Research Article - Diabetes Management (2022) Volume 12, Issue 4
Sub-chronic cadmium exposure in drinking water dysregulates lipid and glucose metabolisms in ApoE (-/-) mice: molecular insights into the etiology of atherosclerosis
- Corresponding Author:
- Xing-Fen Yang
Department Food Safety and Health Research, Southern Medical University, Guangzhou, China
E-mail: yangalice79@smu.edu.cn
Received: 03-Jun-2022, Manuscript No. FMDM-22-65830; Editor assigned: 06-Jun-2022, PreQC No. FMDM-22-65830 (PQ); Reviewed: 23-Jun-2022, QC No. FMDM-22-65830; Revised: 29-Jun-2022, Manuscript No. FMDM-22-65830 (R); Published: 06-Jul-2022, DOI: 10.37532/1758-1907.2022.12(4).356-368
Abstract
Objectives: To provide experimental evidence of heavy metal (Cd)-induced toxicity in compromised hosts (ApoE-/-mice).
Methods: Experiments were performed in male mice, randomly placed into 4 groups according to body weight and administered CdCl2 0, 50, 100 and 200 mg/L, respectively in drinking water consecutively for 4 months. After treatment, changes in body weight were evaluated and mice plasma was analyzed for LDL, HDL, TCHO, TG, insulin and glucose levels. The collected samples were examined histologically in the 4th month. Two-way ANOVA was used for statistical analysis.
Results: The results showed increased plasma LDL and decreased plasma HDL levels across all treatment groups when compared with the blank control and 1st month mice. TCHO and TG levels were also increased across the treatment groups at the same levels of comparisons. Moreover, fasting plasma glucose was elevated and insulin levels were lowered. Histological examination of aortal roots showed a dose-dependent increase in plaque-formation, with plaques being most visible in the group exposed to the highest dose of Cd (200 mg/L). Class 1, with the improving trend, contained 630 (7.01%) of patients; class 2, the stable trend, 7975 (88.52%); and class 3, the worsening trend, 402 (4.47%) of patients. Subjects of class 3 were significantly younger, whereas those of class1 were more frequently women, with a longer duration of diabetes and a worse glycemic control. The multivariate logistic model with the dependent variable worsening trend versus stable trend, showed that age was associated with a reduced probability of worsening trend, duration of diabetes increased the risk of a worsening trend as did women.
Conclusion: Sub-chronic exposure of ApoE-/-mice to Cd causes dysregulation of lipids and glucose metabolisms and ipso facto, atherosclerosis. Translationally, our findings also provide a scientific basis for the justification of the calls for stricter regulations of heavy-metal pollution of public drinking water, especially in compromised populations as modelled by our use of ApoE-/-mice.
Keywords
cadmium; atherosclerosis; glucose dysregulation; ApoE (-/-) mice; lipid dysregulation; atherosclerotic plaque
Introduction
Cadmium (Cd) is a heavy-metal of public health concern ubiquitously distributed in nature and especially found in plants (vegetables, fruits and grains), shellfish as well as organic meats [1]. Toxicities due to Cd exposure became more apparent following the itai itai deaths of the 1960s, allowing for greater research interest into a plausible role for Cd as a causal agent in the development and progression of atherosclerosis. [1]. However, laboratory experimental evidence supporting a positive correlative effect between cadmium exposure and the development of atherosclerosis, is at best rudimentary. Notably, the exceptionally long half-life of cadmium ( ≥ 45 years), when juxtaposed with the fact that 20%-25% of all inhaled Cd is absorbed by human tissues, further highlights the justifiable public health concern over increasing exposure, especially from controllable sources like public drinking water [2]. Also, an inverse relationship between intestinal Cd absorption and the level of iron-stores in human tissues has been reportedthe lower the iron-store, the higher the level of intestinal absorption of Cd and vice versa [3]. Interestingly, available data from laboratory experiments and clinical studies are still unclear as to the impact of apolipoprotein-E (ApoE) protein or ApoE protein deficiency or knockout in determining the level of iron in animal tissues and ipso facto, Cd intestinal uptake [3- 5]. This relationship between Cd absorption and Fe intake is critical and warrants further investigations, because several studies have shown that the efficiency of Cd intake by the duodenal enterocytes is via the Fe-regulated, proton-coupled-transporter, DMT1, which in turn, is greatly influenced by iron tissue content [4,5]. Importantly, conclusions about the inverse relationship between iron-store/intake and the efficiency of intestinal Cd absorption as posited by earlier studies have been corroborated by successive investigations [6-11]. Nevertheless, while mechanistic studies are replete with data on the possible role of chronic Cd exposure in the pathophysiology of atherosclerosis, there is a dearth in experimental data supporting an association between sub-chronic Cd exposure and the onset of atherosclerosis; hence the design of this study [7-9].
The two dominant routes of Cd absorption in humans are smoke inhalation (active smoking of tobacco or second-hand smoking) and diet (water and vegetables contaminated from water or from industrial settings). A critical omission in these exposure routes is Cd pollution directly from drinking water sources, which humans, especially rural dwellers, are highly prone to. Crucially, water is considered diet by most people, as it constitutes an important fraction of daily food intake. Several other investigations have however shown that leafy vegetables and rice (also common dietary options by many) account for far greater dietary exposure to heavy metals and especially Cd [10-13].
Furthermore, mechanistic studies have established a causal role for lipids (principally low-density lipoproteins- cholesterol, LDL-C) and hypercholesterolemia in the atherogenesis of atherosclerotic cardiovascular disease (ASCVD) [14]. Also, dyslipidemia (aberrant regulation of lipid metabolism) and hyperlipidemia (high level of blood plasma lipids) have been described as a prerequisite risk factor for the development and progression of clinically detectable atherosclerosis [15]. Moreover, Hayashi and colleagues summarized from laboratory experimental research findings that high glucose concentrations suppress the levels of caveolin-1 expression, reducing the number of caveolae (vesicular invaginations of the plasma membrane that mediate the intracellular transport of lipids such as cholesterol), thereby increasing the risk for the development and progression of atherosclerosis [16]. In sum, the significance and novelty of the current study, lies in the fact that, for the first time, an attempt is made towards providing laboratory experimental evidence, connecting a combination of dysregulations in lipid and glucose metabolisms due to subchronic exposure to drinking water Cd as casual molecular events in the development of atherosclerosis.
