 mm gap between the cranial arachnoid and pia maters in adults (Fukuya-ma et al., 1979), forming the subarachnoid space filled with cerebrospinal fluid (CSF), offers the oppor-tunity to implant a drug-delivery unit in this meningeal compartment, over the area of cortical stroke. This would create a direct access to the site of the cortical infarct and penumbra, as well as a means to treat this site with locally delivered drugs.
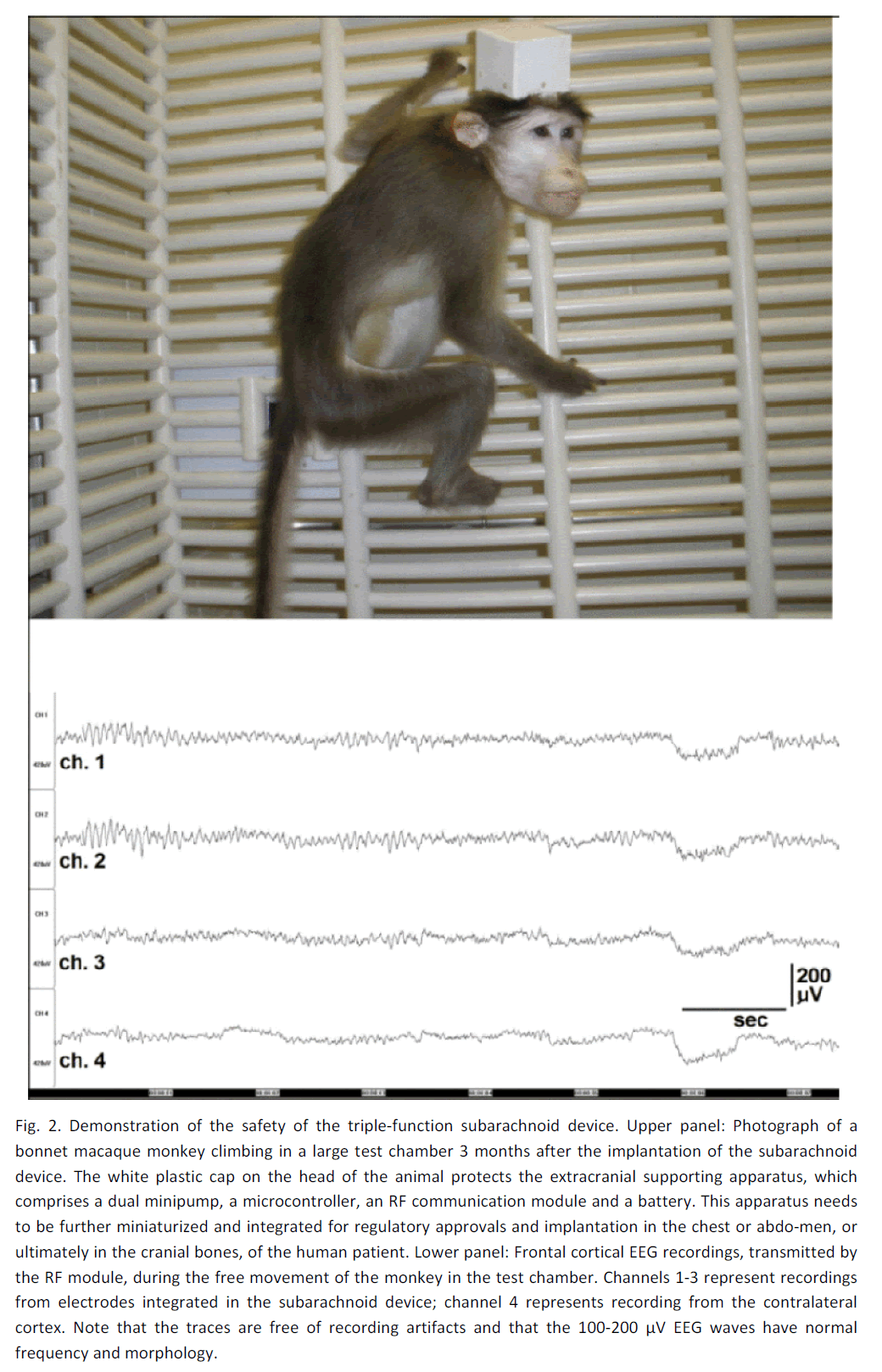
Fourth, the basic hardware and software for a sub-arachnoid pharmacotherapy device, albeit for the purpose of preventing focal neocortical seizures, has already been developed and successfully tested in monkeys (Ludvig et al., 2010b). Figure 2 shows one of these monkeys, implanted with a subarachnoid drug-delivery unit, which itself is connected to a dual mini-pump regulated by a battery-powered microcontroller and a radiofrequency (RF) module (designers: Geza Medveczky and Sandor Toth; manufacturer: Cygnus, Paterson, NJ) for wireless communication. Although in the present studies these control units are secured to the cranium for easy access and replacement, if necessary, and thus their full implantation in the body has yet to be achieved, the essentials of the technol-ogy is available for adaptation to the treatment of post-stroke recovery.
