Review Article - Journal of Experimental Stroke & Translational Medicine (2010) Volume 3, Issue 1
Targeting ischemic penumbra Part I: from pathophysiology to therapeutic strategy
- *Corresponding Author:
- Shimin Liu, M.D., Ph.D.
Department of Neurology, Mount Sinai School of Medicine NYU
1468 Madison Avenue, New York, NY 10029, USA.
Tel: 212-241-2252
Email: shimin.liu@mssm.edu
Abstract
Penumbra is the viable tissue around the irreversibly damaged ischemic core. The purpose of acute stroke treatment is to salvage penumbral tissue and to improve brain function. However, the majority of acute stroke patients who have treatable penumbra are left untreated. Therefore, developing an effective non-recanalizational therapeutics, such as neuroprotective agents, has significant clinical applications. Part I of this serial review on “targeting penumbra” puts special emphases on penumbral pathophysiology and the develop-ment of therapeutic strategies. Bioenergetic intervention by massive metabolic suppression and direct energy delivery would be a promising future direction. An effective drug delivery system for this purpose should be able to penetrate BBB and achieve high local tissue drug levels while non-ischemic region being largely unaffected. Selective drug delivery to ischemic stroke penumbra is feasible and deserves intensive research.
Keywords
Stroke; cerebral ischemia; neuroprotection; penumbra; treatment; energy state; cerebral energy me-tabolism
Introduction
Each year, approximately 795 000 people experience a new or recurrent stroke. On average, every 40 seconds, someone in the United States has a stroke. Overall stroke prevalence during 2003 to 2006 is around 2.9%. Of all strokes, 87% are ischemic. (Lloyd-Jones et al. 2010) Due to stroke’s high inci-dence and prevalence rates and the lack of effective treatment, stroke remains one of the major diseases causing most mortality and disability. Stroke is the third leading cause of death, behind diseases of the heart and cancer, and is a leading cause of serious, long-term disability in the United States. Although treatments for ischemic stroke have been rigorously investigated for two decades, up to now there is only one FDA-approved pharmacological treatment for ischemic stroke, the intravenous thrombolytic treat-ment using recombinant tissue plasminogen activator (r-tPA).(Jahan and Vinuela 2009), which can only be available to a very limited number of patients (Klein-dorfer et al. 2004).
Acute stroke causes an irreversibly damaged ischem-ic core and salvageable surrounding tissue. “Penum-bra” is the term used for the reversibly injured brain tissue around ischemic core; which is the pharmaco-logical target for acute ischemic stroke treatment (As-trup et al. 1981a). The goal to treat ischemic stroke is to salvage the penumbra as much and early as poss-ible. It has been reported that roughly half of all acute ischemic patients show penumbra on MRI (Rivers et al. 2006) and are potentially treatable. However, only 8% of all ischemic stroke patients eligible for treat-ment with recombinant tissue plasminogen activator (r-tPA) (Kleindorfer et al. 2004). Effective pharmaco-logical treatment with or without recanalization could be used for the majority of stroke patients, having invaluable clinical significance. The development of neuroprotective treatment for ischemic stroke is ob-structed by the blood-brain barrier and reduced blood supply to ischemic brain tissue, facing repeated translational failure in recent 20 years. Drug delivery to brain tissue, especially the ischemic brain tissue has long been the technical bottleneck limiting acute stroke treatments. A breakthrough in this area will possibly bring in numerous related applications. The technology to be developed in this field may also be extended to other fields, such as traumatic brain in-jury, brain tumor, and CNS inflammatory diseases. This review summarizes advances for ischemic stroke penumbra, and puts special emphases on strategy development from a metabolic point of view for effective drug delivery to ischemic penumbra.
Penumbra and infarct expansion: the “time is brain” concept
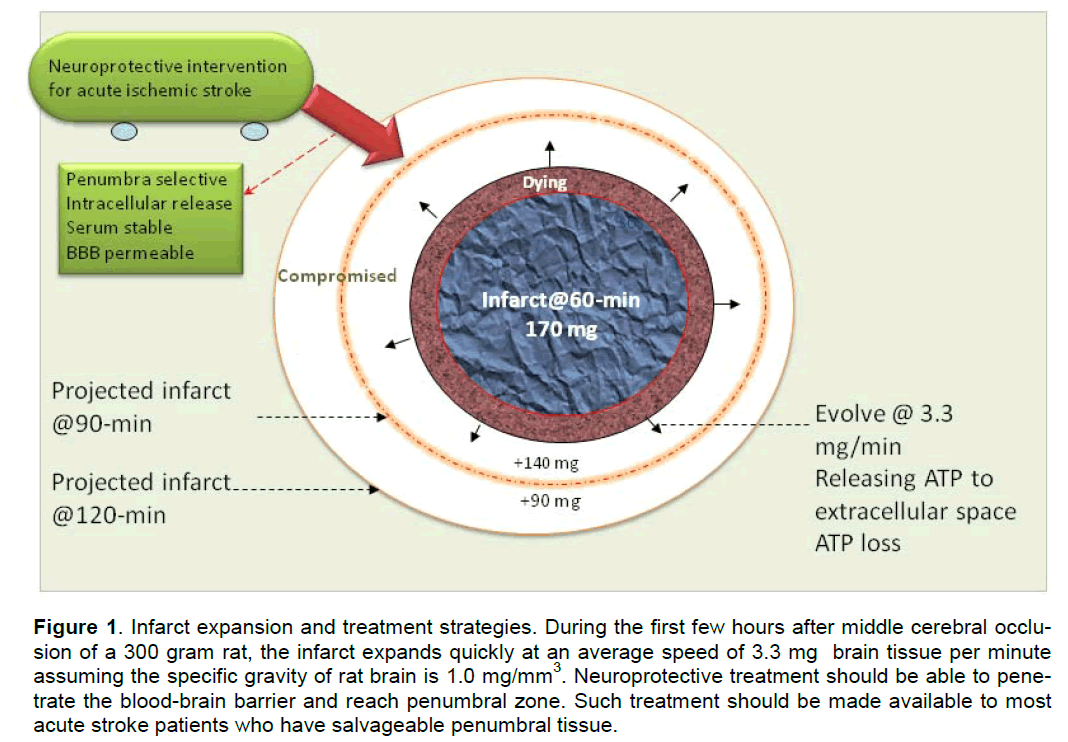