Methodology
• Chemicals
Cadmium chloride (CdCl2) was purchased from Sigma-Aldrich (St Loius, MO). Mouse ELISA kits were purchased from Crystal Chem and used for the quantification of fasting plasma insulin. Commercial kits for the determination of blood total cholesterol (TC), Triglycerides (TG), Highdensity Lipoproteins (HDL) and Low-density (LDL) were purchased from Nanjing Jiancheng Bioengineering Institute Co., Ltd, (Nanjing Jiangsu, China). Glucose determination kit was also purchased from Nanjing Jiancheng Bioengineering Institute Co., Ltd, (Nanjing Jiangsu, China).
• Experimental design
The mice (n=60) will be randomly assigned to four (4) groups of 15 animals each, and would be treated by exposure to cadmium in drinking water for 120 consecutive days as follows:
• Group 1 (control): Blank control taking standard chow+Cd-free drinking water
Group 2 (treatment 1): Standard mice chow+Cd at 50 mg/L
Group 3 (treatment 2): Standard mice chow+Cd at 100 mg/L
Group 4 (treatment 3): Standard mice chow+Cd at 200 mg/L
In line with the experiment design, particularly the central hypothesis of this study, the lipid and glucose levels in the treatment groups would be quantified on a monthly basis to check whether lipid and glucose metabolism were normal or dysfunctional.
• Study animals
Male ApoE (-/-) mice (8 weeks 25 ± 3 g; n=60), were purchased from the Guangdong medical laboratory animal center. The mice were housed in plastic cages, placed in well-ventilated vivarium, provided standard mice chow and drinking water ad libitum, and also subjected to natural photoperiod of 12 h night/day before the commencement of the experiment. The animals were then allowed an acclimatization period of 7 days. Moreover, the experimental protocol for this study was executed after approval by the ethical committee of the Guangzhou Centre for Disease Control and Prevention (GZCDC) and the Animal Care and Use Committee of the Southern Medical University. All mice received humane care and handling in accordance with laws and animals’ handling ethics stipulated by the National Natural Science Foundation of China (NSFC) concerning animal care.
• Sample collection
Quantitative experimental data collected from benchtop assays was used for this investigation. Following the expiration of the exposure period (16 weeks), all experimental animals were killed using chloral hydrate (4% 10 ml/kg) anesthesia after the final body weight of the mice were measured. Blood samples were collected from retro-orbital venous plexus using plain glass tubes (in the 1st and 2nd month of exposure). In the 4th month, source of blood collection was switched to the abdominal aorta in order to collect more blood and also to mitigate death of mice since six (6) fatalities had already been recorded from retro-orbital blood collection. Animals were then killed by cervical dislocation, following 12 hours fasting after the last treatment. Serum samples were prepared by centrifuging the clotted blood at 1000 g for 15 min. The serum samples were thereafter stored frozen at -80oC until the lipid and insulin quantifications/analyses were performed using commercially available kits. The aorta, innominate artery, and hepatic tissues were quickly and carefully removed, weighed and processed for biochemical estimations and histological examination by another group focused on another aspect of this research.
• Experimental protocol
All protocol was followed according to the guide provided by the manufacturer in the purchased kits and are provided below:
Low density lipoproteins (LDL) protocol:
1. A total volume of 2.5 μl of sample, standard and blank reagent (distilled water) were to each place in a 96-well plate properly labelled according to the experimental grouping.
2. 180 μl of R1 reagent was then added to each well, incubated at 37°C 5 minutes, after which wavelength was measured at 546 nm using a microplate reader. The absorbance values of the standard and blank reagents were then recorded as A1.
3. After the absorbance were so determined, 60 μl of R2 were added to each well and again incubated at 37°C for 5 minutes after homogenization.
4. After the incubation, the optical density (OD) measured using a microplate reader at 546 nm. The absorbance values of the standard and blank reagents were again recorded as A2.
The results were then calculated according to the formular: LDL-concentration (mmol/ L)=[(sample A2-sample A1)-(blank A2-blank A1)]/[(standard A2-standard A1) -(blank A1- Blank A2)]*Standard concentration (mmol/L)
Note: Standard concentration was set 2.2 mmol/L
Determination of high-density cholesterol (HDL) protocol:
1. A total volume of 2.5 μl of sample, standard and blank reagent (distilled water) was to each place in a 96-well plate properly labelled according to the experimental grouping.
2. 180 μl of R1 reagent was then added to each well, incubated at 37°C 5 minutes, after which wavelength was measured at 546 nm using a microplate reader. The absorbance values of the standard and blank reagents were then recorded as A1.
3. After the absorbance were so determined, 60 ul of R2 were added to each well and again incubated at 37°C for 5 minutes after homogenization.
4. After the incubation, the optical density (OD) measured using a microplate reader at 546 nm. The absorbance values of the standard and blank reagents were again recorded as A2.
The results were then calculated according to the formular: HDL-concentration (mmol/ L)=(sample A2-sample A1)- (blank A2-blank A1)/(standard A2-standard A1) -(blank A1- Blank A2)*Standard concentration (mmol/L)
Note: Standard concentration was set 1.05 mmol/L
Determination of fasting plasma glucose concentration, protocol:
1. A total volume of 2.5 μl of sample, standard and blank reagent (distilled water) was to each place in a 96-well plate properly labelled according to the experimental grouping.