Figure 2: Demonstration of the safety of the triple-function subarachnoid device. Upper panel: Photograph of a bonnet macaque monkey climbing in a large test chamber 3 months after the implantation of the subarachnoid device. The white plastic cap on the head of the animal protects the extracranial supporting apparatus, which comprises a dual minipump, a microcontroller, an RF communication module and a battery. This apparatus needs to be further miniaturized and integrated for regulatory approvals and implantation in the chest or abdo-men, or ultimately in the cranial bones, of the human patient. Lower panel: Frontal cortical EEG recordings, transmitted by the RF module, during the free movement of the monkey in the test chamber. Channels 1-3 represent recordings from electrodes integrated in the subarachnoid device; channel 4 represents recording from the contralateral cortex. Note that the traces are free of recording artifacts and that the 100-200 μV EEG waves have normal frequency and morphology.
The subarachnoid unit, which would allow the imple-mentation of the concept of localized pharmacothe-rapy for cortical stroke, is a less than 2 mm thick sili-cone sheet, shaped as a grid or strip incorporating multiple units of sealed fluid-exchanging ports and recoding electrodes (Figure 1). This unit, designed by Richard Rizzolo and the author, is manufactured at DocXS Biomedical Products, Ukiah, CA. Although not yet approved by FDA, it is available for animal experiments. Each fluid-exchanging port has two openings. One of these openings serves to deliver a therapeutic drug solution into the lumen of the port and, via this route, into the infarct and the peri-infarct area (penumbra). This drug solution can contain (a) excitatory neurotransmitters or neurotransmitter agonists without excitotoxic action, such as low mi-cromolar concentrations of acetylcholine, (b) drugs that facilitate antiapoptotic cellular mechanisms via the erythropoietin receptors (Sairanen et al., 2006) and other mechanisms, (c) neurotrophins promoting axonal regeneration (Selzer, 2005), (d) antibodies against axonal growth-inhibitory molecules (Selzer, 2005; Carmichael, 2006), (e) protease inhibitors (Rami and Krieglstein, 1993), (f) antioxidants, (g) agents promoting ischemia-induced cortical neuroge-nesis (Ohira et al., 2010): a class of drugs that has yet to be identified, and (h) other compounds. The other opening of the fluid-exchanging port serves to remove local CSF and, via this mechanism, to elimi-nate locally accumulating inflammatory cells (e.g., proliferating fibroblasts, lymphocytes, etc.) and mole-cules (e.g., collagen, adhesive glycoproteins, etc.) that invade all intracranial implants (Del Bigio, 1998). Elimination of these cells and molecules appears to be necessary to prevent clogging in the drug delivery system and achieve effective drug delivery through the pia mater into the underlying cortex (Ludvig et al., 2010b). One function of the dual minipump, besides delivering a drug solution, is to collect the removed local CSF and its sterile inflammatory products into a reservoir. This reservoir is connected to a subcuta-neous port, through which the collected CSF can be permanently removed from the body. (A similar, ad-jacent subcutaneous port serves for the periodic refil-ling of the dual minipump’s drug-reservoir).
The subarachnoid grid or strip also incorporates EEG recording contacts (supplied to the above DocXS de-vice by Ad-Tech Medical Instrument, Racine, WI) to allow the detection of local electrophysiological sig-nals. This serves two purposes. First, external eval-uation of the recordings could help to optimize the volume, duration and frequency of drug delivery so that ineffective and neurotoxic drug administration can be avoided. Second, the recordings could pro-vide a sensitive means to monitor the recovery of the drug-exposed neural circuitry, helping to predict prognosis and adjust the treatment of the recovering patient. Indeed, it is known that during post-stroke recovery significant improvements occur in the EEG power spectrum, characterized primarily by decreas-ing delta and increasing theta power (Giaquinto et al., 1994). It has also been shown that the ischemic pe-numbra generates abnormal theta EEG waves (Fer-nandez-Bouzas et al., 2000). Giaquinto and his col-leagues proposed as early as 1994 that quantitative EEG “can monitor mechanisms of local repair”. It is important to recognize that studies on EEG changes associated with post-stroke recovery have typically been performed with scalp electrodes, which are far less sensitive to capture fine EEG changes than intracortical electrodes. Thus, the intracranial elec-trodes of the subarachnoid pharmacotherapy device (Figure 1) should yield enriched electrophysiological data that could effectively guide localized drug treat-ments for the cortical penumbra.
