Editorial - International Journal of Clinical Rheumatology (2017) Volume 12, Issue 4
Targeted gene therapy in the management of osteoarthritis: back to the future with great expectations
- Corresponding Author:
- Tamer A Gheita
Department of Rheumatology and Clinical Immunology
Cairo University, Egypt
E-mail: gheitamer@hotmail.com
Abstract
Keywords
osteoarthritis, gene therapy, treat to target, joint degeneration
Osteoarthritis (OA) is a degenerative joint disease of the articular cartilage with subchondral bone remodeling and secondary inflammation [1]. OA is the most common joint disease worldwide, a chief cause of chronic disability especially in the elderly. The universal increase in life expectancy will make OA the 4th most important cause of disability by the year 2020 and the single greatest cause by 2030 [2]. It is considered a whole-joint problem that leads to cartilage loss and eventually joint failure [3] with severe pain [4]. There is no cure for OA and modern therapies usually fail to improve symptoms which may lead to an amplified demand for joint replacement surgery [4]. The treatment armamentarium and strategies depend on the disease stage and severity and new insights into OA pathophysiology and novel therapeutics will improve its pharmacologic options [5]. The development of Disease Modifying Anti-osteoarthritic Drugs (DMOADs) now faces important difficulties and has not proven effective in slowing down OA disease progression [6]. Yet, it is also questionable if the non-pharmacological treatment options could help prevent disease progression. When it comes to management of OA, it has been recently advocated by Malemud [7] to think outside the box. Interestingly, the localized nature of OA makes it an ideal candidate for gene therapy [1].
It is becoming apparent that many genes, each with a small effect size, contribute to the risk of development of the disease, though, the genetics of OA pain are starting to be explored. OA was relatively late to enter the GWAS era but the benefits were considerable. Biological insights of the novel robust associations represent the largest translational contribution of GWAS findings to OA [8]. The compound genetic factors that contribute to the incidence and severity of OA may vary in relation to certain joints (hand, hip, knee or spine) involved, gender and ethnicity. There is persuasive verification that there are joint specific genetic factors contributing to the pathogenesis of OA in harmony with the notable reported variations in the prevalence of OA at skeletal sites [9]. In order to properly treat and repair damaged articular cartilage the three main target cells for genetic modification are synovial lining cells, chondrocytes and mesenchymal stem cells [10]. Examples of candidate genes used in gene therapy of OA are shown in Table 1.
| Category | Examples |
|---|---|
| Growth factor | IGF-1, TGF-β, BMP-2, -7, FGF |
| Cytokine/anti-cytokine | IL-4, IL-1Ra, sIL-1R, sTNFR |
| Proteinase inhibitor | TIMP, PAI, Serpin |
| Free radical antagonist | SOD |
| TF/signaling molecule | Sox-5,-6,-9, SMAD |
| Matrix molecule | Type II collagen, COMP |
| Enzyme | GFAT |
| Inhibitor of apoptosis | Bcl-2 |
Table 1: Candidate genes categories for use in osteoarthritis gene therapy.
Huge efforts are on the way to identify genetic and epigenetic factors influencing OA [11]. A candidate gene approach has been used to screen large populations for mutations in genes assumed to be implicated in the occurrence of OA. The most effective candidates are considered in the growth or differentiation pathways essential for the formation and upholding of synovial joints. Signal transduction pathways important in joint formation are stimulated by Bone Morphogenetic Proteins (BMPs), Transforming Growth Factors (TGFs) and Wnt family proteins all of which have been involved in OA [12]. Cartilage Acidic Protein-1 is overexpressed in the OA joint and is produced in chondrocytes and synovial fibroblasts by proinflammatory cytokines. It appreciably slows cartilage degeneration, osteophyte formation and gait abnormalities of post-traumatic OA [13]. Medications used further play a role; steroids and selective cyclo-oxygenase-2 inhibitors decrease Matrix Metalloproteinase (MMP-1) and increase aggrecan gene expression. Increased gene expression of type II collagen was also observed with celecoxib while piroxicam showed no effects [14]. Long noncoding RNAs play critical regulatory roles in many biologic processes, contribute to the Extra-Cellular Matrix (ECM) degradation and are key players in the pathogenesis of OA which makes them also possibly used as potential targets in OA therapy [15].
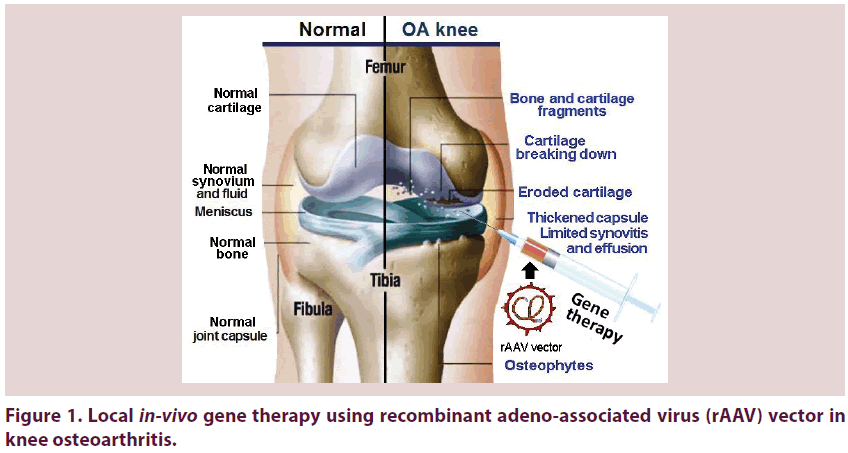
The joints are very complex targets for drug delivery in a specific and selective mode. Gene transfer is the only technology that can resolve the delivery problem in a clinically practical fashion. Large steps are taken in the use of gene transfer to enhance cartilage repair, ligament healing and restoration of different tissues as the tendon and meniscus making it an early clinical success for gene therapy [16]. Genes can be proficiently transferred to tissues within joints by Intra- Articular (IA) injection using an array of variable vectors and strategies. Using the appropriate gene transfer technologies, IA expression of anti-arthritic transgenes at therapeutic levels can be reached [17]. Gene therapy is an emerging therapeutic line that offers a unique treatment option [6] and may help in breakthroughs in the outcome and management of OA. The large steps taken in this therapeutic option will definitely take us ‘back to the future’ with potential ‘great expectations’. Figure 1 shows local in-vivo gene therapy using Recombinant Adeno-Associated Virus (rAAV) vector in knee OA. In spite the fact that there are many applicable genes of potential use in managing OA genetically, substantial proof throws light on Interleukin-1 (IL-1) as a principle target [10] having an outstanding role in the pathophysiology of OA. Gene transfer of its receptor antagonist (IL-1Ra) adds to the promising future towards cure form OA disease [18].
