Review Article - Interventional Cardiology (2022) Volume 14, Issue 0
Sudden cardiac death in COVID-19 Patients
- Corresponding Author:
- Dur-e-Najaf
Aga Khan University,
Stadium Road,
Karachi,
Pakistan
E-mail: durenajaf.najaf@scholar.aku.edu
Received date: 01-Feb-2022, Manuscript No. FMIC-21-41908; Editor assigned: 03-Feb-2022, PreQC No. FMIC-21-41908 (PQ); Reviewed date: 14-Feb-2022, QC No. FMIC-21-41908; Revised date: 21-Feb-2022, Manuscript No. FMIC-21-41908 (R); Published date: 28-Feb-2022, DOI: 10.37532/1755-5310.2022.14(s8).197
Abstract
Sudden cardiac death has been one alarming outcome reported in patients infected by the novel SARS-CoV-2. Cardiac involvement in COVID-19 patients may manifest as myocarditis, arrhythmias, acute coronary artery syndrome, atherosclerosis, dilated cardiac myopathy, myocardial infarction, and congestive heart failure. Elderly, males, and individuals with previous medical history of comorbidities (hypertension, diabetes mellitus, dyslipidemia) are more likely to suffer severe outcomes, sudden cardiac death being one of them. In this review, we highlight the underlying pathophysiology of plausible causes for sudden cardiac death in both pre and post COVID patients keeping in view the presently known viral characteristics. We discuss multiple organ failure due to cytokine storm, electrophysiological, hemodynamic, microscopic, and macroscopic cardiovascular changes for each cardiac pathology presented in SARS-CoV-2 patients. This literature review also enlists possible management and treatment options with their mode of action, uses and adverse effects. 3 major data bases: PubMed, Wiley Online Library and Google Scholar were used to review case reports, case series, letters to editor, narrative reviews, systemic reviews, randomized control trials, observational studies, and meta-analysis. The COVID-19 pandemic has pressured nations and health workers worldwide to control both the spread and deleterious health effects associated with SARS-CoV-2. Comprehensive knowledge of peculiar cardiac manifestations, along with further epidemiological studies and clinical trials is needed for optimum management of patients.
Keywords
Sudden cardiac death • Cardiac arrest • COVID-19 • Cardiac complications • COVID management • treatment • Vaccination
Introduction
The SARS-CoV-2, a new strain of the coronavirus and the most serious global health calamity of the century, was first reported in Wuhan China, December 2019. Ever since its outbreak, the novel virus has significantly affected the public health, economic, social, environmental domains in over 200 countries. As of February, 2022 around 417 million cases have been confirmed and more than 5.85 million lives lost [1]. This virus primarily spreads through droplets of saliva and nasal discharge when an infected individual coughs or sneezes and has variable manifestations. Fever, dry cough, tiredness, diarrhea, conjunctivitis, sore throat, body aches, headaches, loss of taste and smell are the most common amongst COVID-19 patients. However, the clinical spectrum of SARS-Cov-2 is wide, around one third of infected individuals are asymptomatic carriers and of those who show symptoms, 81% develop mild to moderate symptoms, 14% develop severe symptoms and 5% face severe symptoms [2]. Although the virus mainly targets the respiratory system, a new study published in Journal of American Medical Association (JAMA) Cardiology has documented cardiac involvement in around 78% of SARS-CoV-2 patients [3].
SARS-CoV-2 enters host cell by the binding of viral Spike (S) protein to Angiotensin-Converting Enzyme (ACE-II). The cellular ACE-II is first transported to the cell surface to be cleaved by host proteases (disintegrins and metalloproteinase 17) into an enzymatically active and soluble ACE-II, which retains SARS-CoV-2 interaction site; cellular ACE-II is highly expressed in the heart and the soluble ACE-II increases in inflammatory conditions and diseases [4]. A recent study by Kornilov and colleagues, has established a positive correlation between all metabolic syndrome component biomarkers (body mass index, blood pressure, glycemic control, lipid levels) and plasma soluble ACE-II levels, which is indicative of shared pathways in cardiac metabolic diseases and COVID-19 [5]. Another study showed higher soluble ACE-II levels in men compared to women (p=2 × 10-16) and in elderly individuals (p=8.6 × 10-11). These findings not only explain why SARS-CoV-2 is more likely affects elderly males with chronic diseases such as hypertension, diabetes mellitus, cardiovascular and chronic lung diseases, but also suggest the use of soluble ACE-II as a potential biomarker for COVID-19 severity [5].