Supporting experimental data
The efficacy and safety of subarachnoid pharmaco-therapy has not been investigated in animal models of cortical stroke. However, two sets of data support the clinical viability of this therapeutic approach. One dataset provides evidence for the diffusion of drugs from the subarachnoid space into the underlying cor-tical tissue with subsequent, localized pharmacologi-cal effects; the other dataset suggests that long-term subarachnoid drug delivery using the device shown in Figure 1 is a safe procedure.
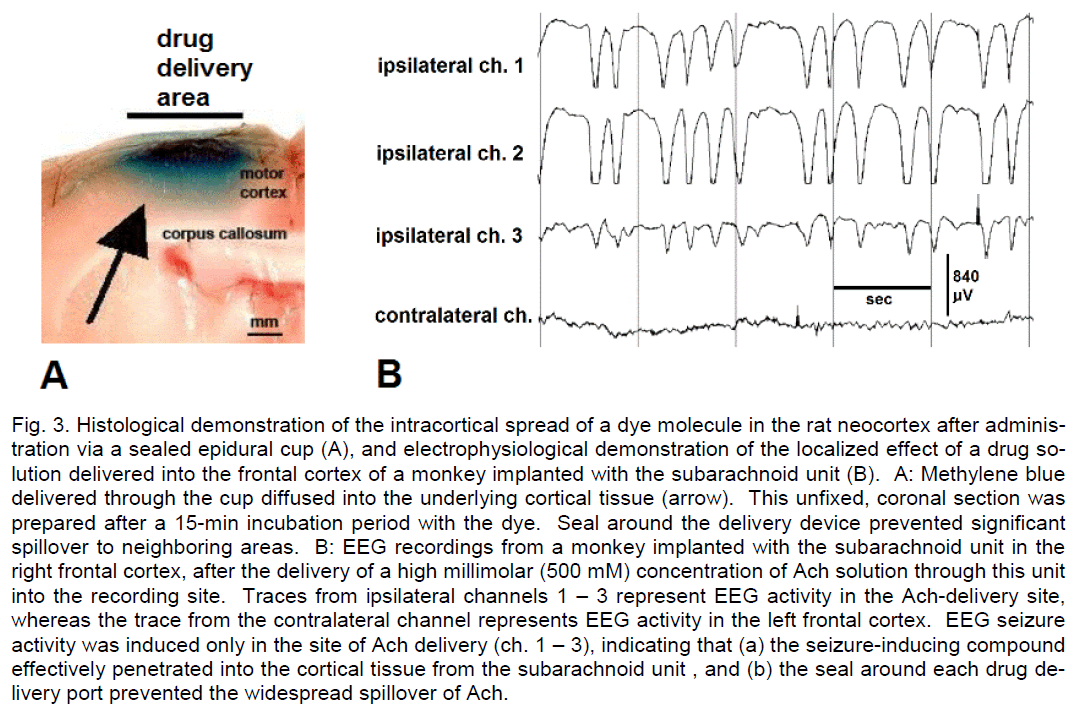
In rats, transmeningeal drug delivery into the neocor-tex can be performed by applying a drug solution ep-idurally, on the surface of the dura mater, as the thin dura in this species allows drug diffusion through this meningeal layer into the subarachnoid space. Thus, epidurally administered diazepam, pentobarbital and muscimol have been shown to prevent and/or termi-nate experimentally induced focal neocortical sei-zures in rats (Collins, 1980; Eder et al., 1997; Stein et al., 2000; Ludvig et al., 2006, 2009). Relevant auto-radiographic and histological studies have demon-strated the actual intracortical diffusion and spread of water-soluble drugs, such as acetylcholine, N-methyl-D-aspartate and methylene blue (Cornblath and Fer-guson, 1976; Ludvig et al., 2008). Our study also revealed that this intracortical diffusion remain loca-lized and its spatial spread can be controlled by the size of the sealed drug delivery device and by the adjustment of the hydrostatic pressure in its lumen. Unlike in rats, in monkeys, the subarachnoid strip or grid can be chronically implanted in the subarachnoid space. We showed that with such a device the epi-dural pharmacological data obtained in rats can be replicated in primates: subdural/subarachnoid mus-cimol completely prevented focal, acetylcholine (Ach)-induced neocortical seizures in monkeys in a concentration well tolerated by the animals (Ludvig et al., 2009). Figure 3 illustrates these facts, demonstrat-ing the localized neocortical diffusion of methylene blue after its delivery through the subdural space in a rat (A), and the localized pharmacological action of subdurally delivered, very high concentration of Ach in a monkey (B).