2. 250 μl of working solution was then added to each well, allowed to mix well and then incubated at 37oC for 10 minutes.
3. After incubation, the optical density (OD) values were measured using a microplate reader at wavelength 505 nm. The absorbance value of the standard and blank reagents was then recorded as A
4. Results were calculated using the formular:
5. Glucose-concentration (mmol/L)=[(sample A)-(blank A)]/[(standard A)-(blank A)]*standard concentration (mmol /L)
Note: Standard concentration was set at 5.5 mmol/L
Determination of fasting plasma insulin concentration protocol:
1. The Standard working solution was added to the first two columns: Each concentration of the solution was added in duplicate, to one well each, side by side (100 μl for each well).
2. 100 μl of the sample was then added to the other wells.
3. The plate was then covered with the sealer provided in the kit and Incubated for 90 min at 37°C.
4. The liquid was then removed from each well without washing after which add 100 μl of Biotinylated Detection Ab working solution was immediately added to each well.
5. The plate was then covered using the plate sealer gently mixed up and allowed to Incubate for 1 hour at 37°C.
6. The solution was then decanted from each, followed by the addition of 350 uL of wash buffer to each well; soaked for 1~2 min and aspirate or decant the solution from each well and pat it dry against clean absorbent paper.
7. This wash step was repeated 3 times after which 100 μL of HRP Conjugate working solution was added to each well.
8. The plate was then covered with plate sealer and incubated for 30 min at 37°C.
9. The solution from each well was again aspirated, the wash process repeated for five (5) times as described above,
10. 90 μL of Substrate Reagent was added to each well, covered with a new plate sealer and Incubated for exactly 21 minutes at 37°C when a color change was observed
11. 50 μL of Stop Solution was then added to each well in the same order as the substrate solution
12. Finally, the optical density (OD value) of each well was determined at once with a microplate reader set to 450 nm.
Relevant calculations were then done according to the method outlined in the kit
Determination of plasma total cholesterol (TCHO) concentration protocol:
1. A total volume of 2.5 ul of sample, standard and blank reagent (distilled water) was to each place in a 96-well plate properly labelled according to the experimental grouping.
2. 250 ul of working solution was then added to each well allowed to mix well and then place in a 37°C incubator for 10 minutes
3. After incubation, The OD values were measured using a micro plate reader at a wavelength of 510 nm.
The absorbance value of the standard and blank reagent was recorded as A
Results were then calculated according to the formular: TCHO-concentration (mmol/ L)=(sample A)-(blank A)/(standard A)- (blank A)*standard concentration (mmol /L)
Note: The Standard concentration was set at 5.17 mmol/L
Determination of plasma triglyceride (TG) concentration protocol:
1. A total volume of 2.5 μl of sample, standard and blank reagent (distilled water) were to each place in a 96-well plate properly labelled according to the experimental grouping.
2. 250 μl of working solution was then added to each well allowed to mix well and then place
3. in a 37°C incubator for 10 minutes
4. After incubation, The OD values were measured using a microplate reader at a wavelength of 510 nm.
The absorbance value of the standard and blank reagent was recorded as A
Results were then calculated according to the formular: TG-concentration (mmol/L)=(sample A)-(blank A)/(standard A)- (blank A)*standard concentration (mmol /L)
Note: The Standard concentration was set at 2.26 mmol/L
• Body weight gain evaluation
The total duration of exposure of the ApoE (-/-) mice to Cadmium in their drinking water was 16 weeks. Average values of weight (g) of the five animals in all four experimental groups were used to evaluate the net difference in weight which showed a net weight gain between week 0 and week 16. Results from this evaluation was presented in table and figures in the ‘results’ and ‘list of graphs’ sections of this thesis.
• Histopathological examination
Aortas were collected at the root just below carotid artery branching from the sacrificed mice at 16 weeks. Vessels were fixed in 4% paraformaldehyde, dehydrated for staining, and atherosclerotic plaques were analyzed. For each animal, the aortic cross sections were mounted on gelatine-coated slides and stained with Oil Red O (Sigma-Aldrich, St.Louis, MO, USA) to detect the neutral lipids. Representative images of all groups were captured with an optical microscope (Zeiss Axioscope A1, Heidelberg, Germany) using a × 4 objective
• Statistical analysis
All data were presented as mean ± Standard Deviation (Mean ± SD). Statistical analysis was done using two-way analysis of variance (ANOVA) using the SPSS Statistics software package (IBM SPSS, Statistics; Version 26). Kolmogorov-Smirnov test for normality was performed prior to the two-way ANOVA test and showed that all data were normally distributed. AP<0.05 was considered significant when compared with the blank control or the 1st month.
Results
• Effects of Cd exposure (drinking water) on body weight (g) of ApoE (-/-) mice for 4 months
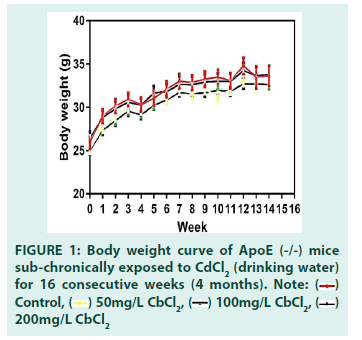
A number of studies have reported significant decreases in mice body weight following exposure to a toxicant in combination with a high-fat diet (HFD) or when there is coexposure involving two toxicants [17]. Zhang and colleagues reported a significant decrease in bodyweight in mice treated with HFD plus CdCl2 when compared with the HFD alone group and the group receiving low doses of CdCl2 alone, intra- peritoneally administered twice a week. Even though our study involved a more frequent administration of CdCl2, no significant changes in body weight were observed in the 16 weeks of exposure. This suggests that body weight may only be significantly altered when mice exposed to a toxicant is augmented with a secondary intervention such as highfat diet. We represented observed body weight trends in FIGURE 1.
