Case Report - Interventional Cardiology (2021) Volume 13, Issue 1
Successful radiofrequency ablation of para-left bundle branch premature ventricular contractions: Aiming at the breakout point to spare the conduction system
- Corresponding Author:
- Leonor Parreira
Department of Cardiology,
Centro Hospitalar de Setúbal–Hospital de
São Bernardo, Rua Camilo Castelo Branco,
175. 2900-400 Setubal,
Portugal,
E-mail: leonor.parreira@gmail.com
Received date: December 02, 2020 Accepted date: December 25, 2020 Published date: January 01, 2021
Abstract
Background: The origin of the arrhythmia and its breakout point may be located apart. This has been described for arrhythmias originating within the His-Purkinje system. Case presentation: We report a case of a 70-year-old man with right bundle branch block and left anterior fascicular block with idiopathic nonsustained ventricular tachycardia originating in the septal left ventricular outflow close to the proximal left bundle branch. Ablation was successfully and safely performed away from the origin of the arrhythmia near the left anterior fascicle, at the breakout point. Arrhythmias from the conduction system may have different preferential exits and meticulous activation sequence mapping is the preferable strategy to select the ablation site.
Keywords
Left ventricular outflow tract • Premature ventricular contractions •Membranous septum • Radiofrequency ablation • Right bundle branch block
Introduction
Idiopathic Premature Ventricular Contractions (PVCs) arise more frequently from the right ventricular outflow tract (RVOT) followed by the Left Ventricular Outflow Tract (LVOT), mainly from the aortic cusps [1]. Less common sites include the tricuspid annulus and the right ventricular inflow free wall region [2] on the right. The basal septum immediately below the Right Coronary Cusp (RCC) is also an uncommon site of origin of idiopathic PVCs. The distinctive electrocardiographic pattern being an inferior lead discordance, with a negative QRS polarity in lead III and positive polarity in lead II, and a positive polarity in lead aVL [3]. The proximity to the left His bundle and the proximal Left Bundle Branch (LBB) is a drawback for ablation at this location due to the risk of Atrioventricular Block (AVB) or LBB block during ablation [3,4].
Case Report
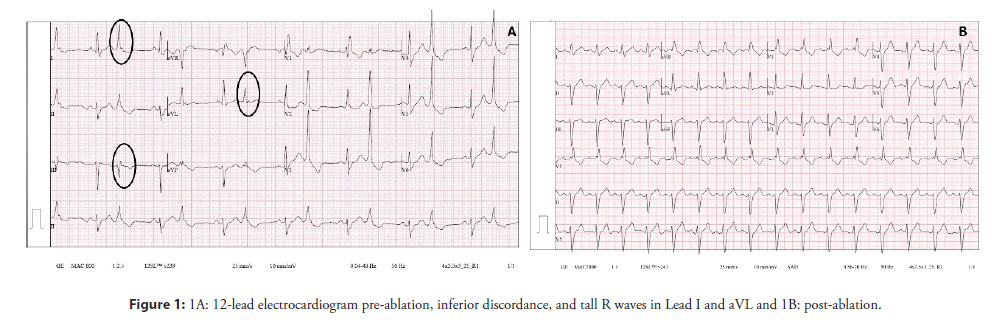
A 70-year-old man was referred to our institution by his primary care physician due to extremely frequent PVCs and runs of Non-Sustained Ventricular Tachycardia (NSVT) refractory to bisoprolol and sotalol. The patient complained of recurrent palpitations and precordial discomfort at rest, but he denied syncope. The episodes were not worsened by exercise or emotional stress. His past history was unremarkable and there was no family history of sudden death or cardiac diseases. The 12-lead ECG in sinus rhythm showed a Right Bundle Branch Block (RBBB) and a left anterior fascicular block with a QRS duration of 160 ms and frequent PVCs with a QRS duration of 130 ms, RBBB morphology, inferior lead discordance (positive lead II and negative lead III), tall R wave in lead I and aVL and QS in aVR (Figure 1A). Transthoracic echocardiogram was normal and the 24-hour Holter on bisoprolol, revealed 42966 multiform PVCs/24 h (1837 PVCs/hour) but with a clear predominance of one morphology, 1051 couplets, 112 triplets and 15 episodes of Non-Sustained Ventricular Tachycardia (NSVT). A cardiac magnetic resonance imaging study (CMR) was performed after the first evaluation at our center and the 24- hour Holter was repeated. The CMR showed mild left and right ventricular enlargement but it was otherwise normal with no late gadolinium enhancement. The repeated 24-hour Holter on sotalol showed an increase in the PVC burden with 49254 PVCs/24 hours (2141 PVCs /hour) same morphologies, 394 couplets, 72 triplets and 8 runs of NSVT. Arrhythmia-induced biventricular dilatation was assumed, and catheter ablation was proposed.