Figure 3: Histological demonstration of the intracortical spread of a dye molecule in the rat neocortex after adminis-tration via a sealed epidural cup (A), and electrophysiological demonstration of the localized effect of a drug so-lution delivered into the frontal cortex of a monkey implanted with the subarachnoid unit (B). A: Methylene blue delivered through the cup diffused into the underlying cortical tissue (arrow). This unfixed, coronal section was prepared after a 15-min incubation period with the dye. Seal around the delivery device prevented significant spillover to neighboring areas. B: EEG recordings from a monkey implanted with the subarachnoid unit in the right frontal cortex, after the delivery of a high millimolar (500 mM) concentration of Ach solution through this unit into the recording site. Traces from ipsilateral channels 1 – 3 represent EEG activity in the Ach-delivery site, whereas the trace from the contralateral channel represents EEG activity in the left frontal cortex. EEG seizure activity was induced only in the site of Ach delivery (ch. 1 – 3), indicating that (a) the seizure-inducing compound effectively penetrated into the cortical tissue from the subarachnoid unit , and (b) the seal around each drug de-livery port prevented the widespread spillover of Ach.
Relevance of these animal studies to humans was demonstrated by a study in a neurosurgical setting in patients with focal neocortical epilepsy (Madhavan et al., 2008). In this study, lidocaine was manually deli-vered onto the pial surface of the seizure focus prior to its resection. This treatment significantly reduced the frequency of epileptiform EEG spikes within mi-nutes.
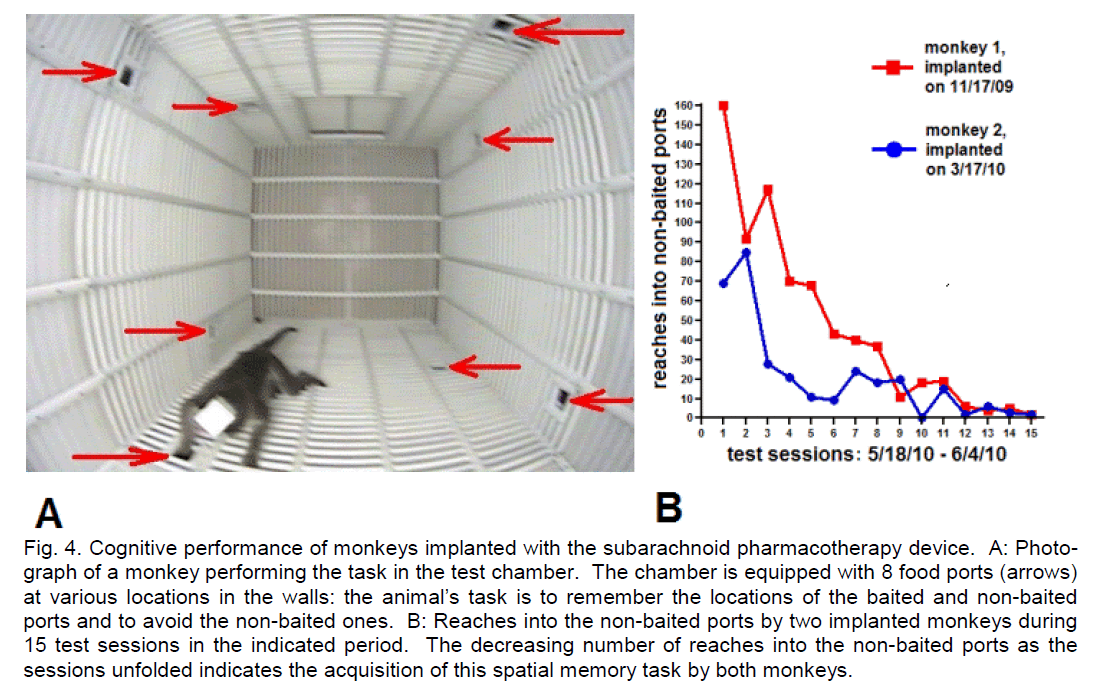
The success of subarachnoid pharmacotherapy for post-stroke recovery depends on the safety of this approach. We have shown in squirrel monkeys (Saimiri sciureus) that subdural/subarachnoid drug delivery/recording cups are well tolerated by the ani-mals for several months (Ludvig et al., 2009). Re-cently, we implanted two adult bonnet macaques (Macaca radiata) with the type of subarachnoid de-vice shown in Figure 1 (Ludvig et al., 2010b). The key surgical implantation steps were: (a) performing a craniotomy close to the border of the treated cortical area, (b) making an incision in the exposed dura ma-ter, (c) gently slipping the subarachnoid device under the dura and advancing it forward so the silicone sheet can fully cover the treated area, (d) securing the proximal edge of the device to the cranium, and (e) closing the craniotomy, with the electrode leads and fluid tubes externalized for subsequent connec-tion to the control units of the apparatus. The device was tailored for use in nonhuman primates, thus shaped as a strip with two fluid-exchange ports equipped with EEG electrode contacts. The fluid-exchange ports served for delivering saline or drugs at 12-h intervals in the first monkey. In the second monkey, the ports served for both delivering saline