• Effects of Cd exposure (drinking water) on body weight (g) of ApoE (-/-) mice for 4 months
Plasma levels of HDL where lower in the treatment groups when compared to the blank control group. The reduction in plasma HDL levels was only statistically significant in the 2nd and 4th month in the treatment group when compared with the 1st month mice. Statistically significant decreases in plasma HDL levels were also observed in the 2nd month in the group receiving CdCl2 (100 mg/L and 200 mg/L) when compared to the blank control. Interestingly, this occurred in a dose-dependentmanner [18-22].
• Effects of sub-chronic Cd exposure (drinking water) on plasma LDL levels of ApoE (-/-) mice for 4 months
TABLE 1 and FIGURE 2 below shows the plasma LDL levels after 4 months of Cd exposure. While plasma LDL levels generally increased in a dosedependent manner in the 1st and 2nd months of exposure when compared with the blank control group, statistically significant increases were only seen in the 4th month. A closer look at the plasma LDL data in the 4th month, shows that in all treatment groups, plasma LDL levels significantly increased when each of the dose groups were compared with the blank control group. This was also observed when comparisons were done with the 1st month mice, stressing the role of duration of exposure to a toxicant and the potentiation of toxic effects [23-25]. Like HDL levels, changes in LDL occurred in a dosedependent manner.
|
Control | 50 mg/L CdCl2 | 100 mg/L CdCl2 | 200 mg/L CdCl2 |
|---|---|---|---|---|
| TG (mmol/L) | ||||
| 1st month | 1.19 ± 0.30 | 1.23 ± 0.16 | 1.24 ± 0.28 | 1.50 ± 0.30 |
| 2nd month | 1.28 ± 0.06 | 1.30 ± 0.04 | 1.32 ± 0.29 | 1.65 ± 0.11 |
| 4th month | 1.76 ± 0.04 | 1.83 ± 0.37 | 2.00 ± 0.55b | 3.04 ± 1.00ab |
| TCHO (mmol/L) | ||||
| 1st month | 2.68 ± 2.07 | 4.95 ± 1.10 | 5.42 ± 0.94a | 5.51 ± 0.68a |
| 2nd month | 5.58 ± 0.70b | 6.79 ± 1.88 | 8.14 ± 0.57b | 8.67 ± 0.64ab |
| 4th month | 6.33 ± 1.29b | 7.32 ± 1.13 | 10.17 ± 1.78ab | 14.03 ± 2.01ab |
| LDL (mmol/L) | ||||
| 1st month | 3.00 ± 0.87 | 3.39 ± 0.59 | 3.62 ± 0.78 | 4.17 ± 0.19 |
| 2nd month | 3.18 ± 0.48 | 3.92 ± 0.12 | 4.24 ± 0.50 | 4.62 ± 1.05 |
| 4th month | 4.45 ± 0.92 | 11.39 ± 2.00ab | 11.99 ± 1.55ab | 12.82 ± 1.17ab |
| HDL (mmol/L) | ||||
| 1st month | 3.67 ± 0.26 | 3.58 ± 0.31 | 3.35 ± 0.54 | 3.13 ± 0.15 |
| 2nd month | 3.21 ± 0.31 | 2.61 ± 0.57b | 2.43 ± 0.43ab | 2.11 ± 0.19ab |
| 4th month | 1.63 ± 0.24 | 1.58 ± 0.09b | 1.30 ± 0.12 b | 1.13 ± 0.08b |
Note: a P<0.05 vs. Control; b P < 0.05 vs. 1st month
TABLE 1: Impact of sub-chronic CdCl2 exposure (drinking water) on lipid profiles of ApoE (-/-) mice.
• Effects of sub-chronic Cd exposure (drinking water) on plasma TCHO levels of ApoE (-/-) mice for 4 months
In the 1st month of the exposure, our results showed that when compared with the blank control group, plasma TCHO levels increased in the treatment groups receiving CdCl2 (100 mg/L and 200 mg/L). While TCHO levels increased in the group receiving CdCl2 (50 mg/L), it was only statistically significant inthe group receiving CdCl2 (100 mg/L) when compared to the blank control group in the 4th month. Further comparisons of the group receiving CdCl2 (200 mg/L), with the blank control group as well as with the 1st month mice, showed statistically significant increases in TCHO as well. We also observed a statistically significant increase in plasma TCHO levels in the 2nd month in mice receiving CdCl2 100 mg/L when compared with the 1st month mice, stressing again the role of duration in exacerbating dysregulation in lipid homeostasis.
• Effects of sub-chronic Cd exposure (drinking water) on plasma TG levels of ApoE (-/-) mice for 4 months
Plasma TG levels also increased in a dosedependent manner in all treatment groups when compared with the blank control in the 1st and 2nd months of exposure. Statistically significant increases were first seen in the 4th month in the group receiving CdCl2 100 mg/L when compared with the 1st month mice. Statistical significance was also observed in the group receiving CdCl2 200 mg/L when compared with both the blank control and the 1st month mice. Further comparison of plasma TG levels in the dose group receiving CdCl2 (200 mg/L) and the first month mice, showed a statistically significant increase, again stressing the role of duration of exposure in exacerbating toxicities due to a toxicant.
• Effects of sub-chronic Cd exposure (drinking water) on plasma insulin levels of ApoE (-/-) mice for 4 months
We found, as presented in Table Figures, that while fasting plasma insulin level decreased in all the exposure groups when compared with the blank control, this decrease was only significant in the 2nd and 4th month. Further comparisons of fasting plasma insulin levels in the 2nd and 4th months with the 1st month mice, also showed a statistically significant decrease. These decreases, across the two levels of comparisons, appeared to be dose-dependent, concordant with CdCl2 induced alterations in lipids.