After written informed consent was obtained, the patient underwent an electrophysiological study and catheter ablation.
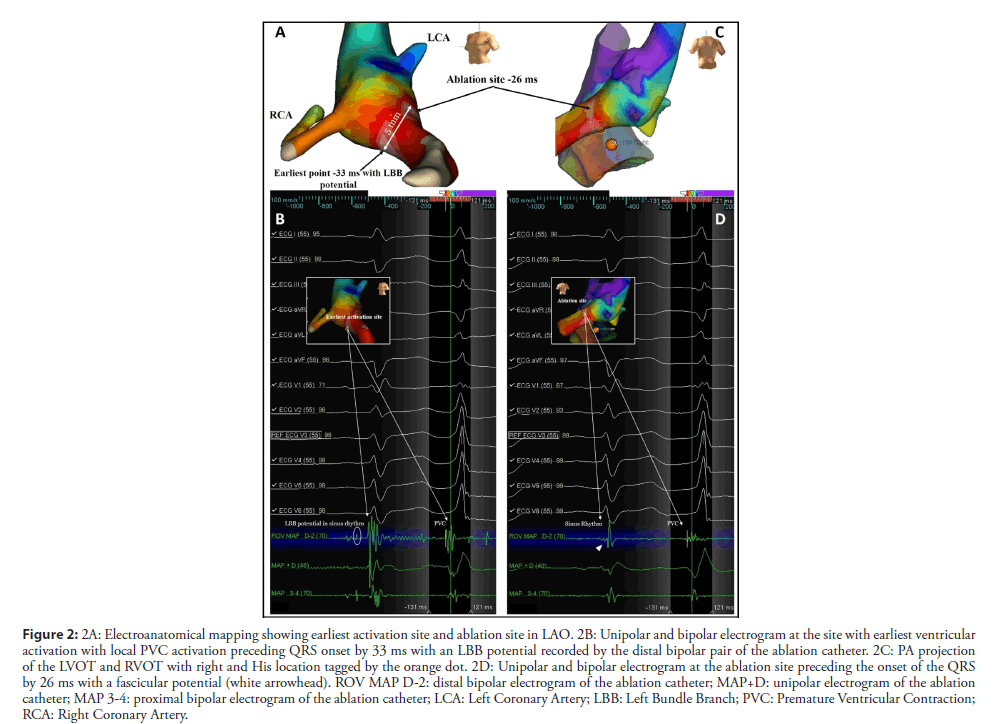
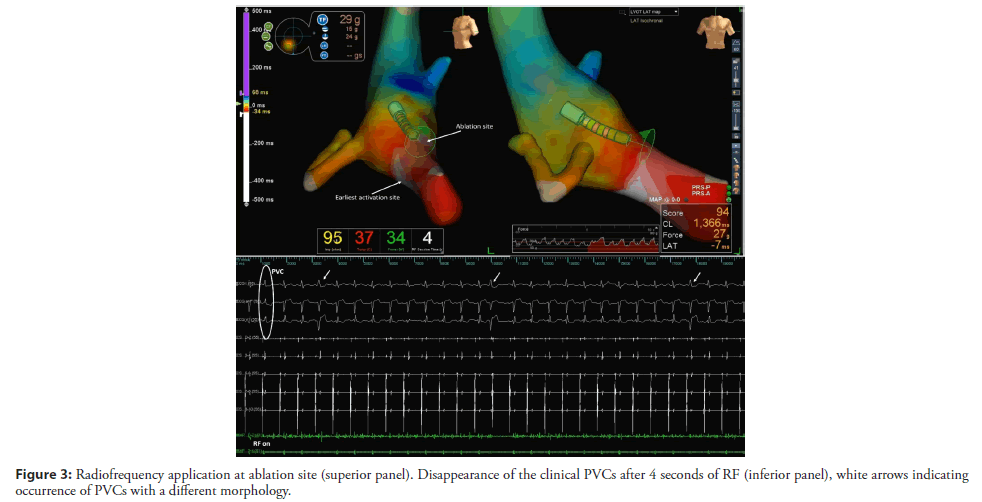
The RVOT and LVOT mapping were performed with the electroanatomical mapping system EnSite Precision (Abbott) using an irrigated tip catheter (Tacticath, Abbott) with a 3.5-mm distal tip electrode and a 1-4-1 interelectrode distance. The RVOT was reached by the right femoral vein and the LVOT was accessed by retrograde approach. The activation map of the PVCs was obtained, showing late activation of the RVOT in relation to the LVOT. The earliest activation was identified in the high left ventricular septum below the RCC (Figure 2A). The local activation time preceded the onset of the QRS by 33 ms and the local electrogram displayed an LBB potential at the distal bipolar electrode pair (Figure 2B). Pacing at this site showed a 12/12 pace-map match. Delivering radiofrequency (RF) to this site would result in complete AV block due to pre-existing RBBB so we kept moving the ablation catheter in the area and found a second point 5 mm away from the first site (Figures 2A and 2C) showing a local electrogram that was equally early preceding the onset of the QRS by 26 ms separated from the first site by points with more delayed local activation times. The local electrogram did not display a LBB potential, but a fascicular potential was present in sinus rhythm (Figure 2D), corresponding to the left anterior fascicle. Unipolar electrograms showed a QS morphology at both sites, although at the first site the descend was sharper. Pacemapping was not possible at the second site due to absence of ventricular capture. Considering that the patient already had a left anterior fascicular block at the beginning of the procedure we decided to apply RF at this site carefully uptitrating energy delivery to a maximal power of 45 watts for 60 seconds. The PVCs disappeared in the first 4 seconds of RF application (Figure 3) and no junctional rhythm or blocked P-waves were observed during ablation, the His-Ventricular interval did not increase significantly (58 ms before and 60 ms after ablation). The QRS developed a more pronounced left anterior fascicular block pattern but the QRS duration did not increase (Figure 1B). At 1 month of follow-up, the patient was asymptomatic, and no PVCs were detected on ECG (Figure 1B). The 24-hour Holter recording without antiarrhythmics detected 2300 multiform PVCs/24 h, all different from the ablated morphology. At 6 months the patient remained asymptomatic and referred an improvement in exercise tolerance and the 24-hour Holter recording detected 2100 multiform PVCs/24 h.