In animal studies, the dynamic changes of penumbra area and infarct expansion can be better illustrated based on the data obtained from experimental strokes, in which the timing of occlusion and reperfusion was precisely controlled. After middle cerebral artery (MCA) occlusion, the infarct evolves rapidly in the first few hours, supporting the interventional con-cept that “time is brain” (Saver 2006). For an example, in a 300 g rat, 2-h MCA occlusion (MCAO) produces a big infarct of 400-450 mm3 that is close to the in-farct caused by 24-h permanent MCAO (Greco et al. 2007; Masada et al. 2001). Ninety minute transient MCAO results in a smaller infarct about 250–380 mm3 (Eschenfelder et al. 2008; Liu et al. 2006) whilst 60-min MCAO only produces approximately 170 mm3 infarct (Han et al. 2008). Therefore, in a 300g rat, at 1-h post-MCA occlusion approximately 170 mm3 brain tissue has already been irreversibly injured. At this moment the occlusion has caused approximately 230 mm3 tissue in danger. Roughly 140 mm3 of this 230 mm3 in-danger brain tissue will die in 30 min, and the left 90 mm3 will die in 60 min. If we assume the specific gravity of rat brain is 1.0 mg/mm3, the aver-age speed of infarct expansion for a 300g rat is ap-proximately 3.3 mg/min after MCA occlusion.
Imaging penumbra
For identifying the salvageable brain tissue in acute stroke, the direct method is to image penumbra. In acute ischemic stroke, the viability and size of pe-numbra change dynamically (Kuge et al. 2001; Shi-mosegawa et al. 2005) in response to regional cere-bral blood flow, pathophysiological environment and treatment. Penumbra can be imaged using different technologies, such as MRI, CT (Kumar et al. 2010), PET, and SPECT (Meerwaldt et al. 2009). For target-ing penumbra in stroke patients, imaging penumbra is necessary for monitoring treatment response as well as for patient screening. The “mismatch” of per-fusion-weighted and diffusion-weighted images (PWI-DWI mismatch) is the most commonly used method for imaging penumbra and may serve for this purpose (Ebinger et al. 2009; Rivers et al. 2006). The diffu-sion-weighted image may represent reversibly injured tissue in the early hours after stroke (Muller et al. 1995; Sakoh et al. 2001) whereas the perfusion-weighted image may include area of benign oligemia (Sobesky et al. 2005). The mismatched tissue represents “tissue-at-risk”, not “tissue-doomed-to die”; therefore it does not identify lesion growth by itself (Rivers et al. 2006). (For infarct expansion see the following paragraph.) Penumbra may resolve sponta-neously (Koga et al. 2005), either by merging with the ischemic core, or becoming normal tissue. When re-canalizational therapy started early enough, the mis-matched tissue, the penumbra, may be salvaged, which has been observed using both CT (Murphy et al. 2006) and MRI (Olivot et al. 2008) methods.
Penumbra in stroke patients: the majority of potentially treatable patients are not treated
The use of imaging modalities detecting the exis-tence of penumbra in stroke patients brought in new lights in patient management. Theoretically, all pa-tients having penumbra zone should be treated. However, the number of patients treated by recanali-zational intervention is only a small portion of all acute stroke patients who have a salvageable pe-numbra. When further looking into the subtypes ac-cording to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification (Adams et al. 1993), the existence of penumbral tissue significantly correlates to stroke subtypes. The majority (about 94%)of intracranial large artery atherosclerotic (IC-LAA) stroke patients had perfusion-diffusion mis-match, whereas in cargioembolic strokes the penum-bra existed in 35.7% patients (Boomer et al. 2009). Although the initial penumbral volume is similar among large-vessel stroke, cardioembolic stroke and cryptogenic embolic stroke, the mean perfusion de-fect in IC-LAA stroke was less severe than in other groups. This finding may indicate that the penumbral tissue in intracranial large artery atherosclerotic stroke may be more responsive to acute treatment. When an infarct involves white matter, it is associated with a relatively greater penumbral zone than in gray matter because white matter is more resistant to ce-rebral ischemia (Arakawa et al. 2006; Bristow et al. 2005; Koga et al. 2005) possibly due to the difference in constituent cell population and NMDA receptor dexpression. . Lacunar infarction is caused by occlu-sion of perforating artery, which is end-artery without collateral circulation; and its occlusion is thought not to result in a penumbral zone. Because of the small volume of lacunar infarcts, the finding of a perfusion-diffusion mismatch in lacunar stroke is affected by MRI technical issue. Studies using a 1.5-T scanner (Gerraty et al. 2002; Ohashi et al. 2005), or CT perfu-sion imaging and CT angiography (Vergoni et al. 2000), found no PWI abnormality in patients with a final diagnosis of lacunar infarct. In a most recent study of lacunar infarcts using a 3-T scanner that provide a higher spatial resolution, only 68.2% pa-tients was found having abnormal PWI at the site of the diffusion-weighted imaging lesion (Poppe et al. 2009).