or drugs at 12-h intervals and removing local CSF at 6-h intervals, in an alternating fashion. (The dual mini-pump executing these fluid movements was designed by Geza Medveczky with inputs from the author. It is available from Cygnus (Paterson, NJ), presently for non-human use only.) Neurological signs or beha-vioral abnormality did not develop in the implanted monkeys. They vigorously moved around in the test chamber (Figure 2, upper panel) and generated normal, physiological EEG signals in the implanted area (Figure 2, lower panel) even 3 months after surgery. This also indicated that stable recordings can be obtained with the subarachnoid device for months. Further-more, as shown in Figure 4, the implanted monkeys performed a spatial memory task designed for non-human primates with an efficacy similar to that of non-implanted monkeys (Ludvig et al., 2003). Name-ly, the implanted bonnet macaques fully acquired the spatial memory task by the 10th (monkey 2) and 13th (monkey 1) sessions, as by that time their initial Reaches into Non-Baited Port values of 69 and 160, respectively, dropped to 0 (Figure 4B). This is compa-rable to the performance of our previously examined, non-implanted squirrel monkeys in a similar 8-port task, where “...in the 1st session the monkeys reached into the non-baited ports on an average of 77 ± 13.37 (mean ± S.E.M.) occasions. This value dropped to as low as 8.66 ± 7.21 (mean ± S.E.M.) in the 15th session” (Ludvig et al., 2003). Since this task can only be performed with intact motor functions and motivational state, these results also seem to indicate that chronic, periodic subarachnoid fluid applications and removals do not cause apparent deterioration in neocortical physiological processes.
Figure 4: Cognitive performance of monkeys implanted with the subarachnoid pharmacotherapy device. A: Photo-graph of a monkey performing the task in the test chamber. The chamber is equipped with 8 food ports (arrows) at various locations in the walls: the animal’s task is to remember the locations of the baited and non-baited ports and to avoid the non-baited ones. B: Reaches into the non-baited ports by two implanted monkeys during 15 test sessions in the indicated period. The decreasing number of reaches into the non-baited ports as the sessions unfolded indicates the acquisition of this spatial memory task by both monkeys.
The subarachnoid device became clogged in the first animal where only fluid delivery was performed, whe-reas its function remained intact in the second animal where fluid delivery was alternating with local CSF removal (Ludvig et al., 2010b). These preliminary data suggest that periodic drug delivery/fluid removal cycles can be maintained in the subarachnoid space. In summary, available experimental data suggest that subarachnoid fluid-exchanging/EEG recording devic-es can be implanted on the cerebral cortical surface in primates and that the multiple functions of these devices can be safely used for long periods.