Methodology
An extensive literature review was carried out on 3 major databases: PubMed, Wiley Online Library and Google scholar. The keywords are “sudden cardiac arrest” OR “cardiac death” AND “COVID 19” OR “coronavirus pandemic” AND “cardiac complications” AND “current COVID-19 treatment” AND “COVID-19 vaccination.” Under this search, all publicly available literature in English ranging from case reports, case series, letter to editors, systemic reviews, narrative reviews, randomized control trials, observational studies, and meta-analysis on these databases was included.
Literature Review
Pathophysiology of plausible causes
Studies have shown that COVID-19 may have direct and indirect effects on human heart including myocarditis, arrhythmias, deep venous thrombosis, pulmonary embolism, acute coronary artery syndrome and heart failure. A meta-analysis of 1527 patients confirmed the incidence of hypertension in 17.1%, cardiocerebrovascular disease in 16.4%, and diabetes in 9.7% of the participants. Approximately 8.0% of COVID-19 patients suffered acute cardiac injury with the prevalence of acute cardiac injury being 13 times higher in Intensive Care Unit (ICU) patients compared to non-ICU patients [6]. Although not much is yet known about long-term complications, a prediction of outcomes similar to patients recovered from acute respiratory stress syndrome due to previous SARS-CoV virus highlight cardiovascular abnormalities (40%), altered glucose metabolism (60%), and altered lipid metabolism (68%) after a 12 year follow up. Histopathological changes along with certain biochemical reactions and mechanisms may explain the pathophysiology of these manifestations and complications [7] (Table 1).
| Cytokine storm |
| • Increased levels of cytokines |
| • Infiltration of lymphocytes and neutrophils in multiple organs |
| • Ferroptosis |
| • Multiple organ failure |
| Hemodynamic Changes |
| • Hypercoagulability |
| • Decreased capillary transit time |
| Electrophysiological Changes |
| • Increased phosphorylation of L-type Ca2+ channels |
| • Reduced function of Na+ voltage gated channels |
| • Reduced outward K+ current |
| • Reduced activity of SERCA |
| • Loss of connexins |
| Thyroid dysfunction |
| • Thyroid follicular epithelium’s destruction and apoptosis |
| Cardiac Myopathy |
| • Ventricular and atrial dilation |
| • Ventricular hypokinesia |
| • Reduced LVEF |
| Dyslipidemia |
| • Decreased ApoA-1 and ApoE |
| • Oxidized HDL and LDL |
| • Accumulation of VLDL and triglycerides |
| Dehydration |
| • Hyperkalemia |
| • Hypernatremia |
| • Hypercoagulability |
| • Hardening of vessel walls |
| Inactivation of anti-aging genes |
| • Sirtuin 1 gene inactivation |
| • Downregulation of xenobiotic metabolism |
| • Increased mitophagy and reactive oxygen species production |
| • Tissue damage and cell death |
Table 1: Causes of sudden cardiac death in COVID-19 patients.
Cytokine storm
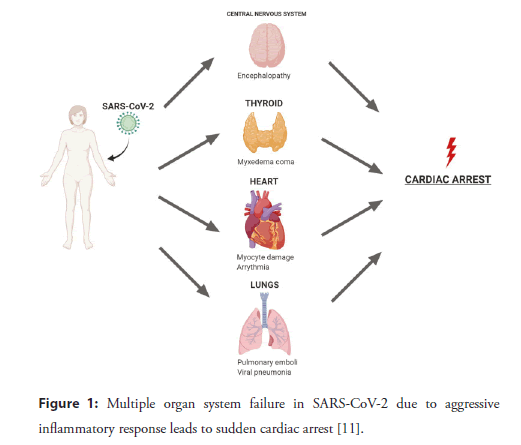
Cytokine storm is a hallmark for patients with COVID-19 as increase in cytokine levels of IL-2, IL-7, IL-10, GCSF, IP-10, MCP- 1, M1P1A and TNF-alpha has been reported [8]. Overproduction of the inflammatory cytokines has positive feedback on immune cells, which increases the rate at which these cells multiply and recruit at the inflamed site further increasing the damage (Figure 1). Histopathological and immunochemical examination of the heart tissue of a 48-year-old patient, who died of COVID-19 in Belgium, showed hypertrophied cardiac tissue infiltrated by lymphocytes and a few individual polymorphic neutrophils [9]. Predominantly lymphocytes interlocked the myocytes resulting in myocardial degeneration and necrosis. 92% of the lymphocytes were CD3+ T lymphocytes, 8% were CD20+ B lymphocytes and the ratio of CD4:CD8 was 1.7. A limited number of CD68+ macrophages and a few plasma cells were also found. Furthermore, on autopsy viral particles and signs of ferroptosis (iron-catalyzed form of cell death due to excessive lipid peroxidation) have been noted [9].