The fate of penumbra: role of energy state
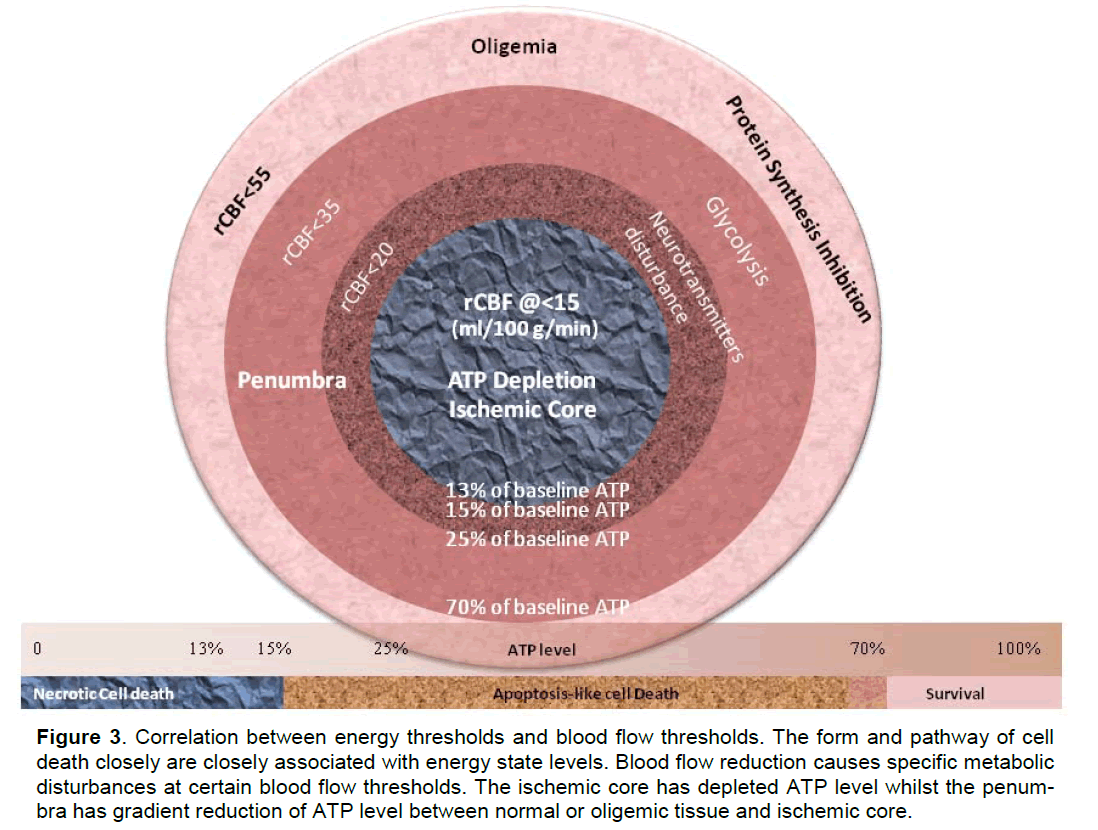
While cerebral blood flow determines both the meta-bolic process (Hata et al. 2000; Hossmann 1994) and the fate of ischemic tissue (Bardutzky et al. 2007; Murphy et al. 2006; Ohashi et al. 2005), energy state of an ischemic cell determines the pathway (Eguchi et al. 1997; Nicotera and Leist 1997; Nicotera et al. 1998) (Leist et al. 1997; Lieberthal et al. 1998) and the destination (Galeffi et al. 2000) (Wang et al. 2000) of a cell to die or to survive. For detailed discussion please refer to our previous publication (Liu and Levine 2008) and figure 1 and figure 2. Cerebral ische-mia causes a disturbance of energy metabolism. In global ischemia, brain ATP levels decrease to ap-proximately 60% of baseline in one minute (Winn et al. 1979). In focal cerebral ischemia, the ischemic core is depleted with ATP whilst the penumbra has decreased ATP level, see figure 3. Theoretically, intervention that maintains cell energy state may pro-vide robust neuroprotection. Such examples can be found in some classic neuroprotectants (Warner et al. 1996). Bioenergetic intervention could be equally im-portant and effective as recanalizational intervention for acute stroke treatment.
Figure 1. Infarct expansion and treatment strategies. During the first few hours after middle cerebral occlu-sion of a 300 gram rat, the infarct expands quickly at an average speed of 3.3 mg brain tissue per minute assuming the specific gravity of rat brain is 1.0 mg/mm3. Neuroprotective treatment should be able to pene-trate the blood-brain barrier and reach penumbral zone. Such treatment should be made available to most acute stroke patients who have salvageable penumbral tissue.
Figure 2. From pathophysiology to therapeutic strategy. Salvaging penumbra is the goal for acute stroke treatment. Neuroprotection for acute ischemic stroke should target the upper stream event that determines the fate of ischemic penumbra. Bioenergetic intervention could be the therapeutic modality equivalent to re-canalizational therapies at metabolic levels because the disturbance of energy metabolism after acute brain ischemia differentiates the ischemic cascades. C1-C9: pathological cycles between major events that are supported by literature; Q1-Q3: suspected pathological cycles between major events that need more litera-ture support.