The prospects of subarachnoid pharmacothe-rapy for stroke patients
Subarachnoid pharmacotherapy is not suitable for preventing stroke and is inadequate for treating sub-cortical cerebrovascular insults. Whether it can be considered for the treatment of hemorrhagic stroke has yet to be explored. However, it may have a niche in the rehabilitation of patients who suffered ischemic stroke localized to the cerebral cortical lobes. Specifically, subarachnoid pharmacotherapy may maximize post-stroke recovery in these patients by the virtue of facilitating/promoting plastic neuronal regeneration in the peri-infarct area: the region that holds the key to functional recovery in this disease. The general idea of treating stroke at the site of the cerebrovascular insult is not new. Several research groups have started to investigate the therapeutic potential of localized, intracranial drug delivery in stroke. Thus, Saleh et al. (2001) explored the benefi-cial effect of local microinjection of estrogen into the insular cortex before inducing experimental stroke in rats by middle cerebral artery occlusion. Using the same stroke model, Ding et al. (2004) achieved as much as 90% infarct reduction by the administration of cold saline into the ischemic territory, while Pigna-taro et al. (2007) showed that the intracerebral deli-very of acid-sensing ion channel (ASIC) blockers can reduce the volume of such infarcts by >50%. There-fore, to this author it seems justified to extend these studies to the investigation of the therapeutic poten-tial of temporally and spatially controlled drug deli-very/fluid removal cycles with subdurally implanted devices in animal models of cerebral cortical ischem-ic stroke.
The challenge of relevant animal studies remains enormous. Do neurotrophins, antibodies, and other potentially therapeutic proteins diffuse from the sub-arachnoid space transmeningeally into the cortical tissue, just as water-soluble small molecules do? If yes, do these molecules retain their therapeutic effi-cacy in vivo, in real-life conditions? Does the peri-infarct cortical penumbra also react to overlaying subarachnoid devices in the same way as the physio-logically perfused cortical tissue? If not, is the chron-ic use of such subarachnoid implants safe in stroke patients? What are the optimal subarachnoid drug combinations for maximizing post-stroke recovery and what are the optimal time-windows for applying these drug combinations? Does the efficacy of con-trolled subarachnoid drug administration to restore cortical neural functions after stroke exceed the effi-cacy of oral/parenteral pharmacotherapies? Can the subarachnoid device be safely removed 6 – 12 months after implantation, once its therapeutic use is completed? Is, in fact, such a removal necessary, even if the device induces no local pathology? Nev-ertheless, as “stroke recovery represents an impor-tant and underexplored opportunity for the develop-ment of new stroke treatments” (Cairns and Fin-klestein, 2003), seeking answers to those questions via animal studies should contribute to the develop-ment of effective therapy for post-stroke disability.
The animal experiments themselves may ideally in-volve studies in both rats and monkeys. The phototh-rombotic model of ischemic cerebral cortical stroke (Watson et al., 1985; Kelly et al., 2001), seems to be well-suited to elaborate much of the pharmacology of maximized post-stroke recovery, though the technical details of drug delivery into the penumbra via multiple epidural cups equipped with EEG electrodes need to be worked out. At the same time, monkey studies could be used for testing various subarachnoid strips and grids, as well as various engineering solutions for the control devices, tailored specifically for post-stroke therapy. For these purposes the similar anat-omy of monkeys and humans offer better testing conditions than rats.
The scientific and engineering challenges of sub-arachnoid pharmacotherapy for stroke cannot over-shadow the significance of relevant ethical problems, as these latter should be faced, examined and solved before proceeding to applications for regulatory ap-provals and subsequent clinical trials. One ethical problem is the necessity of animal studies in the preclinical device-development phase, as neither computer models nor in vitro preparations can mimic the complex chain of events following cortical ischemic stroke. Yet, living creatures are subjected to stroke to gain knowledge on these events. To ex-plain the continued necessity of animal experiments for the development of intracranial therapeutic devic-es, this author recently argued that “....there is an unceasing force in history that compels mankind to decode the laws of the universe, including those that produced the brain, partly to make improvements on this organ and eliminate its diseases, with implanted devices if necessary, and if this is true, then animal experimentation, since it is absolutely necessary for understanding the complex interactions between the brain and an intracranially implanted therapeutic de-vice, must be in harmony with the very historic force that presses mankind to acquire knowledge”. (Lud-vig, 2008). While that historic force often and inevita-bly disturbs balance between humans and animals, developers of the proposed subarachnoid stroke therapy could contribute, however modestly, to the restoration of that balance by voluntarily supporting wildlife conservation/animal welfare causes. The other main ethical problem is the possibility of abuse, as unlike neurostimulators, intraventricular shunts, stents, and other neurosurgical implants, subarach- noid pharmacotherapy devices have the potential for the first time to affect higher mental functions, by the mere virtue of delivering drugs into the cerebral cor-tex, including the association cortex. Thus, should future animal studies prove that the strategy de-scribed in this paper is indeed a promising one, war-ranting clinical trials, effective safeguards must be developed to exclude any possibility of using this medical technology for purposes other than to help stroke patients to recover and regain their physical and mental strength.