Figure 1: Multiple organ system failure in SARS-CoV-2 due to aggressive inflammatory response leads to sudden cardiac arrest [11].
Hemodynamic changes
According to previous studies, SARS-CoV-2, induces activation of coagulation and inhibition of fibrinolysis, which increases the risk of sudden myocardial death. Excess fibrin deposition increases the expression of E-selectins, P-selectins, platelet activating factors, favours the aggregation and adhesions of platelets, and increases the chances of interactions between endothelial cells and neutrophils [10,11]. This aggressive inflammatory response promotes myocarditis and micro-embolism. Blood clots in both the large and microscopic blood vessels of COVID-19 patients can not only cause tissue hypoxia, but also explain the pathophysiology of many other symptoms in these individuals. Leif Ostergaard in his short review explains that capillary occlusion and capillary blood flow disturbances can limit tissue oxidation and blood flow in kidneys, heart, and brain [12]. Blood capillary transit time is reduced and affects the time availability for blood-tissue oxygen exchange. Body senses reduced tissue oxygenation and it activates cytokine release, thus, entering a viscous cycle. Moreover, capillary basement membrane has pericytes embedded for formation, maintenance, and remodeling of the capillaries. These pericytes may be contractile, in which case, hypoxia and oxidative stress may cause the pericytes in the heart to contract and impede erythrocyte passage. It must be noted, the adverse effects on hemodynamics of an individual are subject to risk factors for capillary dysfunction. As a result, in younger patients’ capillary damage is usually asymptomatic, but in elderly individuals with symptomatic preexisting capillary dysfunction, capillary flow is expected to worsen, and life-threatening complications may occur [12].
Electrophysiological changes
It has been hypothesized that hypoxia and hypercapnia may be interfering with the electrophysiological activity of the cardiac myocytes. Acute hypoxia leads to adrenergic stimulation, which increases phosphorylation of L-type calcium channels. This increases calcium influx in cardiac myocytes and prolongs the duration of action potential, thereby, precipitating arrhythmias. Another theorized reason for prolonged action potential is reduced outward K+ current, reduced function of voltage gated Na+ channels, and altered gap junctions responsible for electrical communication between adjacent cardiac myocytes. Furthermore, acidosis due to hypercapnia reduces phase 0 slope of cardiac action potential and influences the release and uptake of calcium ions. Oxygen sensitive cysteine residues in sarcoplasmic reticulum Ca2+- ATPase are reduced, which leads to calcium accumulation in the cytoplasm of cardiac cells and less is sequestered back. In addition, sustained hypoxia, uncouples endothelial nitric oxide synthase, which increases the production of reactive oxygen species. Therefore, the variability in concentration of different ions inside and outside the cardiac myocytes, alter the electrophysiology of normally functioning cells to cause atrial and ventricular arrhythmias [13].
Thyroid dysregulation
A case report of 69-year-old woman, from David Geffen School of Medicine, discusses how SARS-CoV-2 may have caused multiple organ failure to present as sudden cardiac arrest [10] (Table 2). While the patient demonstrated many clinical findings of myxedema coma, the team states it must have been an outcome of multiple organ failure (cardiac injury, pulmonary embolism, viral pneumonia, myxedema coma) due to high inflammatory state. It was also speculated that previous history of hypothyroidism in their patient may be a contributing factor in the vulnerability of the patient to get infected by COVID-19 and later suffer from complication like myxedema coma. This is primarily because thyroid hormone regulates the immune system by regulating the function and proliferation of lymphocytes, macrophages, and dendritic cells. SARS-CoV-2 enters hosts cells via ACE-IIreceptors and these receptors are highly expressed in thyroid tissue which may explain the damage. In support of this argument, autopsy results from patients infected by SARS-CoV-1 in 2000s include thyroid follicular epithelium’s destruction and apoptosis [10].
| 1) 69-year-old woman with history of small cell lung cancer, presented with hypothermia, hypotension and decreased respiratory rate. |
| Lab investigation |
| • Raised thyrotropin, low free thyroxin, elevated thyroid peroxidase (pre-existing undiagnosed hypothyroidism) |
| • Marked raised serum inflammatory markers |
| Cause of death |
| • Multiple organ failure (including myxedema coma) and sudden cardiac arrest |
| 2) 28-year-old woman Gravida 2 para 1 African American female with morbid obesity, presented with severe shortness of breath 24-hour post-partum |
| Investigation |
| • Chest X-ray: Cardiomegaly and bilateral pulmonary opacities |
| • Transthoracic Echocardiogram: Global hypokinesis with reduced LVEF of 15% |
| • Cardiac Magnetic Resonance Imaging: Severely dilated left ventricle and LVEF-10%, moderately dilated right ventricle and RVEF-17% |
| Cause of death |
| • Dilated cardiac myopathy (either due to post-partum changes or COVID 19 infection |
| 3) 65-year-old woman with COVID-19 infection and past medical history of obesity, DM2, hypertension, hyperlipidemia, transient ischemic attack, and left sided breast cancer, presented after 2 weeks of cough, fever, and shortness of breath |
| Investigation |
| • Chest X-ray: Bilateral pulmonary opacities |
| • Transthoracic Echocardiography: Severe global right and left ventricular hypokinesia, LVEF of 25% |
| • Electrocardiography: Sinus tachycardia with shift in Q-wave inversion and QRS complex |
Table 2: Case reports included.
Cardiac myopathy
Dilated cardiac myopathy and decreased systolic function may be other causes for sudden cardiac death. A case report of a 65-yearold woman, who had been experiencing worsening cough, fever, shortness of breath for over a week, has been the first to report acute biventricular heart failure complicated by cardiogenic shock. Transthoracic echocardiography of the patient showed severe right and left ventricular hypokinesis with paradoxical septal motion and Left Ventricular Ejection Fraction (LVEF) of 25% [14]. In other case report, of 28-year-old pregnant African American female, chest X-ray showed cardiomegaly and bilateral pulmonary infiltrates, transthoracic echocardiography showed global hypokinesia with LVEF of 15%, and myocardial core biopsy showed marked inflammation and necrosis [15]. An up-regulation of IL-6 (a pro-inflammatory cytokines) may have reduced myocardial contractility to result in depression of global systolic function in these patients. Additionally, it must also be highlighted that elevated BNP levels were noted in both the patients. This may be explained by multiple organ system failure and buildup of fluid (edema) due to failing heart and kidneys. Insufficient glomerular filtration rate and decreased pumping of the heart may contribute towards congestive heart failure.
Dyslipidemia
Dyslipidemia, a condition characterized by abnormal amounts of lipids or lipoproteins in the blood, was also seen in COVID-19 patients. Decreased apoproteins on HDL (responsible for transport of excess cholesterol from cells to liver for excretion), elevated triglycerides, increased lipid peroxidation have been reported in patients suffering from COVID-19 [16,17]. This may increase the chance of sudden cardiac arrest due to atherosclerosis, blood vessel stenosis, and thrombosis. A possible explanation of the mechanism behind dyslipidemia in COVID-19 patients is that persistent inflammation results in decreased apolipoprotein A-I (ApoA-I), Apolipoprotein E (ApoE), and increased serum amyloid [17]. This affects the anti-inflammatory and antioxidant properties of High-Density Lipoproteins (HDL), which leads to further lipid oxidation. Oxidized Low-Density Lipoproteins (LDL) and oxidized High-Density Lipoproteins (HDL) lead to altered lipoprotein transportation. Moreover, low levels of ApoE on HDL decrease lipoprotein lipase activity, resulting in Very-Low-Density Lipoproteins (VLDL) and triglycerides accumulation [17].
Dehydration
Previous data suggests that COVID-19 patients may present with Gastrointestinal (GI) symptoms including anorexia, vomiting, diarrhea, and abdominal pain; out of which diarrhea is the most common. GI manifestations in COVID-19 can be explained by widely expressed ACE-II along the gastrointestinal tract (esophagus, stomach, small intestines, colon, rectum). COVID-19 related diarrhea is characterized by loose-watery stools and the average frequency of bowel movements is in the range of 3.3- 4.3 times per day; however, some patients may experience severe diarrhea with a bowel frequency of 18-30 times per day [18]. Severe dehydration due to diarrhea, disrupts the body’s ionic balance leading to hyperkalemia (especially in diabetic ketoacidosis) and raised NaCl concentration. According to a case report, hyperkalemia may result in peaked T-waves, flattened or absent P-waves, widened QRS complex; thus, promoting sudden cardiac arrest in such patients [19]. It must also be noted that elevated serum sodium levels due to dehydration increase plasma viscosity and affects genes included in cell adhesion, proliferation, leukocyte and lymphocyte activation, coagulation, and angiogenesis. Risk of atherosclerotic plaque formation in aortic root and thickening of walls of coronary arteries is increased in dehydrated individuals and may lead to sudden cardiac arrest [20].
Discussion
Inactivation of anti-aging genes
An association between anti-aging genes and the immune system has been proposed to play a crucial role in the lifespan of individuals [21]. It has been emphasized that an anti-aging gene Sirtuin-1 (Sirt 1), responsible for deacetylation of p53 transcription factor, is also essential for the maintenance of heat shock protein 70 that regulates the immune system and programs cell death [22]. Regression of Sirt 1, downregulates the transcription factors critical for metabolic activity, insulin resistance, mitophagy, and inflammation. Furthermore, nuclear receptors such as Peroxisome Proliferatoactivated Receptor gamma (PPAR gamma) and Liver X Receptors (LXR), involved in xenobiotic metabolism, are regulated by Sirt 1. Therefore, attack on Sirt 1 by SARS-CoV-2 is the most serious threat to human body as it not only leads to mitochondrial apoptosis in many tissues with relevance to diabetes and Multiple Organ Disease Syndrome (MODS), but also increases levels of xenobiotics in plasma and several tissues, which favours production of reactive oxygen species [23]. Thus, inactivation of Sirt 1 by SARS-CoV-2 damages several tissues and organs, including the heart.
Possible treatment options
COVID-19 pandemic has emerged with its unique challenges as medical institutes globally struggle for prevention, control, and treatment without compromising health care. An understanding of complex interplay of SARS-CoV-2 with the multiple organs and possible mechanisms has helped carry out epidemiological studies and randomized trials to control the spread of the novel virus. It is crucial to evaluate all systems and manage the patient accordingly.
• NSAIDS/Acetaminophen
• Corticosteroids
• Anti-viral agents
• Anti-thrombotic agents
• Angiotensin converting enzyme inhibitors and angiotensin receptor blockers
• Low molecular weight heparin
• Omega 3 polysaturated fatty acids
• Statins
Early diagnosis
The incubation period for COVID-19 is 2-14 days after exposure and most infected individuals develop symptoms between 5-6 days of contracting the virus. According to a recent meta-analysis the most common symptoms in infected individuals to be fever (81.2%), cough (58.5%), fatigue (38.5%), dyspnea (26.1%) and presence of sputum (25.8%) [24]. Signs and symptoms with laboratory findings are used for early confirmation compared to radiological investigations, which may take longer to manifest. However, it is important for both the patients and health workers to look for early signs and symptoms for better prevention of complications. Following a nasopharyngeal swab PCR or blood/sputum cultures, clinicians look for lymphopenia, eosinophilia, elevated AST/ALT, elevated CRP, elevated D-dimer, elevated troponin, and elevated ESR. For further clarity, imaging including chest X-ray (variable, bilateral patchy opacities) and chest CT (ground glass opacities with or without consolidation) are considered. In addition, COVID-19 infected individuals should get tested for anti-aging protein Sirt 1, as low levels of Sirt 1 are indicative of defective immune system and foresee an uncontrolled COVID-19 infection [21]. These results may help individualize clinical decisions and monitor patient over the course of the disease.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Pathophysiology of SARS-CoV-2 involves exaggerated inflammatory response of the body; therefore, use of NSAIDs is a management option. NSAIDs inhibit cyclooxygenase enzyme responsible for prostaglandin production to provide anti-inflammatory and anti-analgesic effects. Previous evidence emphasizes the therapeutic potential of NSAIDs, particularly naproxen, in treating respiratory infections cause by influenza and rhinovirus. Indomethacin has also shown in vitro RNA synthesis inhibition in SARS-CoV-1 [25]. Nevertheless, the risks and benefits of NSAIDs in COVID-19 are still a debate and it is suggested to use the substitute, acetaminophen, for anti-pyretic and anti-inflammatory purposes.
Corticosteroids
Another class of drug responsible for immunosuppression is corticosteroids. According to a recent systemic review, corticosteroids not only reduce the duration of mechanical ventilation but also decrease disease progression towards respiratory failure and death. Significant improvement in alveolarcapillary membrane permeability, tissue repair and reduction in inflammatory markers has been reported [26]. On the other hand, prolonged use of corticosteroids may present with delayed viral clearance, hyperglycemia, osteoporosis, emotional disturbances, central fat deposition, secondary infections, and avascular necrosis. The risk of opportunistic infections (mucormycotic, aspergillus) and reactivation of latent infections increases on using systemic corticosteroids for longer or on combination or systemic corticosteroids and other immunosuppressants [27]. Hence, the beneficial and detrimental outcomes of corticosteroids in treatment of SARS-CoV-2 require more clinical trials before a consensus is reached.
Anti-viral drugs
Since SARS-CoV-2 requires RNA dependent RNA polymerase (RdRp) to multiply after gaining entry into host cells, drugs inhibiting RdRp may prove helpful. As per available literature, remdesivir and chloroquine exhibit anti-viral properties in vivo and in vitro. Remdesivir is a nucleotide analog inhibitor of RdRp, while chloroquine inhibits viral replication by interfering with ACE2 and by inhibiting the production and release of TNF and IL-6 [28]. Hydroxychloroquine has a similar mode of action as chloroquine but has proved more potent and has better safety profile than chloroquine in treatment of COVID-19 patients [29]. A combination of aspartate protease inhibitors (lopinavir and ritonavir) normally used for HIV infections may be considered to treat severe COVID-19 patients after further clinical trials [30]. Primitive clinical results regarding the effect of favipiravir in COVID-19 patients have shown decreased median time for viral clearance and improvement in chest CT [31].
Anti-thrombotic therapy
Virus mediated endothelial dysfunction and cytokine storm promote diffuse microvascular thrombi in multiple organs, therefore, contributing to sudden cardiac arrest in COVID-19 patients. Antithrombotic drugs, such as anti-coagulants may help prevent clot formation in these patients. A case of 107 patients showed that Intensive Care Unit (ICU) patients with COVID-19, had high frequency of pulmonary embolism (20.6%), almost twice as high as was noted in influenza patients admitted in ICU for respiratory failure in 2019 [32]. Though prophylactic use to anticoagulants in pro-thrombotic COVID-19 patients is still under consideration, a retrospective study of 449 severe COVID-19 patients showed that 99 patients (22.0%) received Low Molecule Weight Heparin (LMWH) for around 7 days [33]. Another study points out that patients with elevated D-dimer (greater than 6 folds above the upper normal limit) and were on prophylactic heparin showed approximately 20% lower mortality when compared to patients who did not receive heparin. Moreover, phase 1 clinical trials have demonstrated positive effects of fibrinolytic therapy i.e., reduced mortality and improved oxygenation [34]. A study has also discussed heparin’s anti-inflammatory properties by dampening NF-κB, a pro-inflammatory transcription factor involved in SARS-CoV-2 [35]. Despite anti-coagulant properties, use of anti-thrombotic drugs as therapeutic pharmacological agents in COVID-19 individuals still awaits systemic evaluation.
Other
A retrospective study of 51 hypertensive COVID-19 patients, Angiotensin Converting Enzyme Inhibitors (ACEI) and Angiotensin Receptor Blockers (ARB) have decreased IL-6 and viral load levels compared to patients taking other antihypertensives. However, both ACEI and ARB upregulate ACE2 expression and may increase disease severity, so the use of these agents to treat COVID-19 patients is arguable [36]. Another study suggests use of Low Molecular Weight Heparin (LMWH) to prevent Deep Venous Thrombosis (DVT) in COVID-19 patients as statistics show larger proportion of LMWH administration in surviving (18/22) than deceased (18/33) patients [11]. Omega 3 polysaturated fatty acids are precursors for Specialized Pro-resolving Mediators (SPMs) and help combat inflammation. A combined therapy of omega 3 polysaturated fatty acids and aspiring may have anti-coagulant properties [12]. Use of statins to treat dyslipidemia and for antiinflammatory properties was also pointed out in a meta-analysis [37].
Vaccination
Life threatening SARS-CoV-2 has led to early development of COVID-19 vaccines that intend to reduce the spread and severity of the infection. Different strategies in the development of such vaccines include, nucleic mRNA-based vaccines (Pfizer and Moderna), viral vector vaccines (Oxford-AstraZeneca, Gamaleya, CanSino and Johnson and Johnson), whole pathogen inactivated viral vaccines (Sinopharm, Sinovac and Bharat Biotech’s vaccine) and subunit vaccines. These vaccines primarily target the S-protein amongst the virus surface elements with the goal of helping susceptible population subgroups, for example health workers, elderly, pregnant women, immunocompromised individuals. Though, observational studies indicate self-limiting side effects of COVID-19 vaccines including pain/swelling at site of injection 29%-85% of participants), fever (0.2%-95%), myalgia, fatigue (8.4%-55%), and headache [38], a peculiar finding highlighted by a meta-analysis was the presence of thrombosis and thrombocytopenia in 23 patients, with no previous prothrombotic condition, 6-24 days after receiving AstraZeneca. Most patients had more than one site of thrombosis, the common sites being cerebral venous system, arterial thrombosis, pulmonary emboli, and hepatic-portal vein [39]. Furthermore, myocarditis has also been reported in young adult and adolescent males after second dose of COVID-19 mRNA vaccinations in around 12.6 per million doses. These presented with chest pain, had elevated cardiac troponin levels and abnormal ECG (ST segment elevation) [40]. Following this, intensive reviews for the risk of thromboembolism associated with COVID-19 were run by U.K. Medicines and Healthcare Products Regulatory Agency and European Medicines Agency, which confirmed the risk was not higher than background risk for deep venous thrombosis in general population [40]. Moreover, a recent systemic review and meta-analysis detected 8,926 published articles on COVID-19 vaccines to evaluate the efficacy, immunogenicity, and safety of COVID-19 vaccines. Seven studies included in the meta-analysis highlighted that the efficacy of adenovirus vector vaccine was 73% (95% Cl=69-77) and that of messenger RNA (mRNA) vaccine was 85% (95% Cl=82-88) in participants aged ≥ 18 years. Moreover, production of neutralizing antibodies against receptorbinding domains in greater than 90% of the vaccinated individuals was noticed within 30 days [38]. Thus, it can be concluded that currently available vaccines and potential booster doses are the most effective interventions to combat the deadly virus.
Conclusion and Clinical Perspective
An understanding of possible pathophysiological mechanisms behind sudden cardiac death in COVID-19 patients together with the potential management and treatment, as has been highlighted in this review, will enable health care workers globally to reduce both the spread and severity of COVID-19. This knowledge is particularly significant for primary caregivers to deliver timely and individualized care to COVID-19 patients, keeping in view the currently known viral characteristics and indications. While much has been hypothesized and proven through case reports, clinical trials, meta-analysis, and observational studies from previous SARS-CoV-2 outbreaks, the need for further research and more effective therapeutic approach remains. The safety and efficacy of diagnostic tools and treatment options can be further improved to save lives in this global pandemic.
References
- Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus Pandemic (COVID-19). Our World in Data. (2022).
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. NEJM. 382(18): 1708-1720 (2020).
- Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5(11): 1265-1273 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur Heart J. 41(19) 1810-1817 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Kornilov SA, Lucas I, Jade K, et al. Plasma levels of soluble ACE2are associated with sex, Metabolic Syndrome, and its biomarkers in a large cohort, pointing to a possible mechanism for increased severity in COVID-19. Crit Care. 24(1): 1-3 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 109(5): 531-538 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Goha A, Mezue K, Edwards P, et al. COVID-19 and the heart: An update for clinicians. Clin Res Cardiol. 43(11): 1216-1222 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Song P, Li W, Xie J, et al. Cytokine storm induced by SARS-CoV-2. Clinica Chimica Acta. 509: 280-287 (2020)
[CrossRef] [Google Scholar] [Pubmed]
- Jacobs W, Lammens M, Kerckhofs A, et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature. ESC Heart Fail. 7(6): 3772-3781 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Dixit NM, Truong KP, Rabadia SV, et al. Sudden cardiac arrest in a patient with myxedema coma and COVID-19. J Endocr Soc. 4(10): bvaa130 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Zhang P, Qu Y, Tu J, et al. Applicability of bedside ultrasonography for the diagnosis of deep venous thrombosis in patients with COVID-19 and treatment with low molecular weight heparin. J Clin Ultrasound. 48(9): 522-526 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep. 9(3): e14726 (2021).
[CrossRef] [Google Scholar] [Pubmed]
- Lee S, Li G, Liu T, et al. COVID-19: Electrophysiological mechanisms underlying sudden cardiac death during exercise with facemasks. Med Hypotheses. 144: (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Chitturi KR, Thacker S, Al-Saadi MA, et al. Successful treatment of acute heart failure in COVID-19-induced cytokine storm with tocilizumab: A case report. Eur Heart J Case Rep. 4(FI1): 1-6 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Akintayo AA, Addo B, Soleye SO, et al. Diagnostic dilemma: COVID-19 related cardiomyopathy or peripartum cardiomyopathy? J Cardiol Cases. 24(5): 206-209 (2021).
[CrossRef] [Google Scholar] [Pubmed]
- Sorokin AV, Karathanasis SK, Yang ZH, et al. COVID-19-Associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 34(8): 9843-9853 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Hariyanto TI, Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 14(5): 1463 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Megyeri K, Dernovics Á, Al-Luhaibi ZII, et al. COVID-19-associated diarrhea. World J Gastroenterol. 27(23): 3208 (2021).
[CrossRef] [Google Scholar] [Pubmed]
- Mattu A, Brady WJ, Robinson DA. Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med. 18(6): 721-729 (2000).
- Dmitrieva NI, Burg MB. Elevated sodium and dehydration stimulate inflammatory signaling in endothelial cells and promote atherosclerosis. PLOS ONE. 10(6): e0128870 (2015).
[CrossRef] [Google Scholar] [Pubmed]
- Martins IJ. Acta scientific nutritional health biotherapy and the immune system in ageing science. (2018).
- Martins IJ. COVID-19 infection and anti-aging gene inactivation. (2022).
- Martins IJ. Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. (2022).
- Alimohamadi Y, Sepandi M, Taghdir M, et al. Determine the most common clinical symptoms in COVID-19 patients: A systematic review and meta-analysis. J Prev Med Hyg. 61(3): E304 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Yousefifard M, Zali A, Zarghi A, et al. Non-steroidal anti-inflammatory drugs in management of COVID-19; A systematic review on current evidence. Int J Clin Pract. 74(9): e13557 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Raju R, Prajith V, Biatris PS, et al. Therapeutic role of corticosteroids in COVID-19: A systematic review of registered clinical trials. Futur J Pharm Sci. 7(1): 67 (2021).
[CrossRef] [Google Scholar] [Pubmed]
- van Paassen J, Vos JS, Hoekstra EM. Corticosteroid use in COVID-19 patients: A systematic review and meta-analysis on clinical outcomes. Crit Care. 24(1): (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30(3): 269-271 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Yao X, Ye F, Zhang M, et al. In Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 71(15): 732-739 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 382(19): 1787-1799 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID-19: An open-label control study. Engineering (Beijing). 6(10): 1192-1198 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with COVID-19: Awareness of an increased prevalence. Circulation. 142(2): 184-186 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 18(5): 1094-1099 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Wang J, Hajizadeh N, Moore EE, et al. Tissue Plasminogen Activator (tPA) treatment for COVID-19 associated Acute Respiratory Distress Syndrome (ARDS): A case series. J Thromb Haemost. 18(7): 1752-1755 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Li X, Li L, Shi Y, et al. Different signaling pathways involved in the anti-inflammatory effects of unfractionated heparin on lipopolysaccharide-stimulated human endothelial cells. J Inflamm (Lond). 17(1): 5 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5(9): 1020-1026 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 134: 153-155 (2020).
[CrossRef] [Google Scholar] [Pubmed]
- Sharif N, Alzahrani KJ, Ahmed SN, et al. Efficacy, immunogenicity and safety of COVID-19 vaccines: A systematic review and meta-analysis. Front Immunol. 12: 714170 (2021).
[CrossRef] [Google Scholar] [Pubmed]
- Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 384(23): 2202-2211 (2021).
[CrossRef] [Google Scholar] [Pubmed]
- Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 144(6): 471-484 (2021).
[CrossRef] [Google Scholar] [Pubmed]