Research Article - Journal of Medicinal and Organic Chemistry (2022) Volume 5, Issue 4
Synthesis and Characterization of Fe(III)-Ion with N-(4-Hydroxyphenyl) Acetamide
Aida Smajlagic1*, Majda Srabovic1, Zahida Ademovic2, Ekrem Pehlic3, Melita Huremovic1, Edina Huseinovic1
1University of Tuzla, Faculty of Natural Sciences and Mathematics, Department of Chemistry, Bosnia and Herzegovina 2University of Tuzla, Faculty of Technology, Bosnia and Herzegovina 3University of Bihać, Faculty of Biotechnical Engineering, Bosnia and Herzegovina
Received: 29-Aug-2022, Manuscript No. JMOC-22-73164; Editor assigned: 01-Sep-2022, PreQC No. JMOC-22- 73164 (PQ); Reviewed: 15-Sep-2022, QC No. JMOC-22-73164; Revised: 22-Sep-2022, Manuscript No. JMOC- 22-73164 (R); Published: 29-Sep-2022 DOI: 10.37532/jmoc.2022.5(4).73-77
Abstract
Objective: The molecule N-(4- hydroxyphenyl) acetamide (paracetamol) has several potential centres where reaction can be initiated, and it is benzene ring, hydroxyl (-OH), amino (-NH) and keto (-C=O) group. The aim of this research paper is to establish complex between defence of molecule and Fe(III)-ion. Different researches of complex ligands for iron are very important in medicine, because molecules are used as antibiotics or medicinal reagents.
Methods: Identification and characterization of the complex Fe (III)-N-(4-hydroxyphenyl) acetamide was done with FTIR, UV and MS spectroscopy.
Results: After the spectrum analysis of the synthetized complex utilizing FTIR, UV and MS method, the results indicate the coordination between ligands and metals through adequate functional groups.
Conclusion: Based on the obtained results, it may be confirmed that Fe (III)-ion achieves interaction through free electronic pair of oxygen atom (-OH) group and nitrogen atom (-NH) group N-(4- hydroxyphenyl) acetamide and accordingly indicates that the molecule of N-(4- hydroxyphenyl) acetamide behaves as bidentate ligand.
Keywords
N-(4- hydroxyphenyl) acetamide • Metal complexes, iron (III)-ion • Bidentate ligand • FTIR •UV •MS
Introduction
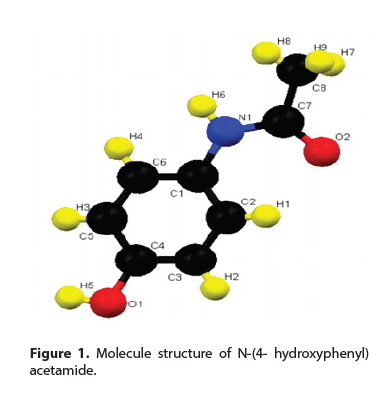
N-(4- hydroxyphenyl) acetamide (paracetamol) was synthetized in 1878. by Morse, and it was clinically used for the first time by Von Mering in 1887 [1]. It is usually grouped with nonsteroid anti-inflammatory drugs (NSAID), but it is not considered to be the one, because it shows poorer anti-inflammatory activities. As one of the most frequently used (OTC) drugs, its main activity mechanism is inhibition of cyclooxygenases (COX), the enzyme responsible for production of prostaglandin, important mediators of inflammation, pain and temperature [2]. Molecule structure paracetamol comprises aromatic rings with two main activating hydroxyl and amide groups (Figure 1). Paracetamol is a derivative of aniline compounds [3],while present hydroxyl groups (-OH) in para position differentiates molecule from other aniline derivatives.
The researches showed that in the last century, attention was paid to researching the metal complexes utilising drugs as ligand [4]. Metal complexes became important in detection of drugs widely used as therapy compounds in treatment of illnesses such as cancer, lymphoma, infectious diseases, diabetes, anti-inflammatory and neurological disorders. Biometal cations in biological systems may interact with water molecules and different parts of various organic and non-organic biomolecules [5], which may be taken in the organism through food or pharmaceutical drugs. Building of complexes with drugs through O-, N- and S-donor atoms of appropriate functional groups (-OH, -COOH, -SH, -NH2), leads to improvement of stability and overall biological importance of molecule. N-(4- hydroxyphenyl) acetamide coordinates as bidentate ligand through oxygen atom of hydroxyl group located on benzene ring (H-O-M) and the atom of carbonyl group (C=O-M). Coordination is also possible through nitrogen atom from amino group (-NH). Iron (Fe) is one of the most important elements in organism and is integral part of many proteins, which is found in several oxidation conditions and forms stable coordination complexes. This metal participates in catalysis of different oxide-reduction reactions and due to that features it changes oxidation conditions. Iron is taken into the organism in inorganic form or in relation to chemical.