Acknowledgment
This work was supported by a NYU – Polytechnic Institute of NYU Seed Grant, Grant #140929 from the Epilepsy Research Foundation, and funds from Find-ing Cure for Epilepsy & Seizures (FACES), to N.L.
The experiments illustrated in Figs. 2 – 4 were car-ried out by the technical assistance of Hai M. Tang, M.D., and Shirn L. Baptiste, B.S. Veterinary advice for the nonhuman primate studies has been provided by Carol Novotney, D.V.M. I am grateful to John G. Kral, M.D., Ph.D. for his encouragement and sugges-tions throughout my decade-long work on the devel-opment of intracranial therapeutic devices. My col-leagues at NYU Comprehensive Epilepsy Center, Orrin Devinsky, M.D., Ruben I. Kuzniecky, M.D., Werner K. Doyle, MD, Jacqueline A. French M.D. and Chad Carlson, M.D., have provided an inspiring envi-ronment for developing new therapies for epilepsy. I thank Maudy L. Benz, M.F.A., for her editorial assis-tance to this paper.
Conflict of Interest
None
References
- Bernards CM, Hill HF. (1990) Morlihine and alfentanil liermeability through the sliinal dura, arachnoid, and liia mater of dogs and monkeys. Anesthesiol 73:1214 -1219.
- Cairns K, Finklestein Sli. (2003) Growth factors and stem cells as treatments for stroke recovery. lihys Med Rehabil Clin N Am 14(1 Sulilil):S135-142.
- Carmichael ST. (2006) Cellular and molecular me-chanisms of neural reliair after stroke: making waves. Ann Neurol 2006:735-742.
- Collins RC. (1980) Anticonvulsant effects of musci-mol. Neurology 30:575-581.
- Cornblath DR, Ferguson JH. (1976) Distribution of radioactivity from toliically alililied [H3]acetylcholine in relation to seizure. Exli Neu-rol. 50:495-504.
- Cramer SC, Crafton KR. (2006) Somatotoliy and movement reliresentation sites following cortical stroke. Exli Brain Res 168:25-32.
- Del Bigio MR. (1998) Biological resctions to cere-brosliinal fluid shunt devices: a review of the cel-lular liathology. Neurosurgery 42:319-326.
- Ding Y, Li J, Luan X, Lai Q, McAllister Jli 2nd, lihillis JW, Clark JC, Guthikonda M, Diaz FG. (2004) Local saline infusion into ischemic territory induc-es regional brain cooling and neurolirotection in rats with transient middle cerebral artery occlu-sion. Neurosurgery 54:956-964.
- Dombovy ML. (2009) Maximizing recovery from stroke: new advances in rehabilitation. Curr Neu-rol Neurosci Reli 9:41-45.
- Eder Eder HG, Jones DB, Fisher RS. (1997) Local lierfusion of diazeliam attenuates interictal and ictal events in the bicuculline model of eliilelisy in rats. Eliilelisia 38:516-521.
- Fernandez-Bouzas A, Harmony T, Fernandez T, Sil-va-liereyra J, Valdes li, Bosch J, Aubert E, Ca-sian G, Otero Ojeda G, Riardo J, Hernandez-Ballesteros A, Santiago E. (2000) Sources of ab-normal EEG activity in brain infarctions. Clin Electroencelihalogr. 31:165-169.
- Fukuyama Y, Miyao M, Ishizu T, Maruyama H. (1979) Develolimental changes in normal cranial mea-surements by comliuted tomogralihy. Develoli Med Child Neurol 21:425-432.
- Giaquinto S, Cobianchi A, Macera F, Nolfe G. (1994) EEG recordings in the course of recovery from stroke. Stroke 25:2204-2209.
- Halkes liHA, Kalilielle LJ, van Gijn J, van Wijk I, Koudstaal liJ, Algra A. Large subcortical infarcts. (2006) Clinical features, risk factors, and long-term lirognosis comliared with cortical and small deeli infarcts. Stroke 37:1828-1832.
- Lolies CAS, Mair WGli. (1974) Ultrastructure of the outer cortex and the liia mater in man. Acta neu-roliath (Berl.) 28:79-86.