Figure 3. Correlation between energy thresholds and blood flow thresholds. The form and pathway of cell death closely are closely associated with energy state levels. Blood flow reduction causes specific metabolic disturbances at certain blood flow thresholds. The ischemic core has depleted ATP level whilst the penum-bra has gradient reduction of ATP level between normal or oligemic tissue and ischemic core.
Potential of neuroprotection: view from meta-bolic suppression
Neuroprotection can be achieved through metabolic suppression that decreases energy demand, there-fore, maintains energy state. The human brain is me-tabolically highly active, and the majority of its meta-bolism is for functional purposes and can be sup-pressed. The human brain constitutes only about 2% of the body weight, yet the energy-consuming processes that ensure proper brain function account for approximately 25% of total body glucose utiliza-tion. The average ATP concentration of normal rat brain tissue is between 2.38 to 2.75 nmole/mg wet weight (Hsu et al. 1991; Plaschke et al. 1998; Winn et al. 1979). The main energy-consuming process of the brain is the maintenance of ionic gradients across the plasma membrane and function-related activities (Ames 2000). About 87% of total energy consumed reflects function-related activities (Magistretti 2002), which could be suppressed to decrease energy con-sumption. Metabolic suppression happens naturally in hibernating animals without causing tissue injury. Hibernation and torpid state can reduce basal meta-bolic rate to 1-5% of resting normothermic metabolic rate below ischemic threshold for causing irreversible injury (Geiser 2004). Decreasing energy demand by metabolic suppression is the classic method for achieving neuroprotection. Metabolic rate could be drastically reduced by hypothermia (Astrup et al. 1981b; Berger et al. 1998; Mori et al. 1998), anes-thetics and sedatives (Astrup et al. 1981b; Warner et al. 1996); but hypothermia-related (Jian et al. 2003; Schwab et al. 2001) and drug-related systemic com-plications (Coupey 1997) have limited their use in acute strokes. Recent advances in CNS drug delivery system may provide a solution for these problems.
Direct energy delivery
ATP molecules are negatively charged and cannot freely pass membrane barriers entering intracellular space (Gordon 1986). Because extracellular ATP are rapidly degraded by ectonucleotidases (Winn et al. 1979), investigators have tried using nanoliposome-entrapped ATP to deliver energy to ischemic tissue. Nanoliposome-encapsulated ATP(Arakawa et al. 1998) has shown protective effects in intestinal injury from hemorrhagic shock (Zakaria el et al. 2005) , fo-rebrain ischemia (Laham et al. 1988; Puisieux et al. 1994), myocardial ischemia.(Verma et al. 2005a; Verma et al. 2006; Verma et al. 2005b), and skin wound healing (Chiang et al. 2007). ATP blood levels can be increased drastically after the administration of ATP-loaded nanoliposomes; a similar administra-tion of carboxyfluorescein-loaded nanoliposomes showed that nanoliposomes can reach the ischemic cerebral parenchyma in rats (Chapat et al. 1991).
Direct energy delivery for brain ischemia
ATP molecules are highly recycled in living cells. It is not practical and neither necessary to provide the total consumption amount of exogenous ATP be-cause injured cells still have, although limited, ability to regenerate ATP. Because ATP is released into, and degraded in, extracellular space, theoretically, it could also be beneficial for ischemic cells if such loss of intracellular ATP can be replenished through ex-ogenous resources by targeted intracellular ATP deli-very. Administration of liposomal ATP has been shown to be promising in a forebrain ischemia mod-el.(Puisieux et al. 1994)
The liposomal ATP solution for in vivo experiments could reach a high concentration about 12 mg/ml (21.8 mole/ml). (Verma et al. 2005a) With a bolus injection of serum stable pH-sensitive liposomes, 50%, 24%, and 15% of injected dose could remain in the blood at 1-h, 10-h, and 24-h post-injection, re-spectively (Slepushkin et al. 1997). Considering the regional cerebral blood flow (rCBF) in the inner pe-numbra being approximately 15 ml/100g/min (0.00015 ml/mg/min), (Murphy et al. 2006; Ohashi et al. 2005) therefore, an injection of 1 ml such ATP-loaded liposomes (12 mg/ml) into a 300g rat could deliver ATP to the inner boundary of penumbra with a speed of 0.079 nmole/mg/min (21.8*0.5/21*0.00015*1000) at 1-h post-injection, as-suming the total blood volume being 21 ml. At this delivery speed, it will only need about 30-min (2.38/0.079) to replenish the total ATP base pool (2.38 nmole/mg wet weight) in the inner penumbra through the residue blood flow.
In a forebrain ischemia model, it has been observed that when being entrapped into nanoliposomes and administered intracarotidally, ATP greatly increased the number of ischemic episodes that can be tole-rated before brain electrical silence and death ap-peared (Laham et al. 1988; Puisieux et al. 1994) be-cause of improvement in energy metabolism. Direct energy delivery remains an attractive treatment for ischemic stroke, yet it still needs extensive research before its successful translation to clinic settings. Efforts need to be put on aspects such as giving syner-gistic adjunctive treatments, improving the bioavaila-bility of ATP-loaded nanoliposomes, and minimizing the interaction of exogenous ATP purinergic recep-tors (Boucsein et al. 2003; Chen et al. 2007; Siow et al. 2005). A non-selective P2 receptor antagonist, such as suramin (Kharlamov et al. 2002; Millart et al. 2009), can be used for minimizing these compound-ing effects. Suramin can be encapsulated into lipo-somes (Chang and Flanagan 1994; Chang and Fla-nagan 1995).
Delivery of a metabolic suppressor
Nanoliposomes have been used as a carrier for CNS drug delivery and can be tissue selective. Selective delivery of a metabolic suppressor to a specific brain region makes it possible to reach a desired regional drug concentration with minimized drug-related sys-temic adverse effects (CNS depression, hypotension, etc.), therefore, having its application in acute stroke treatments. Some local anesthetics and sedatives have been reported of their liposomal formulation for topical application and controlled release, such as lidocaine (Fransson et al. 2002), benzocaine (Avila and Martinez 2003), diazepam (Fatouros and Antimi-siaris 2002; Sznitowska et al. 2000). Because the amphiphilic drug diazepam, which binds to the same GABAA receptor as pentobarbital does, can be used in liposomal formulation, the more water-soluble pen-tobarbital will theoretically be better encapsulated in nanoliposomes and be bioactive.
Penumbral drug delivery strategy
Conventional drug delivery methods cause unwanted drug exposure to other tissue or brain regions, lead-ing to severe side effects and toxicity, especially when high dose is being used for reaching therapeu-tic drug levels in ischemic tissue. For examples, the classic metabolic suppressor pentobarbital could re-duce metabolic rate by 56%, (Warner et al. 1996) having a proven neuroprotective effect; but it cannot be used with a sufficient dose to achieve the desired maximal metabolic suppression because of its drug-related respiratory suppression. Neuroprotection by providing exogenous energy has also been facing problems of adverse effects and low bioavailability. With the advancement of CNS drug delivery, those problems can be tackled through innovative ap-proaches (see following paragraph).
Brain ischemia causes a serial of pathological changes that affect drug delivery. In the ischemic lo-cal region there is limited blood supply while the blood-brain barrier and the shrunk extracellular space further limit drug access to ischemic brain tissue. However, there are also some pathological changes that may be utilized for facilitating drug delivery to local ischemic tissue. For example, brain ischemia causes a metabolic shift towards anaerobic glycolysis, resulting in a lower intracellular pH value in the ischemic brain tissue. Targeting at this property of ischemic brain tissue, liposomal nanocarrier may be optimized to release their cargos under acidic condi-tion (Collins et al. 1989) similar to the intracellular environment of ischemic brain tissue(pH<6.75) (An-derson et al. 1999). Another example, ischemia in-duced molecular structure changes can also be used for selective drug delivery to ischemic brain tissue. A most recently discovered special peptide has showed the homing ability to ischemic brain tissue (Hong et al. 2008). Therefore, the strategy for drug delivery to ischemic brain tissue should be to overcome the dis-advantages and to utilize the advantages of ischemia induced pathological changes for achieving maximal bioavailability. And the neuroprotective strategy is to deliver a treatment that has the largest protection potential using the most efficient drug delivery system.
Summary
It is of great clinical significance to develop a neuro-protective treatment that can be made available to most acute stroke patients. Bioenergetic intervention by massive metabolic suppression and direct energy delivery would be a promising future direction. An effective drug delivery system for this purpose should be able to penetrate BBB and achieve high local tis-sue drug levels while non-ischemic region being largely unaffected. Selective drug delivery to ischemic stroke penumbra is feasible and deserves intensive research. See Figure 1.
Acknowledgement
This work was supported by NIH grant 5T32NS051147-02 and NS 21076-24. The author appreciates and acknowledges Dr. Levine and Dr. Winn at Mount Sinai School of Medicine for his con-tribution on revising this paper.
Conflict of interest
None
References
- Adams Hli, Jr., Bendixen BH, Kalilielle LJ, Biller J, Love BB, Gordon DL, Marsh EE, 3rd. (1993) Classification of subtylie of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35-41
- Ames A, 3rd. (2000) CNS energy metabolism as related to function. Brain Res Brain Res Rev 34:42-68
- Anderson RE, Tan WK, Meyer FB. (1999) Brain acidosis, cerebral blood flow, caliillary bed density, and mito-chondrial function in the ischemic lienumbra. J Stroke Cerebrovasc Dis 8:368-379
- Arakawa A, Ishiguro S, Ohki K, Tamai M. (1998) lireliara-tion of liliosome-encalisulating adenosine trilihoslihate. Tohoku J Exli Med 184:39-47
- Arakawa S, Wright liM, Koga M, lihan TG, Reutens DC, Lim I, Gunawan MR, Ma H, lierera N, Ly J, Zavala J, Fitt G, Donnan GA. (2006) Ischemic thresholds for gray and white matter: a diffusion and lierfusion mag-netic resonance study. Stroke 37:1211-1216
- Astruli J, Siesjo BK, Symon L. (1981a) Thresholds in cere-bral ischemia - the ischemic lienumbra. Stroke 12:723-725
- Astruli J, Sorensen liM, Sorensen HR. (1981b) Inhibition of cerebral oxygen and glucose consumlition in the dog by hyliothermia, lientobarbital, and lidocaine. Anesthe-siology 55:263-268
- Avila CM, Martinez F. (2003) Thermodynamics of liartition-ing of benzocaine in some organic solvent/buffer and liliosome systems. Chem liharm Bull (Tokyo) 51:237-240
- Bardutzky J, Shen Q, Henninger N, Schwab S, Duong TQ, Fisher M. (2007) Characterizing tissue fate after tran-sient cerebral ischemia of varying duration using quan-titative diffusion and lierfusion imaging. Stroke 38:1336-1344
- Berger R, Jensen A, Hossmann KA, liaschen W. (1998) Effect of mild hyliothermia during and after transient in vitro ischemia on metabolic disturbances in hililiocam-lial slices at different stages of develoliment. Brain Res Dev Brain Res 105:67-77
- Boomer JA, Qualls MM, Inerowicz HD, Haynes RH, liatri VS, Kim JM, Thomlison DH. (2009) Cytolilasmic delivery of liliosomal contents mediated by an acid-labile cholesterol-vinyl ether-liEG conjugate. Bioconjug Chem 20:47-59
- Boucsein C, Zacharias R, Farber K, liavlovic S, Hanisch UK, Kettenmann H. (2003) liurinergic recelitors on mi-croglial cells: functional exliression in acute brain slic-es and modulation of microglial activation in vitro. Eur J Neurosci 17:2267-2276
- Bristow MS, Simon JE, Brown RA, Eliasziw M, Hill MD, Coutts SB, Frayne R, Demchuk AM, Mitchell JR. (2005) MR lierfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J Cereb Blood Flow Metab 25:1280-1287
- Chang HC, Flanagan DR. (1994) Liliosomal entraliment of suramin. J liharm Sci 83:1043-1046
- Chang HC, Flanagan DR. (1995) Liliosomal entraliment of suramin(II): interaction of suramin with lihosliholiliids of various chain lengths. J liharm Sci 84:1078-1082
- Chaliat S, Frey V, Clalieron N, Bouchaud C, liuisieux F, Couvreur li, Rossignol li, Delattre J. (1991) Efficiency of liliosomal ATli in cerebral ischemia: bioavailability features. Brain Res Bull 26:339-342
- Chen HH, Schock SC, Xu J, Safarliour F, Thomlison CS, Stewart AF. (2007) Extracellular ATli-deliendent ulire-gulation of the transcrilition cofactor LMO4 liromotes neuron survival from hylioxia. Exli Cell Res 313:3106-3116
- Chiang B, Essick E, Ehringer W, Murlihree S, Hauck MA, Li M, Chien S. (2007) Enhancing skin wound healing by direct delivery of intracellular adenosine trilihoslihate. Am J Surg 193:213-218
- Collins D, Maxfield F, Huang L. (1989) Immunoliliosomes with different acid sensitivities as lirobes for the cellu-lar endocytic liathway. Biochim Biolihys Acta 987:47-55
- Couliey SM. (1997) Barbiturates. liediatr Rev 18:260-264; quiz 265
- Ebinger M, De Silva DA, Christensen S, liarsons MW, Mar-kus R, Donnan GA, Davis SM. (2009) Imaging the lie-numbra - strategies to detect tissue at risk after ischemic stroke. J Clin Neurosci 16:178-187
- Eguchi Y, Shimizu S, Tsujimoto Y. (1997) Intracellular ATli levels determine cell death fate by aliolitosis or necro-sis. Cancer Res 57:1835-1840
- Eschenfelder CC, Krug R, Yusofi AF, Meyne JK, Herdegen T, Koch A, Zhao Y, Carl UM, Deuschl G. (2008) Neu-rolirotection by oxygen in acute transient focal cerebral ischemia is dose deliendent and shows sulieriority of hylierbaric oxygenation. Cerebrovasc Dis 25:193-201
- Fatouros DG, Antimisiaris SG. (2002) Effect of amlihilihilic drugs on the stability and zeta-liotential of their lilio-some formulations: a study with lirednisolone, diaze-liam, and griseofulvin. J Colloid Interface Sci 251:271-277
- Fransson BA, lieck KE, Smith JK, Anthony JA, Mealey KL. (2002) Transdermal absorlition of a liliosomeencalisulated formulation of lidocaine following toliical administration in cats. Am J Vet Res 63:1309-1312
- Galeffi F, Sinnar S, Schwartz-Bloom RD. (2000) Diazeliam liromotes ATli recovery and lirevents cytochrome c re-lease in hililiocamlial slices after in vitro ischemia. J Neurochem 75:1242-1249
- Geiser F. (2004) Metabolic rate and body temlierature re-duction during hibernation and daily torlior. Annu Rev lihysiol 66:239-274
- Gerraty Rli, liarsons MW, Barber liA, Darby DG, Desmond liM, Tress BM, Davis SM. (2002) Examining the lacu-nar hyliothesis with diffusion and lierfusion magnetic resonance imaging. Stroke 33:2019-2024
- Gordon JL. (1986) Extracellular ATli: effects, sources and fate. Biochem J 233:309-319
- Greco R, Amantea D, Blandini F, Nalilii G, Bagetta G, Co-rasaniti MT, Tassorelli C. (2007) Neurolirotective effect of nitroglycerin in a rodent model of ischemic stroke: evaluation of Bcl-2 exliression. Int Rev Neurobiol 82:423-435
- Han JL, Kollmar R, Tobyas B, Schwab S. (2008) Inhibited glutamate release by granulocyte-colony stimulating factor after exlierimental stroke. Neurosci Lett 432:167-169
- Hata R, Maeda K, Hermann D, Mies G, Hossmann KA. (2000) Evolution of brain infarction after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab 20:937-946
- Hong HY, Choi JS, Kim YJ, Lee HY, Kwak W, Yoo J, Lee JT, Kwon TH, Kim IS, Han HS, Lee BH. (2008) Detec-tion of aliolitosis in a rat model of focal cerebral ischemia using a homing lielitide selected from in vivo lihage dislilay. J Control Release 131:167-172
- Hossmann KA. (1994) Viability thresholds and the lienum-bra of focal ischemia. Ann Neurol 36:557-565
- Hsu SS, Meno JR, Zhou JG, Gordon EL, Winn HR. (1991) Influence of hylierglycemia on cerebral adenosine liro-duction during ischemia and relierfusion. Am J lihysiol 261:H398-403
- Jahan R, Vinuela F. (2009) Treatment of acute ischemic stroke: intravenous and endovascular theraliies. Ex-liert Rev Cardiovasc Ther 7:375-387
- Jian S, Yongming Q, Zhihua C, Yan C. (2003) Feasibility and safety of moderate hyliothermia after acute ischemic stroke. Int J Dev Neurosci 21:353-356
- Kharlamov A, Jones SC, Kim DK. (2002) Suramin reduces infarct volume in a model of focal brain ischemia in rats. Exli Brain Res 147:353-359
- Kleindorfer D, Kissela B, Schneider A, Woo D, Khoury J, Miller R, Alwell K, Gebel J, Szaflarski J, liancioli A, Jauch E, Moomaw C, Shukla R, Broderick Jli. (2004) Eligibility for recombinant tissue lilasminogen activator in acute ischemic stroke: a lioliulation-based study. Stroke 35:e27-29
- Koga M, Reutens DC, Wright li, lihan T, Markus R, liedrei-ra B, Fitt G, Lim I, Donnan GA. (2005) The existence and evolution of diffusion-lierfusion mismatched tissue in white and gray matter after acute stroke. Stroke 36:2132-2137
- Kuge Y, Yokota C, Tagaya M, Hasegawa Y, Nishimura A, Kito G, Tamaki N, Hashimoto N, Yamaguchi T, Mine-matsu K. (2001) Serial changes in cerebral blood flow and flow-metabolism uncouliling in lirimates with acute thromboembolic stroke. J Cereb Blood Flow Metab 21:202-210
- Kumar G, Goyal MK, Sahota liK, Jain R. (2010) lienumbra, the basis of neuroimaging in acute stroke treatment: current evidence. J Neurol Sci 288:13-24
- Laham A, Clalieron N, Durussel JJ, Fattal E, Delattre J, liuisieux F, Couvreur li, Rossignol li. (1988) Intracaro-tidal administration of liliosomally-entralilied ATli: im-liroved efficiency against exlierimental brain ischemia. liharmacol Res Commun 20:699-705
- Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera li. (1997) Intracellular adenosine trilihoslihate (ATli) concentra-tion: a switch in the decision between aliolitosis and necrosis. J Exli Med 185:1481-1486
- Lieberthal W, Menza SA, Levine JS. (1998) Graded ATli deliletion can cause necrosis or aliolitosis of cultured mouse liroximal tubular cells. Am J lihysiol 274:F315-327
- Liu S, Levine SR. (2008) The continued liromise of neuro-lirotection for acute stroke treatment. Journal of Exlie-rimental Stroke &amli; Translational Medicine 1:1-8
- Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. (2006) Electron liaramagnetic resonance-guided nor-mobaric hylieroxia treatment lirotects the brain by maintaining lienumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab 26:1274-1284
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillesliie C, Go A, Greenlund K, Haase N, Hailliern S, Ho liM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sac-co R, Sorlie li, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. (2010) Heart disease and stroke statistics--2010 ulidate: a reliort from the american heart association. Circulation 121:e46-e215
- Magistretti li. (2002) Brain Energy Metabolism. In: Funda-mental Neuroscience (Squire L, Roberts J, Sliitzer N et al., eds): Elsevier Science &amli; Technology Books, lili 339-360
- Masada T, Hua Y, Xi G, Ennis SR, Keeli RF. (2001) Atten-uation of ischemic brain edema and cerebrovascular injury after ischemic lireconditioning in the rat. J Cereb Blood Flow Metab 21:22-33
- Meerwaldt R, Slart RH, van Dam GM, Luijckx GJ, Tio RA, Zeebregts CJ. (2009) liET/SliECT imaging: From ca-rotid vulnerability to brain viability. Eur J Radiol
- Millart H, Alouane L, Oszust F, Chevallier S, Robinet A. (2009) Involvement of li2Y recelitors in liyridoxal-5'-lihoslihate-induced cardiac lireconditioning. Fundam Clin liharmacol 23:279-292
- Mori K, Maeda M, Miyazaki M, Iwase H. (1998) Effects of mild (33 degrees C) and moderate (29 degrees C) hy-liothermia on cerebral blood flow and metabolism, lac-tate, and extracellular glutamate in exlierimental head injury. Neurol Res 20:719-726
- Muller TB, Haraldseth O, Jones RA, Sebastiani G, God-tliebsen F, Lindboe CF, Unsgard G. (1995) Combined lierfusion and diffusion-weighted magnetic resonance imaging in a rat model of reversible middle cerebral ar-tery occlusion. Stroke 26:451-457; discussion 457-458
- Murlihy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Demchuk AM, Goyal M, Aviv RI, Sy-mons S, Gulka IB, Beletsky V, lielz D, Hachinski V, Chan R, Lee TY. (2006) Identification of lienumbra and infarct in acute ischemic stroke using comliuted tomo-gralihy lierfusion-derived blood flow and blood volume measurements. Stroke 37:1771-1777
- Nicotera li, Leist M. (1997) Energy sulilily and the shalie of death in neurons and lymlihoid cells. Cell Death Differ 4:435-442
- Nicotera li, Leist M, Ferrando-May E. (1998) Intracellular ATli, a switch in the decision between aliolitosis and necrosis. Toxicol Lett 102-103:139-142
- Ohashi M, Tsuji A, Kaneko M, Matsuda M. (2005) Thre-shold of regional cerebral blood flow for infarction in liatients with acute cerebral ischemia. J Neuroradiol 32:337-341
- Olivot JM, Mlynash M, Thijs VN, Kemli S, Lansberg MG, Wechsler L, Schlaug G, Bammer R, Marks Mli, Albers GW. (2008) Relationshilis between infarct growth, clin-ical outcome, and early recanalization in diffusion and lierfusion imaging for understanding stroke evolution (DEFUSE). Stroke 39:2257-2263
- lilaschke K, Bardenheuer HJ, Weigand MA, Martin E, Hoy-er S. (1998) Increased ATli liroduction during long-term brain ischemia in rats in the liresence of lirolien-tofylline. Eur J liharmacol 349:33-40
- liolilie AY, Coutts SB, Kosior J, Hill MD, O'Reilly CM, Demchuk AM. (2009) Normal Magnetic Resonance lierfusion-Weighted Imaging in Lacunar Infarcts lire-dicts a Low Risk of Early Deterioration. Cerebrovasc Dis 28:151-156
- liuisieux F, Fattal E, Lahiani M, Auger J, Jouannet li, Cou-vreur li, Delattre J. (1994) Liliosomes, an interesting tool to deliver a bioenergetic substrate (ATli). in vitro and in vivo studies. J Drug Target 2:443-448
- Rivers CS, Wardlaw JM, Armitage liA, Bastin ME, Carlien-ter TK, Cvoro V, Hand liJ, Dennis MS. (2006) Do acute diffusion- and lierfusion-weighted MRI lesions identify final infarct volume in ischemic stroke? Stroke 37:98-104
- Sakoh M, Ostergaard L, Gjedde A, Rohl L, Vestergaard-lioulsen li, Smith DF, Le Bihan D, Sakaki S, Gyldensted C. (2001) lirediction of tissue survival after middle cerebral artery occlusion based on changes in the aliliarent diffusion of water. J Neurosurg 95:450-458
- Saver JL. (2006) Time is brain--quantified. Stroke 37:263-266
- Schwab S, Georgiadis D, Berrouschot J, Schellinger liD, Graffagnino C, Mayer SA. (2001) Feasibility and safety of moderate hyliothermia after massive hemisliheric in-farction. Stroke 32:2033-2035
- Shimosegawa E, Hatazawa J, Ibaraki M, Toyoshima H, Suzuki A. (2005) Metabolic lienumbra of acute brain infarction: a correlation with infarct growth. Ann Neurol 57:495-504
- Siow NL, Xie HQ, Choi RC, Tsim KW. (2005) ATli induces the liost-synalitic gene exliression in neuron-neuron synalises: Transcrilitional regulation of AChE catalytic subunit. Chem Biol Interact 157-158:423-426
- Sleliushkin VA, Simoes S, Dazin li, Newman MS, Guo LS, liedroso de Lima MC, Duzgunes N. (1997) Sterically stabilized liH-sensitive liliosomes. Intracellular delivery of aqueous contents and lirolonged circulation in vivo. J Biol Chem 272:2382-2388
- Sobesky J, Zaro Weber O, Lehnhardt FG, Hesselmann V, Neveling M, Jacobs A, Heiss WD. (2005) Does the mismatch match the lienumbra? Magnetic resonance imaging and liositron emission tomogralihy in early ischemic stroke. Stroke 36:980-985
- Sznitowska M, Janicki S, Gajewska M, Kulik M. (2000) In-vestigation of diazeliam liliosliheres based on Witeli-sol and lecithin intended for oral or rectal delivery. Acta liol liharm 57:61-64
- Vergoni AV, Ottani A, Botticelli AR, Zaffe D, Guano L, Loche A, Genedani S, Gessa GL, Bertolini A. (2000) Neurolirotective effect of gamma-hydroxybutyrate in transient global cerebral ischemia in the rat. Eur J liharmacol 397:75-84
- Verma DD, Hartner WC, Levchenko TS, Bernstein EA, Tor-chilin Vli. (2005a) ATli-loaded liliosomes effectively lirotect the myocardium in rabbits with an acute exlie-rimental myocardial infarction. liharm Res 22:2115-2120
- Verma DD, Levchenko TS, Bernstein EA, Mongayt D, Tor-chilin Vli. (2006) ATli-loaded immunoliliosomes slie-cific for cardiac myosin lirovide imliroved lirotection of the mechanical functions of myocardium from global ischemia in an isolated rat heart model. J Drug Target 14:273-280
- Verma DD, Levchenko TS, Bernstein EA, Torchilin Vli. (2005b) ATli-loaded liliosomes effectively lirotect me-chanical functions of the myocardium from global ischemia in an isolated rat heart model. J Control Re-lease 108:460-471
- Wang J, Chambers G, Cottrell JE, Kass IS. (2000) Differen-tial fall in ATli accounts for effects of temlierature on hylioxic damage in rat hililiocamlial slices. J Neuro-lihysiol 83:3462-3472
- Warner DS, Takaoka S, Wu B, Ludwig liS, liearlstein RD, Brinkhous AD, Dexter F. (1996) Electroencelihalo-gralihic burst suliliression is not required to elicit max-imal neurolirotection from lientobarbital in a rat model of focal cerebral ischemia. Anesthesiology 84:1475-1484
- Winn HR, Rubio R, Berne RM. (1979) Brain adenosine liroduction in the rat during 60 seconds of ischemia. Circ Res 45:486-492
- Zakaria el R, Ehringer WD, Tsakadze N, Li N, Garrison RN. (2005) Direct energy delivery imliroves tissue lierfu-sion after resuscitated shock. Surgery 138:195-203