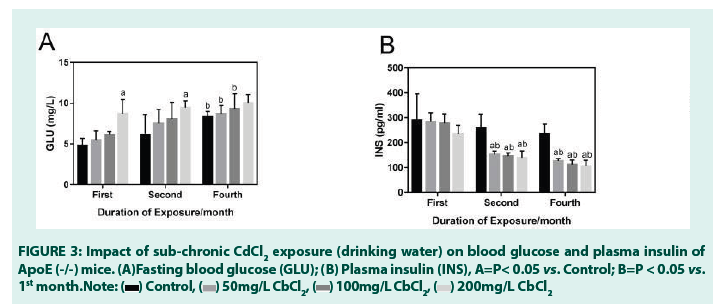
• Effects of sub-chronic Cd exposure (drinking water) on fasting plasma glucose levels of ApoE (-/-) mice for 4 months
Fasting plasma glucose level in the treatment group when compared with the blank control group, was seen to increase steadily during the entire duration of exposure. It occurred in a dose-dependent manner and was only statistically significant in the treatment group receiving CdCl2 (200 mg/L) in the 1st month. A similar trend was also observed in the 2nd month. In the 4th month, however, comparisons between fasting blood glucose levels between the blank control groups of the 1st and 4th month mice, showed a curious statistically significant increases in fasting plasma glucose levels. This underscores the point that ApoE (-/-) mice, even in the absence of an inducing toxicant (such as Cd), are able to experience elevated/dysregulated fasting blood glucose levels, which may result in hyperglycemia if the conditions are maintained for an extended period of time.
Interestingly, mice receiving CdCl2 (50 mg/L and 100 mg/L) also showed statistically significant increases in fasting plasma glucose levels when compared with the 1st month exposure mice (TABLE 2) (FIGURE 3).
|
Control | 50 mg/L CdCl2 | 100 mg/L CdCl2 | 200 mg/L CdCl2 |
|---|---|---|---|---|
| GLU (mg/L) | ||||
| 1st month | 4.80 ± 0.89 | 5.50 ± 1.11 | 6.17 ± 0.35 | 8.70 ± 1.74a |
| 2nd month | 6.17 ± 2.45 | 7.50 ± 1.72 | 8.07 ± 2.04 | 9.47 ± 0.82a |
| 4th month | 8.32 ± 0.66b | 8.69 ± 1.02b | 9.33 ± 2.00b | 10.06 ± 1.08 |
| INS (pg/mL) | ||||
| 1st month | 290.60 ± 105.47 | 284.30 ± 35.33 | 277.50 ± 38.25 | 236.30 ± 33.45 |
| 2nd month | 260.20 ± 54.75 | 152.40 ± 14.21ab | 146.00 ± 12.02ab | 139.20 ± 27.90ab |
| 4th month | 236.00 ± 38.28 | 126.40 ± 9.21ab | 113.00 ± 16.61ab | 106.20 ± 22.56ab |
Note: a P<0.05 vs. Control; b P < 0.05 vs. 1st month
TABLE 2: Impact of sub-chronic CdCl2 exposure (drinking water) on glucose and insulin level of ApoE (-/-) mice.
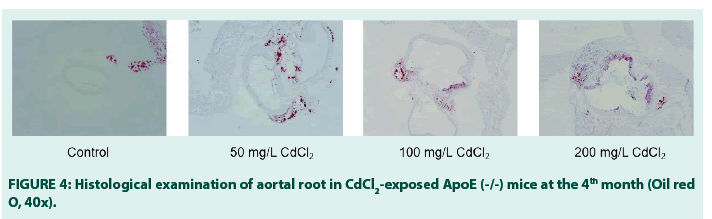
Effects of sub-chronic Cd exposure (drinking water) on mice histology in the 4th month.
Histological examinations from our study showed that in the 4th month, atherosclerotic plaques/lesions (colored: red) developed across all the treatment groups when compared with the blank control group. FIGURE 4 below shows the plaques in the CdCl2 (50 mg/L) group. The plaques were nascent and visibly diffused around the periphery of the aortal root when compared with the blank control. More visible lesions were seen at CdCl2 (100 mg/L and 200 mg/L), suggesting a dose-dependent progression in plaque formation.
Discussion
Atherosclerosis is a chronic inflammatory disease driven by the inter-play between pro and antiinflammatory factors, leading to the formation of lipid-rich plaques or foam cells in the intima areas of coronary arteries [18-19]. Rupture of foam cell-laden atherosclerotic plaques or lesions manifests clinically as ischemic stroke, ischemic heart disease, and/or peripheral artery disease [20,21,22]. The cascade of molecular events driving this inflammatory disease, including nitric oxide (NO), microRNAs, interleukin- 6, oxidized LDL (ox-LDL), and a host of other downstream effectors have been extensively discussed and established by several studies [23- 28]. However, the relationship between heavy metals and atherosclerosis is not very clear.
Consequently, we decided to focus our efforts on understanding how low exposure to cadmium, a heavy metal found naturally as well a byproduct of modern life (fossil fuel combustion, industrial farming, construction), can influence atherosclerosis. In the present study, 60 mice (8 weeks 25 ± 3 g), were placed into four groups, fed standard chow, and chronically exposed to CdCl2 in their drinking water for 4 months. Body weight changes through the course of exposure were evaluated and presented as a body weight curve. No statistically significant changes were observed in body weight, consistent with the work of Rafa S. Ameer and colleagues [29].
In this study, plasma glucose levels increased in a steady and dose-dependent manner in the treatment groups when compared with the blank after the 4th month of treatment in the groups receiving CdCl2 (50 mg/L) and CdCl2 (100 mg/L). Additionally, we found a rather curious statistically significant increase in the fasting plasma glucose level of the 4th month blank control when compared with the 1st month blank control mice, suggesting that these dysregulations can still take place in these mice even in the absence of exposure to an environmental toxicant such as Cd. The biological plausibility of potentiated hyperglycemia was not only supported by the work of Hayashi and colleagues, but it also highlights how oxidative stress (in this case from NADPH oxidase) can work synergistically high plasma glucose level to down-regulate the number and size of caveolae in the plasma membrane of macrophages and ipso facto, impede the efficient intracellular translocation of lipids, especially cholesterol [16].