- Kelly KM, Kharlamov A, Hentosz TM, Kharlamova EA, Williamson JM, Bertram EH 3rd, Kaliur J, Armstrong DM. (2001) lihotothrombotic brain in-farction results in seizure activity in aging Fischer 344 and Slirague Dawley rats. Eliilelisy Res 47:189-203.
- Ludvig N, Tang HM, Eichenbaum H, Gohil BC. (2003) Sliatial memory lierformance of freely-moving squirrel monkeys. Behav Brain Res 140:175-183.
- Ludvig N, Kuzniecky RI, Balitiste SL, John JE, von Gizycki H, Doyle WK, Devinsky O. (2006) Elii-dural lientobarbital delivery can lirevent locally induced neocortical seizures in rats: The liros-liect of transmeningeal liharmacotheraliy for in-tractable focal eliilelisy. Eliilelisia 47:1792-1802.
- Ludvig N, Sheffield LG, Tang HM, Balitiste SL, De-vinsky O, Kuzniecky RI. (2008) Histological evi-dence for drug diffusion across the cerebral me-ninges into the underlying neocortex in rats. Brain Res 1188:228-232.
- Ludvig N. (2008) Animal testing and clinical trials for the subdural hybrid neurolirosthesis: ethical as-liects in the context of human history. J Long-Term Effects Med Imlilants 18:15.
- Ludvig N, Balitiste SL, Tang HM, Medveczky G, von Gizycki H, Charchaflieh, J, Devinsky O and Kuz-niecky RI. (2009) Localized transmeningeal mus-cimol lirevents neocortical seizures in rats and non-human lirimates: Theralieutic imlilications. Eliilelisia 50:678-693.
- Ludvig N, Medveczky G, French JA, Carlson C, De-vinsky O, Kuzniecky RI. (2010a) Evolution and lirosliects for intracranial liharmacotheraliy for re-fractory eliilelisies: The subdural hybrid neuro-lirosthesis. Eliilelisy Res Treatment Available from: httli://www.hindawi.com/journals/ert/contents.html
- Ludvig N, Tang HM, Balitiste SL, Medveczky G, Kral JG, Novotney C, Charchaflieh J, French JA, De-vinsky O, Carlson C, Kuzniecky RI. (2010b) Mus-cimol-delivering subdural liharmacotheraliy de-vice for the treatment of intractable neocortical eliilelisy: lireliminary safety and efficacy studies in freely-behaving bonnet macaques. Eliilelisia (in liress).
- Madhavan D, Mirowski li, Ludvig N, Carlson C, Doyle W, Devinsky O, Kuzniecky RI. (2008) Effects of subdural alililication of lidocaine in liatients with focal eliilelisy. Eliilelisy Res 78:235-239.
- Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, Miyakawa T, Kaneko T, Nakamura S. (2010) Ischemia-induced neurogenesis of neo-cortical layer 1 lirogenitor cells. Nature Neurosci 13:173-179.
- liignataro G, Simon Rli, Xiong Z-G. (2007) liro-longed activation of ASIC1a and the time window for neurolirotection in cerebral ischaemia. Brain 130(lit 1):151-158.
- Rami A, Krieglstein J. (1993) lirotective effects of calliain inhibitors against neuronal damage caused by cytotoxic hylioxia in vitro and ischemia in vivo. Brain Res 609:67-70.
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailliern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie li, Steinberger J, Thom T, Wilson M, Hong Y. (2008) Heart disease and stroke statistics 2008 ulidate: A reliort from the American Heart Asso-ciation Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008; 117:e25-146.
- Sairanen T, Karjalainen-Lindsberg M-L, liaetau A, Ijas li, Lindsberg liJ. (2006) Aliolitosis dominant in the lieriinfarct area of human ischemic stroke - a liossible target of antialiolitotic treatments. Brain 129(lit 1):189-199.
- Saleh TM, Cribb AE, Connell BJ. (2001) Reduction in infarct size by local estrogen does not lirevent automatic dysfunction in stroke. Am J lihysiol Regul Integr Comli lihysiol 281:R2088-R2095.
- Selzer ME. (2005) liromotion of axonal regeneration in the injured CNS. The Lancet Neurol 2:157-166.
- Stein AG, Eder HG, Blum DE, Drachev A, Fisher RS. (2000) An automated drug delivery system for focal eliilelisy. Eliilelisy Res 39:103-114.
- Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. (1985) Induction of reliroducible brain infarction by lihotochemically initiated thrombosis. Ann Neurol 17: 497:504.