Figure 2: 2A: Electroanatomical mapping showing earliest activation site and ablation site in LAO. 2B: Unipolar and bipolar electrogram at the site with earliest ventricular activation with local PVC activation preceding QRS onset by 33 ms with an LBB potential recorded by the distal bipolar pair of the ablation catheter. 2C: PA projection of the LVOT and RVOT with right and His location tagged by the orange dot. 2D: Unipolar and bipolar electrogram at the ablation site preceding the onset of the QRS by 26 ms with a fascicular potential (white arrowhead). ROV MAP D-2: distal bipolar electrogram of the ablation catheter; MAP+D: unipolar electrogram of the ablation catheter; MAP 3-4: proximal bipolar electrogram of the ablation catheter; LCA: Left Coronary Artery; LBB: Left Bundle Branch; PVC: Premature Ventricular Contraction; RCA: Right Coronary Artery.
Results and Discussion
In this report we describe a case of frequent PVCs with a relatively narrow QRS originating from the left ventricular septum underneath the aortic valve, in a patient with RBBB and left anterior fascicular block. Idiopathic PVCs arising from the high left ventricular septum are uncommon [5]. The septum at this level is partially fibrous and is adjacent to the bifurcating atrioventricular bundle and the origin of the LBB [6]. A His-bundle or a LBB potential can be recorded at this site in a high percentage of cases, contraindicating ablation due to the risk of damage to the conduction system. In a series of 8 patients with PVCs from this location, ablation was not attempted in almost 40% of the cases3. The concern about LBB damage was higher in our patient due to the presence of a baseline RBBB. The electrocardiographic pattern of the PVC with a negative polarity in lead III and a tall R in aVL and lead I raised concern about this specific location [3] differentiating it from an RCC origin. This electrocardiographic pattern can also be observed in PVCs originating from the His bundle region in the right septum [4,7] except for the R wave in V1 and V2 which is less frequent for right sided focus, and besides, electrocardiographic algorithms are not reliable due to their low accuracy [8]. The patient was refractory to medical therapy and the ventricles were already dilated, so the benefits were balanced against the risks of atrioventricular conduction block, and ablation was contemplated. An LBB potential was recorded at the earliest activation site which totally contraindicated ablation. The narrow QRS width of the PVCs suggested an origin in the conduction system. Arrhythmias originating within the His-Purkinje system do often show preferential conduction with single or multiple exit points leading to single or multiple morphologies of the PVCs. This has been demonstrated by Itoh et al [9] in a series of 30 consecutive patients that underwent ablation of arrhythmias from the His-Purkinje system. The authors also observed that most patients had disease of the conduction system associated to the PVCs from the His-Purkinje system. For arrhythmias from within the conduction system the risk of damage to the conduction system is a major concern regarding catheter ablation.
Whenever the arrhythmia has its origin in the proximal segment of a bundle branch or atrioventricular node or His bundle, the presence of a complex net connecting the proximal with the distal parts of the system may be perceived as a possibility to map at more distal segments without such a dramatic impact on conduction when compared to ablation at more proximal sites. Successful ablation at a site 5 mm away from the earliest activation site, at a location displaying a fascicular potential in sinus rhythm, and a QS pattern during the PVC, suggests the presence of preferential conduction within the conduction system from the origin of the PVC near the proximal LBB to a more distal exit point near the left anterior fascicle. Marinheiro et al [10] previously described a similar case of PVCs originating from the His bundle in a patient with conduction system disease that underwent successful ablation of the PVCs at a distal site at the level of the slow pathway region. Our patient had a left fascicular block before ablation so we thought that ablating near the left anterior fascicle would not affect overall conduction. Since we were mapping the PVCs with an RF catheter, we didn’t change to cryoablation and decided to apply RF energy with careful titration of power delivery and careful monitoring for accelerated junctional rhythm or conduction block. Although cryoablation is usually considered as a safer energy when ablating near the conduction system, it is also associated with a higher recurrence rate [11] and besides, complete atrioventricular block has also been reported in the case of parahisian PVCs [12].
We believe that in this patient we ablated the main breakout point located at a secure distance from the LBB potential that allowed successful ablation without conduction block. This property of preferential conduction may explain the variable electrocardiographic morphologies of the PVCs arising from the His-Purkinje system, according to the breakout sites, that may be multiple [9].
Although we observed a decrease of over 95% in the PVC burden during 24-Holter recording at 1‐month and at 6-months followup, the persistence of multiform PVCs may indicate either that the principal breakout site was ablated but there were still secondary breakouts accounting for the persistence of multiform PVCs or that the remaining PVCs may be unrelated to the ones previously ablated and were allowed to emerge due to the elimination of the more prevalent ones. Nonetheless, the patient is asymptomatic and the reduction in PVC burden was significant, discouraging any repeated procedures.
Conclusion
Successful RF ablation of a PVCs originating near the LBB in a patient with RBBB and left anterior fascicular block could be successfully ablated at a site with less precocity, distally within the conduction system, sacrificing the left anterior fascicle but without risking occurrence of complete atrioventricular block.
Acknowledgments
The authors thank Lia Marques for her help with figures representative of the electro-anatomical mapping.
Financial Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors have no conflicts to disclose.
Informed Consent
The patient described in the case report had given informed consent for the case report to be published.
Author Contributions
Each author has individually been involved in and has made substantial contributions to case selection, case presentation, discussion, drafting manuscript and revision and final approval.
Data Availability
All data and information regarding this manuscript are present within the text.
References
- Lerman B. Mechanism, Diagnosis, and Treatment of Outflow Tract Tachycardia. Nat Rev Cardiol. 12(10): 597-608 (2015).
- Nair K, Namboodiri N, Gopalakrishnan A, Valaparambil A. Radiofrequency ablation of premature ventricular contractions originating from uncommon sites of right ventricle. Indian Pacing Electrophysiol J. 18(2): 84-86 (2018).
- Yamada T, Plumb V, McElderry H, et al. Focal ventricular arrhythmias originating from the left ventricle adjacent to the membranous septum. Europace. 12(10): 1467–1474 (2010).
- Enriquez A, Pathak R, Santangeli P, et al. Inferior Lead Discordance in Ventricular Arrhythmias: A Specific Marker for Certain Arrhythmia Locations. J Cardiovasc Electrophysiol. 28(10): 1179-1186 (2017).
- Yamada T, McElderry T, Doppalapudi H, et al. Idiopathic Ventricular Arrhythmias Originating From the Aortic Root. J Am Coll Cardiol. 52(2): 139–47 (2008).
- Ouyang F, Fotuhi P, Ho S, et al. Repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp. J Am Coll Cardiol. 39(3): 500-508 (2002).
- Briceño D, Liang J, Shirai Y, et al. Clinical and electrophysiological characteristics of idiopathic ventricular arrhythmias originating from the slow pathway region. Heart Rhythm. 16(9): 1421-1428 (2019).
- Jastrzebski M, Kukla P, Czarnecka D, et al. Comparison of five electrocardiographic methods for differentiation of wide QRS-complex tachycardias. Europace. 14(8): 1165–1171 (2012).
- Itoh T, Yamada T. Multifocal ventricular arrhythmias originating from the his-purkinje system incidence, characteristics, and outcome of catheter ablation. J Am Coll Cardiol EP. 4(9): 1248–60 (2018).
- Marinheiro R, Parreira L, Amador P, et al. Slow pathway region as the breakout site of parahisian premature ventricular contractions: Why choose safety over earliest activation? J Cardiovasc Electrophysiol. 31(1): 267–270 (2020).
- Hanninen M, Yeung-Lai-Wah N, Massel D, et al. Cryoablation versus RF ablation for AVNRT: A Meta‐analysis and systematic review. J Cardiovasc Electrophysiol. 24(12): 1354–1360 (2013).
- Miyamoto K, Kapa S, Mulpuru S, et al. Safety and efficacy of cryoablation in patients with ventricular arrhythmias originating from the para-hisian region. J Am Coll Cardiol EP. 4(3): 366-373 (2018).