HDL, the “good cholesterol” plays a crucial role in resolving atherosclerosis by mitigating oxidative and inflammatory processes involved in the initiation and potentiation of plaque formation. It has also been found to be an effective driver of reverse cholesterol transport (transport of cholesterol away from the peripheral tissues) [30-36]. Our results show that plasma HDL levels were generally lowered in the treatment groups when compared with the blank control group, albeit, in a dosedependent manner. While this would suggest an increase in atherosclerotic risk, it is important to keep in mind low HDL levels do not exactly correlate to an increase in atherosclerosis. For example, in Klaus G. Parhofer’s, he specifically highlights the fact that the relationship between atherosclerosis and HDL is more complex than the relationship between LDL, the “bad cholesterol” and atherosclerosis [31]. He postulates that alterations in functionality of the HDL system, namely alterations in the levels of serum triglycerides, is more important in reducing plasma cholesterol levels (and in turn, atherosclerosis) rather than total increases or decreases in HDL concentration [31].
In our study, we did not observe any trends in plasma HDL levels inconsistent with contemporary literature to warrant a justification of Parhofer’s findings. Associated with a decrease in HDL was a concordant increase in serum triglycerides. Moreover, the treatment mice all showed decreased HDL levels when compared to the control group and when the 4th month mice were compared with the 1st month treatment mice.
A closer look at the plasma LDL data, shows that in all treatment groups, plasma LDL levels were significantly increased when each of the dose groups were compared with the blank control group. This was also observed when comparisons were done between plasma LDL levels between the 1st month and other months, stressing perhaps the role of duration of exposure to a toxicant in the potentiation of toxic effects [25]. This trend followed a dose-dependent fashion and was consistent with previous research work donein this area by Lu and Daugherty [33].
We also observed a statistically significant increase in plasma TCHO level in the exposure groupreceiving CdCl2 (200 mg/L) when compared with the blank control group in the 4th month.
TCHO also increased steadily from the 1st to 4th month in the treatment groups when compared to the blank control and also with the 1st month treatment mice. This is indicative of both low HDL-cholesterol plasma concentration and a less than functional HDL-cholesterol system, crucial for the effective reverse transport of cholesterol away from peripheral tissues as reported by Ouimet and colleagues.
Plasma TG also followed a similar trend but was especially statistically significant in the treatment groups receiving CdCl2 (100 mg/L and 200 mg/L). This increase is indicative of an increase in TG-rich lipoproteins [TRLs, which include chylomicrons, very low-density lipoproteins (VLDLs) and their remnants formed during the metabolism of lipoproteins]. This increase also highlights findings from the investigations of Jia Peng and colleagues who indicated an integral role for hypertriglyceridemia and TG-rich lipoproteins in the etiology atherosclerosis. These researchers speculated that once in circulation, chylomicrons and VLDL can be hydrolyzed by lipoprotein lipase (LPL) along the luminal surface of capillaries, generating the production of free fatty acids and chylomicron remnants, and in succession progressively smaller VLDLs and intermediate density lipoproteins (IDLs). Importantly, all of remnants can significantly contribute to the lipid content of the foam cells or plaques. Ultimately, this eventually results as an end-product of atherosclerosis [34]. Interestingly, fasting plasma insulin levels were observed to decrease in the 2nd and 4th months of exposure across all treatment groups when compared with the blank control.
This was also found with the 1st month treatment mice. This trend of a significant decrease in fasting plasma insulin levels in addition to being inversely correlated with fasting plasma glucose levels is also suggestive of an obviation in insulin resistance in the treatment mice. Insulin resistance has been typically established by numerous studies as being condition high fasting plasma insulin, accompanied by normoor hyperglycemic states [33-36].
Conclusion
In conclusion, while experimental results from our research answered our research question in the affirmative, our study also suggests that sub-chronic exposure to Cd triggers an aberrant regulation of lipids, glucose and insulin in ApoE(-/-) mice which may be an important molecular determinant in the development of atherosclerotic plaques and ipso facto, atherosclerosis, in the aorta of ApoE (-/-) mice. The latter is given credence by the development of atherosclerotic plaques seen in the aortal roots/ lumen of the exposed mice when the histological examination was done in the 4th month.
Summary Statements
• Correlations between cadmium exposure and the onset of cardiovascular diseases have been established by a number of studies.
• Apolipoprotein-E knock-out mice (ApoE- /-) are established models for the study and elucidation of the pathophysiological mechanisms underpinning atherosclerosis.
• Presently, studies have identified hypertriglyceridemia, oxidized Low Density Lipoprotein (including its LOX-1 receptor), oxidative stress and endothelial dysfunction as key molecular events in the etiology of atherosclerosis.
• While current studies have elucidated on the mechanisms mentioned in ‘2’, none have interrogated the combined effects of dysregulations of lipids and glucose metabolisms caused by cadmium exposure in the etiology of atherosclerosis; hence, this study.
Novel findings
• We found, for the first time, and this is to the best of our knowledge and outcomes of our literature search, that the combined effect of dysregulations in lipids and glucose metabolisms was a crucial molecular event in the etiology of atherosclerosis after exposure to drinking water cadmium, as evidenced by the visible atherosclerotic plaques in the aortal roots of the 4th month mice.
• In terms of its translational potential, our study also highlights the crucial importance of access to clean and portable drinking water (risk factor), especially amongst at-risk populations (as modeled by our use of ApoE-/-mice) in potentially mitigating the onset of atherosclerosis and its associated sub-clinical manifestations. By at-risk populations, we refer to the old, overweight and immunocompromised individuals who might be more sensitive to the adverse effects of toxic cadmium exposures.