Inorganic iron must be in divalent Fe(II) form to be transported through membranes utilizing adequate transport proteins such as transferrin transport protein 1 for divalent metals [6]. naturally important helat ligands, with high affinity for FeIII are siderophores. These natural ligands are isolated from bacteria, yeast and fungus.
Material and Methods
Chemicals
In laboratory researches, the used chemicals for synthesis are iron(III) chloride-FeCl3 (Semikem), N-(4- hydroxyphenyl) acetamide -C8H9NO2( 99% Kemig) and distilled water.
Synthesis of Fe(III)-N-(4- Hydroxyphenyl) Acetamide Complex
Solution of N-(4- hydroxyphenyl) acetamide or paracetamol is prepared with 0,51g iron (III)– chloride (FeCl3) in 0,91 g of distilled water of molar ratio (2:1) in favour of ligand with pH=7.
After that, the solution of N-(4- hydroxyphenyl) acetamide is mixed under reflux for 6 hours at the temperature of 60°C with metal chloride. Following the completion of the synthesis, the solutions rest for 48 hours until the crystals are formed [2,8].
Ftir, Uv/Vis and Ms Characterization
Synthetized complex is recorded with spectrophotometer Thermo Scientific Nicolet I S10 in range from 4000-450 cm-1, resolution 2 cm-1.
UV/VIS spectrophotometric analysis of synthetized complex is done on Perkin Elmer Lambda UV/VIS 25 spectrophotometer within the wave range from 200 to 400 nm.
Metal complex is analysed on spectrometer LCMS/ MS Agilent Technologies
Result and Discussion
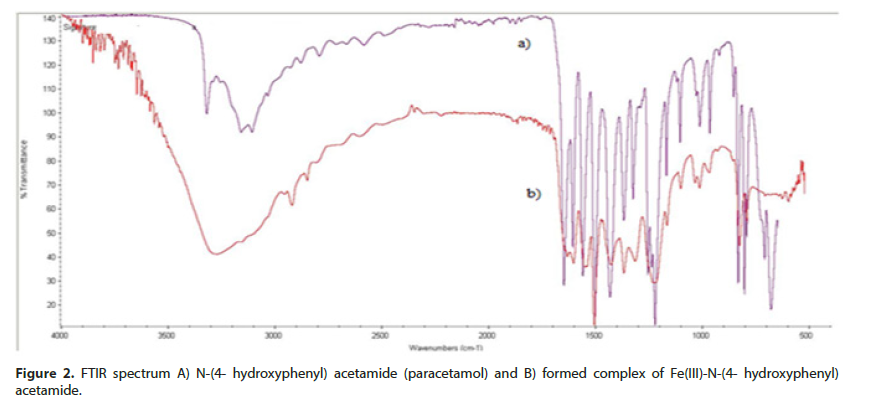
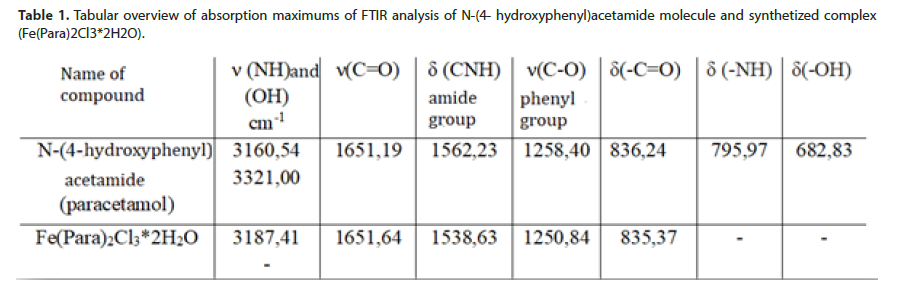
N-(4-hydroxyphenyl) acetamide molecule coordinates through appropriate functional groups with Fe(III)-ion in stoichiometry ratio 2:1.FTIR spectrum of free N-(4-hydroxyphenyl) acetamide and formed complex between Fe (III)-ion and N-(4- hydroxyphenyl) acetamide is presented (Figure 2). Due to interaction between Fe(III)-ion and N-(4- hydroxyphenyl) acetamide through nitrogen atom of amino group (-NH), the wave number moves to 3187,41 cm-1 in the spectrum of the development complex towards the bigger numbers compared to the spectrum of free N-(4- hydroxyphenyl) acetamide. The ribbon originates from valence (-NH) vibrations. Due to interaction of Fe(III)-ion with oxygen atom of hydroxyl (-OH) group, the ribbon disappears (Figure 2).