Recent years have seen an explosion in the volume and quality of genomic studies in the field of OA. A minority have set out to identify genes that regulate OA joint pain. In future studies of the genetic architecture of OA pain, the use of endophenotypes and a focus on epigenetic processes will help clarify the role of genetic factors in its development. Advances in understanding contribute to an effective treatment design to relieve the substantial burden of OA pain [8]. An in-depth understanding of the gene expression profiling of OA may help out in enlightening the mysteries of the pathogenesis of OA and aid in detecting the precise targets for OA treatment [19]. Use of advanced drug delivery systems involving cartilage-specific miRNAs may further accelerate the application of these new findings in arthritis therapy [20]. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene vectors for gene therapy of OA have been developed. These nanoparticles with improved transfection efficiency and low cytotoxicity may be safe and efficient for gene delivery to both chondrocytes and synoviocytes [21].
References
- Ruan MZ, Cerullo V, Cela R et al. Treatment of osteoarthritis using a helper-dependent adenoviral vector retargeted to chondrocytes. Mol. Ther. Methods Clin. Dev. 3, 16008 (2016).
- Tesche F, Miosge N. Perlecan in late stages of osteoarthritis of the human knee joint. Osteoarthr. Cartil. 12(11), 852–862 (2004).
- Jones G. Osteoarthritis: Where are we for pain and therapy in 2013? Aust. Fam. Physician. 42(11), 766–769 (2013).
- Panoutsopoulou K, Zeggini E. Advances in osteoarthritis genetics. J. Med. Genet. 50(11), 715–724 (2013).
- Hinz B, Brune K. Pain and osteoarthritis: new drugs and mechanisms. Curr. Opin. Rheumatol. 16(5), 628–633 (2004).
- Wang HJ, Yu CL, Kishi H et al. Suppression of experimental osteoarthritis by adenovirus-mediated double gene transfer. Chin. Med. J. (Engl). 119(16), 1365–1373 (2006).
- Malemud CJ. The medical therapy of osteoarthritis: “thinking outside the box”. Int. J. Clin. Rheumatol. 11(1), 3–5 (2016).
- Thakur M, Dawes JM, McMahon SB. Genomics of pain in osteoarthritis. Osteoarthr. Cartil. 21(9), 1374–1382 (2013).
- Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthr. Res. Ther. 11(3), 227 (2009).
- Evans CH, Gouze JN, Gouze E, Robbins PD. Ghivizzani SC. Osteoarthritis gene therapy. Gene Ther. 11(4), 379–389 (2004).
- Arden N, Richette P, Cooper C et al. Can we identify patients with high risk of osteoarthritis progression who will respond to treatment? a focus on biomarkers and frailty. Drugs Aging. 32(7), 525–535 (2015).
- Sandell LJ. Etiology of osteoarthritis: genetics and synovial joint development. Nat. Rev. Rheumatol. 8(2), 77–89 (2012).
- Ge X, Ritter SY, Tsang K et al. Sex-specific protection of osteoarthritis by deleting cartilage acid protein 1. PLoS One. 11(7), e0159157 (2016).
- Cho H, Walker A, Williams J, Hasty KA. Study of osteoarthritis treatment with anti-inflammatory drugs: cyclooxygenase-2 inhibitor and steroids. Biomed Res. Int. 2015, 595273 (2015).
- Liu Q, Zhang X, Dai L et al. Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheumatol. 66(4), 969–978 (2014).
- Evans CH. Gene therapies for osteoarthritis. Curr. Rheumatol. Rep. 6(1), 31–40 (2004).
- Evans CH, Ghivizzani SC, Robbins PD. Arthritis gene therapy and its tortuous path into the clinic. Transl. Res. 161(4), 205–216 (2013).
- Wang G, Evans CH, Benson JM et al. Safety and biodistribution assessment of sc-rAAV2.5IL-1Ra administered via intra-articular injection in a mono-iodoacetate-induced osteoarthritis rat model. Mol. Ther. Methods Clin. Dev. 3, 15052 (2016).
- Rao ZT, Wang SQ, Wang JQ. Exploring the osteoarthritis-related genes by gene expression analysis. Eur. Rev. Med. Pharmacol. Sci. 18(20), 3056–3062 (2014).
- Asahara H. Current status and strategy of microRNA research for cartilage development and osteoarthritis pathogenesis. J. Bone Metab. 23(3), 121–127 (2016).
- Lu H, Dai Y, Lv L, Zhao H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS One. 9(1), e84703 (2014).