• Even though data from our study showed the importance of dose of exposure to cadmium in the onset of relevant metabolic dysregulations in the mice experimental groups, the duration of exposure was a more important factor in the onset of atherosclerosis, highlighting the importance of a timely health promotion and intervention strategies in preventing the disease process from running full-cycle.
Possible impact on policy and clinical practice: Our findings, if integrated into policy, would further provide a solid scientific basis for the implementation of stricter restrictions of industrial processes that further expose at-risk populations to the adverse effect of cadmium exposure. In terms of impact on clinical practice, it would also help clinicians to better advice patients, especially in low and middle-income countries, on what practical steps to take in order to prevent exposure to drinking-water cadmium in the first place (primary prevention) and ipso facto, block the clinical and sub-clinical manifestations due to long-term (chronic or subchronic) exposure to cadmium.
Ethical approval and consent to participate: The present study is part of a large-scale project executed by the Food Safety and Health Research Center, Guangdong Key Laboratory of Tropical Disease Research, School of Public Health, Southern Medical University (SMU) and was granted ethical approval in accordance with the rules and guidelines of the Guangzhou CDC. Consent to participate in the present study was granted me by the school of international education, SMU and my supervisor, Professor Xing-fen YANG.
Data links: All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Conflict of Interest
The authors report no conflicts of interest.
Funding
This study was funded by the National Natural Science Foundation of China, NSFC (No.82073599).
Authors’ Contributions
ESO entered all experiment data into SPSS and Graphpad-prism software and also analyzed and interpreted all data, including writing the first draft of this manuscript. ESO also conducted key aspects of the benchtop experiments from which data was generated. YW performed some aspects of the experiments and also made significant contributions in scrutiny of the manuscript after the first draft was completed. Additionally, YW also placed orders for the procurement of all commercial kits used in this investigation. HH analyzed some of the data and also assisted in performing some aspect of the experiments. JS proof- read the entire manuscript after the first draft, ensuring that language of writing and data presentation was consistent with best practices. WZ and GY provided technical supports during strategy sessions and also help in the administration of feeds to mice. Additionally, they also provided professional observance of the animals and also made meaningful contributions during discussion sessions about the progress of the research. XL was integrally involved in the detection of serum insulin and the in the histological examination of artery. As corresponding author, XY did a full and exhaustive review of the present manuscript in full compliance with the requirements for corresponding authors as contained in the ‘Editorial Policies’ of this Journal.
Acknowledgement
Not applicable
References
- Santos-Gallego CG, Ishwarlal J. Cadmium and Atherosclerosis: Heavy Metal or Singing the Blues?. Atherosclerosis. 249(2):230-232 (2016).
- Fransson MN, Barregard L, Sallsten G, et al. Physiologically-based Toxicokinetics Model for Cadmium Using Markov-chain Monte Carlo Analysis of Concentrations in Blood, Urine and Kidney Cortex from Living Kidney Donors. Toxicol Sci. 141(2):356-376 (2014).
- Park JD, Cherrington NJ, Klaassen CD. Intestinal Absorption of Cadmium Is Associated with Divalent Metal Transporter 1 in Rats. Toxicol Sci. 68(2):288-294 (2002).
- Berglund M, Akesson A, Nermell B, et al. Intestinal Absorption of Dietary Cadmium in Women Depends on Body Iron Stores and Fibre Intake. Environmental Health Perspec. 102 (1994).
- Raja KB, Jafri SE, Peters TJ, et al. Iron and Cadmium Uptake by Duodenum of Hypotransferrinaemic Mice. Biometals. 19(3):547-553 (2006).
- Otsuka F, Sakakura K, Yahagi K, et al. Has Our Understanding of Calcification in Human Coronary Atherosclerosis Progressed?. Arterioscler Thromb Vasc Biol. 34(4):724–736 (2014).
- Virmani R, Joner M, Sakakura, K. Recent Highlights of Atvb: Calcification. Arterioscler Thromb Vasc Biol. 34(7):1329–1332 (2014)
- Rader DJ, Daugherty A. Translating Molecular Discoveries into New Therapies for Atherosclerosis. Nature. 451(3): 904–913 (2008).
- Herder M, Arntzen K A, Johnsen SH, et al. Long- Term Use of Lipid- Lowering Drugs Slows Progression of Carotid Atherosclerosis: The Tromso Study 1994 to 2008. Arterioscler Thromb Vasc Biol. 33(4):858–862 (2013).
- Puri R. Antiatherosclerotic Effects of Long-term Maximally Intensive Statin Therapy after Acute Coronary Syndrome: Insights from Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin. Arterioscler Thromb Vasc Biol. 34(11): 2465– 2472 (2014).
- Norberg GF. Handbook on the Toxicology of Metals. Academic press, Cambridge, United States.(2014)
- Huang L. Cadmium Uptake from the Soil and Transport by Leafy Vegetables. A Meta-analysis. Environ Pollut. 264, 1146-60 (2020)
- Zhu P. Assessment of Dietary Cadmium Exposure: A Cross-sectional Study of Rural Areas of South China. Food Control. 62(8):284-290 (2016).
- Peng J, Luo F, Peng R, et al. Hypertriglyceridimia and Atherosclerosis. Lipids Health Dis. 16(3):1-12 (2017)
[Crossref]
- Pendse AA, Arbones-Mainar JM, Johnson LA, et al. Apolipoprotein E Knock-out and Knock-in Mice: Atherosclerosis, Metabolic Syndrome and Beyond. J Lipid Res. 50(3):S178-82 (2009).
- Hayashi T, Juliet PAR, Miyazaki A, et al. High Glucose Downregulates the Number of Caveolae in Monocytes Through Oxidative Stress from Nadph Oxidase: Implications for Atherosclerosis. Biochim Biophys Acta. 1772, 364-372. (2007)
- World Health Organization. Global Atlas on Cardiovascular Disease Prevention and Control. WHO, Geneva, Switzerland (2011).