The ribbons not presenting the spectrum, arising from deformation (-OH) vibrations outside of the plain, as well as deformation (-NH) vibrations are also the consequence of metal-ligand interaction. Based on these facts, it may be confirmed that Fe(III)-ion achieves interaction through free electronic pair of oxygen atom (-OH) group and nitrogen atom (-NH) group N-(4- hydroxyphenyl) acetamide, which complies with the literature that the molecule of N-(4- hydroxyphenyl) acetamide behaves as bidentate ligand [7].
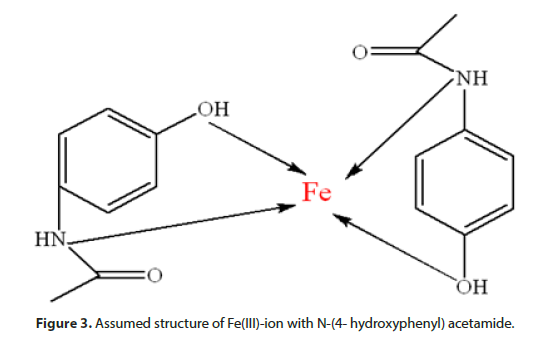
Absorption ribbon at 1651,64 cm-1 originates from valence (-C=O) vibrations, without change of wave number compared to the free N-(4- hydroxyphenyl) acetamide, as well as deformation (-C=O) vibration to 835,37 cm- 1, with no significant movement compared to N-(4- hydroxyphenyl) acetamide. Results of the research indicate that Fe(III)-ion does not coordinate with oxygen atom from carbonyl (-C=O) group N-(4- hydroxyphenyl) acetamide. Absorption maximum of FTIR analysis of synthetized complex is presented (Table 1). The assumed structure of Fe(III)-ion with N-(4- hydroxyphenyl) acetamide is shown (Figure 3).
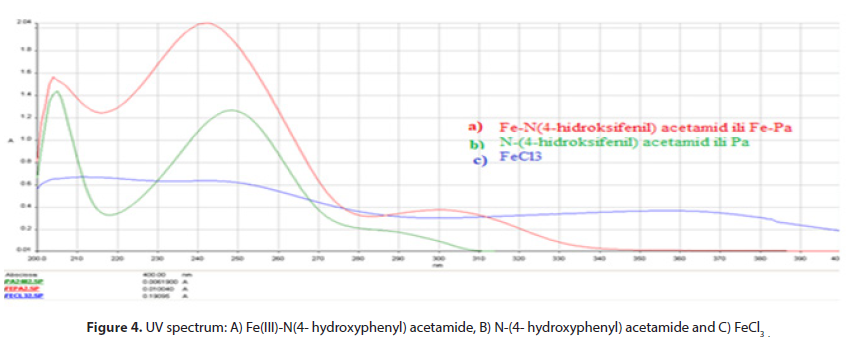
The (Figure 4) Shows UV spectrum of formed complex of Fe(III)-ion and N-(4- hydroxyphenyl) acetamide. The spectrum N-(4- hydroxyphenyl) acetamide shows absorption maximum at 248,10 nm. Spectrum of metal-ligand product is present, where the ribbon of high intensity at 242,19 nm leads to smaller values of wave lengths (blue progress) compared to the spectrum of free (N-(4- hydroxyphenyl) acetamide and it refers to participation of free pairs of nitrogen atom (-NH) group and oxygen (-OH) group in development of coordinative bond.