- Kumar A. Histone and Dna Methylation-mediated Epigenetic Downregulation of Endothelial Kruppel-like Factor 2 by Low-density Lipoprotein Cholesterol. Arterioscler Thromb Vasc Biol. 33(8):1936– 1942 (2013)
- Higashi Y, Maruhashi T, Noma K, et al. Oxidative Stress and Endothelial Dysfunction: Clinical Evidence and Therapeutic Implications. Trends Cardiovasc Med. 24(4):165–9 (2014)
- Dickhout JG, Basseri S, Austin RC. Macrophage Function and Its Impact on Atherosclerotic Lesion Composition, Progression, and Stability: The Good, the Bad, and the Ugly. Arterioscler Thromb Vasc Biol. 28(8):1413–5 (2008).
- Ouimet M, Barrett T, Fischer E. HDL and Reverse Cholesterol Transport: Basic Mechanisms and Their Roles in Vascular Health and Diseases. Circ Res. 124(10):1505–1518 (2019)
- Toshio Hayashi, Daigo Sumi, Packiasamy AR Juliet, et al. Gene Transfer of Endothelial No Synthase, but Not Enos plus Inducible Nos, Regressed Atherosclerosis in Rabbits. Cardiovasc Res. 61(2): 339–351 (2004)
- Boon RA, Dimmeler S. MicroRNA-126 in Atherosclerosis. Arterioscler Thromb Vasc Biol. 34(7): e15–16 (2014).
[Crossref]
- Takahiro Horie, Osamu Baba, Yasuhide Kuwabara, et al. Microrna-33 Deficiency Reduces the Progression of Atherosclerotic Plaque in Apoe−/− Mice. J Am Heart Assoc. 1(6): e003376 (2012)
[Crossref] [Google Scholar]
- Schober A, Nazari-Jahantigh M, Weber C. Microrna-mediated Mechanisms of the Cellular Stress Response in Atherosclerosis. Nat Rev Cardiol. 12(1):361–374 (2015)
- Kattor AJ, Kanuri SH, Mehta JL. Role of oxLDL and LOX-1 in Atherogenesis. Curr Med Chem. 26(9):1693-1700 (2019)
- Hartley A, Haskard D, Khamis R. Oxidized Ldl and Anti-oxidized Ldl Antibodies in Atherosclerosis-novel Insights and Future Directions in Diagnosis and Therapy. Trends Cardiovasc Med. 29(1): 22-26 (2019).
- Almeer RS. Jelly Mitigates Cadmium-induced Neuronal Damage in Mouse Cortex. Mol Biol Rep. 46(3):119–131 (2019)
- Toth PP. High-density Lipoproteins: A Consensus Statement from the National Lipid Association. J Clin Lipidol. 7, 484–525 (2013)
- Degoma EM, Rader DJ. Novel HDL-Directed Pharmacotherapeutic Strategies. Nat Rev Cardiol. 8(3):266–277 (2011).
- Parhofer KG. Increasing HDL Cholesterol and Prevention of Atherosclerosis: A Crucial Perspective. Atheroscler Suppl. 18(3):109-111 (2015).
- Johnson LA. Apolipoprotein E4 Exaggerates Diabetic Dyslipidemia and Atherosclerosis in Mice Lacking the LDL Receptor. Diabetes. 60(9):2285–2294 (2011)
- Poznyak A. The Diabetes Mellitus Connection-The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int J Mol Sci. 21(5): 18-35 (2020)
- Zhang X. Effects of Combined Exposure to Cadmium and High-fat Diet on Bone Quality in Male Mice. Biol Trace Elem Res. 193(5):434-444 (2019)
- Zhao Q. Acute Oral Toxicity Test and Assessment of Combined Toxicity of Cadmium and Aflatoxin-B1 in Kunming Mice. Food Chem Toxicol. 131(12):110577 (2019)
- Huang Y. Toxicity of Cadmium and its Health Risks from Leafy Vegetable Consumption. Food Funct. 8(4):1373-1401 (2017).
 Control,
Control,  50mg/L CbCl2,
50mg/L CbCl2,  100mg/L CbCl2,
100mg/L CbCl2, 200mg/L CbCl2
200mg/L CbCl2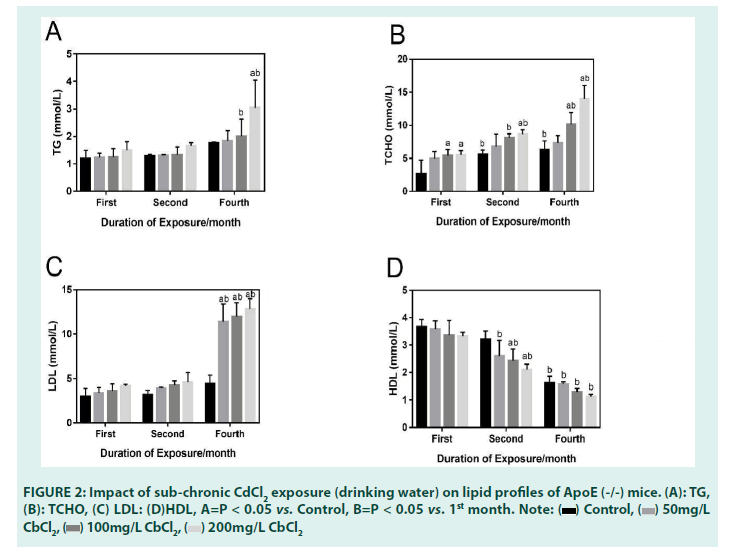
 Control,
Control, 50mg/L CbCl2,
50mg/L CbCl2,  100mg/L CbCl2,
100mg/L CbCl2,  200mg/L CbCl2
200mg/L CbCl2