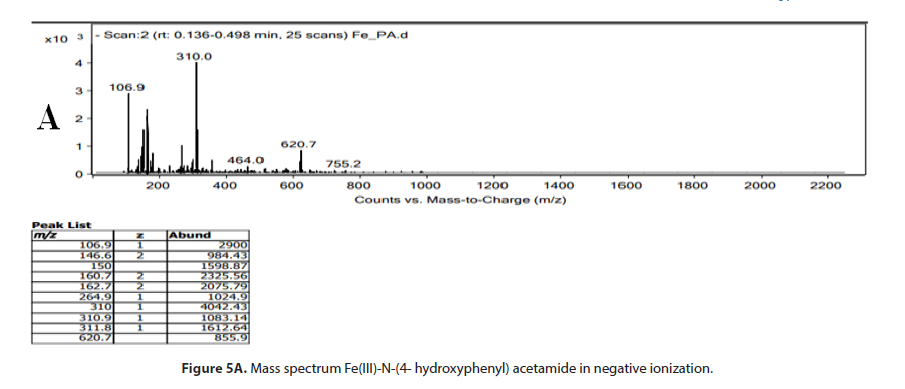
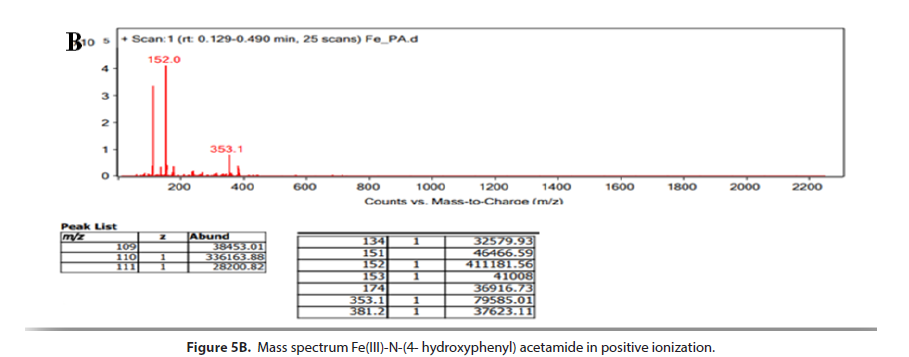
The (Figure 5A and 5B) show the mass spectrum of formed complex between N-(4- hydroxyphenyl) acetamide and Fe(III)-ion in positive and negative ionization. The spectrogram (Figure 5A) shows the peak of smaller intensity, which represents the main ion m/z=464,0 arising from the made complex (2 : 1) in favour of ligand N-(4- hydroxyphenyl) acetamide and salt FeCl3. Emphasised fragment peak at spectrogram m/z=310 arises due to separation of one molecule of N-(4- hydroxyphenyl) acetamide. In further fragmentation procedure, characteristic fragment peak is obtained at m/z =150, which could originate from molecule ion N-(4- hydroxyphenyl) acetamide. There is also a fragment peak at m/z=106,9 arising from separation of acetilium ion. It is assumed that the peaks of small intensity m/z=620,7 and m/ z=755,2 occur during electro-spray ionization process. The (Figure 5B) shows mass spectrum of the made complex in positive ionization, where the peak of higher intensity is present m/z=152,0 which belongs to the molecule ion N-(4- hydroxyphenyl) acetamide, and which in further fragmentation process leads to occurrence of fragment peak at m/z=110. The peak of smaller intensity m/z = 353,1, the most probably arises from fragmented ion made in metal-ligand interaction.
Conclusion
Results of experimental researches indicate development of the complex between molecule and Fe(III)-ion defence. Synthetized complex is analysed utilizing FTIR, UV and MS method. Based on the analysed data, coordination of N-(4- hydroxyphenyl) acetamide through free electronic pair of oxygen atom (-OH) group and nitrogen atom (-NH) group may be noticed. With establishment of the complex between ligands and Fe(III)-ion, stability of the compound is improved aimed at further research.
Acknowledgement
The authors extend their gratitude to the Institute Ruđer Bošković in Zagreb for the analysis of synthetized complex utilizing MS method.
References
- Bertolini A, Ferrari A, Ottani A et al. Paracetamol: New Vistas of an Old Drug. CNS Drug Reviews. 12, 250–275 (2006).
- Refat MS, Mohamed GG, El-Sayed MY et al. Spectroscopic and thermal degradation behaviour of Mg(II), Ca (II), Ba (II) and Sr (II) complexes with paracetamol drug. Arab J Chem. 10, 2376-2387 (2013).
- Nichols G, Frampton CS Physicochemical characterization of the orthorhombic polymorph of paracetamol crystallized from solution. J Pharm Sci. 87, 684-693 (1998).
- Hariprasath K, Deepthi B, Sudheer BI et al. Metal complexes in drug research - A review. J Chem Pharm Res. 2, 496-499 (2010).
- Krstić NS, Nikolić RS, Stanković MN et al. Coordination Compounds of M(II) Biometal Ions with Acid Type Anti-inflammatory Drugs as Ligands – A Review. Trop J Pharm Res. 14, 337-349 (2015).
- Car Ž. 3-Hydroxypiridine-4-ions (II part) (2017): Biological use as iron chelator. Chem Ind. 66, 17−28.
- Sultan MA, Karim AE, Kandory A et al. Synthesis and characterization of Al(III) complex with paracetamol. Int J Pharm Qual Assur. 10,156-159 (2020).
- John Akwu Faruna, Elaoyi David Paul, Yakubu Ali Dallatu Synthesis, Characterization and Evaluation of Anti-Inflammatory Activity of Acetaminophen Complexes with Copper (II) and Zinc (II) Ions. J Biomed Mater Res. 5, 78-83 (2017).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref