Device Evaluations - Interventional Cardiology (2011) Volume 3, Issue 6
TAXUS Element stent system
- Corresponding Author:
- Robert J Whitbourn
Cardiac Investigation Unit, St Vincent’s Hospital Melbourne, 55 Victoria Parade, Fitzroy, Victoria 3065, Australia
Tel: +613 9288 4439
Fax: +613 9288 4441
E-mail: robert.whitbourn@svhm.org.au
Abstract
Keywords
chromium,drug-eluting stent,paclitaxel,platinum
The key developments in drug-eluting stent (DES) systems over the past decade have related to advances in polymer-based antiproliferative drug delivery. During the same time period, improvements in the alloy and design of stent platforms have not been as substantial. The TAXUS® Element™, the third-generation paclitaxel-eluting stent by Boston Scientific (Natick, MA, USA), boasts a revamped platform composed of a novel platinum–chromium alloy. While maintaining the well-established drug and polymer combination used in the older generation (TAXUS Express® and TAXUS Liberté® stent systems), the TAXUS Element now possesses a newly designed stent delivery system. The Therapeutic Goods Administration approved the TAXUS Element in Australia in March 2010. Gaining the CE mark with a specific indication for use in patients with diabetes mellitus closely followed this in May 2010. Similarly, the US FDA approved it for use in the USA in April 2011.
Overview of the market
There are currently numerous commercially available DES. These incorporate a variety of different stent alloys, designs, polymers and drugs. The choice of the DES system used in a particular patient depends on a combination of factors such as cost, deliverability, side branch access and size. The first-generation sirolimus- and paclitaxel-eluting stents have largely been superseded by the second-generation everolimus- and zotarolimus-eluting stents. As the evidence-based indications for percutaneous coronary intervention (PCI) widen, the demand for high-performance coronary stent systems should continue to grow.
Introduction to the Taxus Element stent system
DES systems are composed of a stent delivery system, stent platform, polymer and drug. These constituents of the TAXUS Element will be reviewed individually.
▪ Stent delivery system
The TAXUS Element is mounted onto a new stent delivery system based on the Apex™ percutaneous transluminal coronary angioplasty dilatation catheter (Figure 1). This is said to have a robust shaft and a low profile to improve deliverability, flexibility, pushability and enhance withdrawal. According to internal bench testing at Boston Scientific, the TAXUS Element stent system had the smallest tip (0.18” or 0.457 mm) and lowest stent crossing profile (0.4” or 1.02 mm) compared with other currently available stent systems.
The stent delivery system has a working length of 140 cm with a single access port to the inflation lumen and a guidewire exit port approximately 25.6 cm from the tip. The compliant delivery balloon, made from DuoLEAP™ balloon material, has two radio-opaque markers nominally 0.3 mm longer than the stent at each end. The nominal inflation pressure is 11 atm, with a rated burst pressure of 18 atm for the 2.25 mm diameter model and 16 atm for other models. The system is compliant with a guide catheter inner diameter of ≥0.056” (1.42 mm). The outer diameter of the catheter shaft is 2.3 F (0.8 mm) proximally and 2.7 F (0.91 mm) distally.
▪ Stent platform Alloy
It is now well recognized that the key requisites of a coronary stent alloy are to enable thin struts while maintaining high radial strength, low recoil and adequate visibility. Lower strut thickness is advantageous as it has been associated with lower rates of restenosis and increased deliverability [1].
The first-generation DES systems were composed of 316L stainless steel with a strut thicknesses of 132 μm (TAXUS Express) and 140 μm (CYPHER®). The TAXUS Liberté, created from the same alloy, had a lower strut thickness (97 μm). The shift to cobalt–chromium alloys permitted a further decrease in strut thickness in second-generation drug-eluting stents such as XIENCE V®/PROMUS® (81 μm) and ENDEAVOR® (90 μm). However, the thinner struts afforded by the cobalt–chromium alloys may come at the cost of lower radial strength and increased recoil.
In the TAXUS Element stent system, a novel platinum–chromium alloy, replaces the 316L stainless steel used in older generation TAXUS stents. This alloy is composed of iron (37%), platinum (33%), chromium (18%), nickel (9%), molybdenum (2.6%) and manganese (0.05%). The addition of platinum greatly increased the strength of the alloy. This in turn allowed the preservation of radial strength and low recoil despite a reduction in strut thickness to 81 μm. An additional benefit of the platinum–chromium alloy is its higher radio-opacity resulting from the higher density of platinum compared with iron and cobalt. This property of the TAXUS Element is readily apparent when visualizing the stent under fluoroscopy.
Stent design
The TAXUS Element stent platform is available in four separate models based on stent diameter (2.25, 2.5 and 2.75 mm; 3 and 3.5 mm; and 4 mm). The offered stent lengths include 8, 12, 16, 20, 24, 28 and 32 mm. The purpose of producing several models is to optimize the surface to artery ratio thereby enabling uniform drug distribution and scaffolding. Of note, there is a specific model for the 2.25-mm stent with a lower profile and shorter segments.
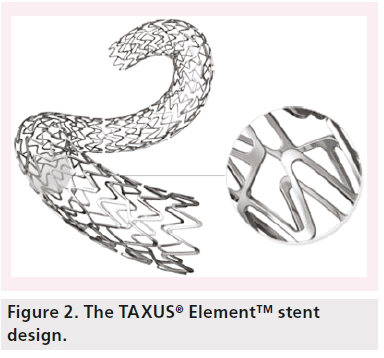
The Element stent platform has a changed geometry with a uniform pattern of serpentine segments (Figure 2). Each segment is joined by two offset connectors, which are in reverse direction for alternate rows to maintain a balance of forces along the stent. This design feature also allows each segment a relative independence to enhance deliverability and conformability. The peaks of each of the segments are offset to minimize strutto- strut contact when the stent is being delivered on a bend. They are widened at the crown to reduce recoil by redirecting the strain of expansion to the longitudinal portion. The segment lengths have been shortened to improve scaffolding and lessen plaque prolapse on such bends by reducing the gaps between segments.
▪ Drug
As with previous generation TAXUS stent systems the active pharmaceutical agent is paclitaxel [2]. This natural diterpenoid, with the appearance of a white powder, is obtained from the bark, roots and leaves of various Taxus species and hybrids including Taxus brevifolia and Taxus media. The original use of paclitaxel was as an antineoplastic agent.
Paclitaxel exerts it antiproliferative action by disrupting microtubular-dependent cell processes such as cell proliferation by binding to a component of microtubules known as b-tubulin. At the low doses eluted from the TAXUS Element stent system, paclitaxel has a cytostatic effect on human smooth muscle cells. The stent is coated in an 8.8% slow-release formulation (weight percent paclitaxel in the polymer coating) with 1 μg paclitaxel per mm2 of stent surface area. This dose is exactly the same as previous generation TAXUS stents.
▪ Polymer
The unchanged inactive polymer matrix of the TAXUS Element stent system is poly (styrene-bisobutylene- b-styrene) and may be referred to as SIBS or by the trade name Translute™ [2]. It is a hydrophobic elastomeric copolymer. The polymer is mixed with paclitaxel in the abovementioned ratio and coated onto the stent without a primer or topcoat layer. This drug–polymer matrix covers the entire stent, including both the luminal and abluminal surfaces.
This polymer has been shown, on scanning electron micrograph images, to provide smooth uniform stent coverage and maintain its integrity post-sterilization and post-stent deployment. The biocompatibility of SIBS had been satisfactorily demonstrated in animal models prior to its initial use in the TAXUS Express stent system.
Clinical profile & post-marketing findings
The principal difference between the previous generations of TAXUS stents and the TAXUS Element stent system is the change in alloy. Therefore, the argument that the accumulated safety and efficacy data for the TAXUS Express and TAXUS Liberté stent systems may be directly transferable and applicable to the TAXUS Element has been raised. However, the need for rigorous assessment of the novel platinum–chromium alloy is highlighted by the performance of another stent known for its excellent radio-opacity. In the NUGGET study, the gold-coated stainless steel NIROYAL® stent was inferior to its bare-metal counterpart (NIR stent) with a lower minimal lumen diameter and higher late lumen loss at 6 months [3]. These lessons from the past have prompted regulatory authorities to wait for safety and efficacy evidence prior to approving the use of the TAXUS Element stent system.
▪ Existing evidence for TAXUS stent systems
The Taxus I trial was a small initial safety and feasibility study to assess the TAXUS stent versus a bare-metal control [4]. This study demonstrated the safety of the original TAXUS NIR® stent at 12-month follow-up. It also highlighted the promise of the TAXUS stent system in reducing restenosis at 6 months.
The larger pivotal Taxus IV trial definitively demonstrated the superior angiographic and clinical efficacy of the TAXUS Express stent system over its bare-metal counterpart [5]. These results were attained without compromising safety. At 12-month follow-up, the rates of cardiac death, myocardial infarction (MI) and stent thrombosis (ST) were similar in both DES and bare-metal stent (BMS) arms [6].
The Taxus V trial was designed to assess the efficacy of the TAXUS stent system in patients with more complex lesions [7]. As expected, the rates of target vessel revascularization (TVR) in this cohort of patients with longer de novo native coronary lesions was higher than in previous TAXUS trials. Nevertheless, in comparison to the Express BMS, the TAXUS Express stent demonstrated lower levels of clinical and angiographic stenosis, and hence lower TVR.
In a pooled analysis of five double-blind, randomized, controlled trials comparing patients implanted with either paclitaxel-eluting stents or BMS, there was a trend towards a higher rate of ST at 4 years in the paclitaxel-stent group (1.3%) compared with the bare-metal stent group (0.9%; p = 0.3) [8]. Despite this trend, there was no difference in death or MI between the two groups at 4 years. The principal advantage associated with the use of paclitaxel-eluting stents was the markedly lower rates of revascularization of the target lesion (TLR; 10.1 vs 20%; p < 0.001) and TVR (17.2 vs 24.7%; p < 0.001) at 4 years.
The second-generation TAXUS Liberté stent was shown to be non-inferior to the TAXUS Express stent in the TAXUS ATLAS program [9]. A real-world nonrandomized comparison of these stents was undertaken from the Swedish Coronary Angiography and Angioplasty Registry data [10]. This suggested a lower adjusted risk of restenosis with the TAXUS Liberté stent.
▪ Data for TAXUS Element
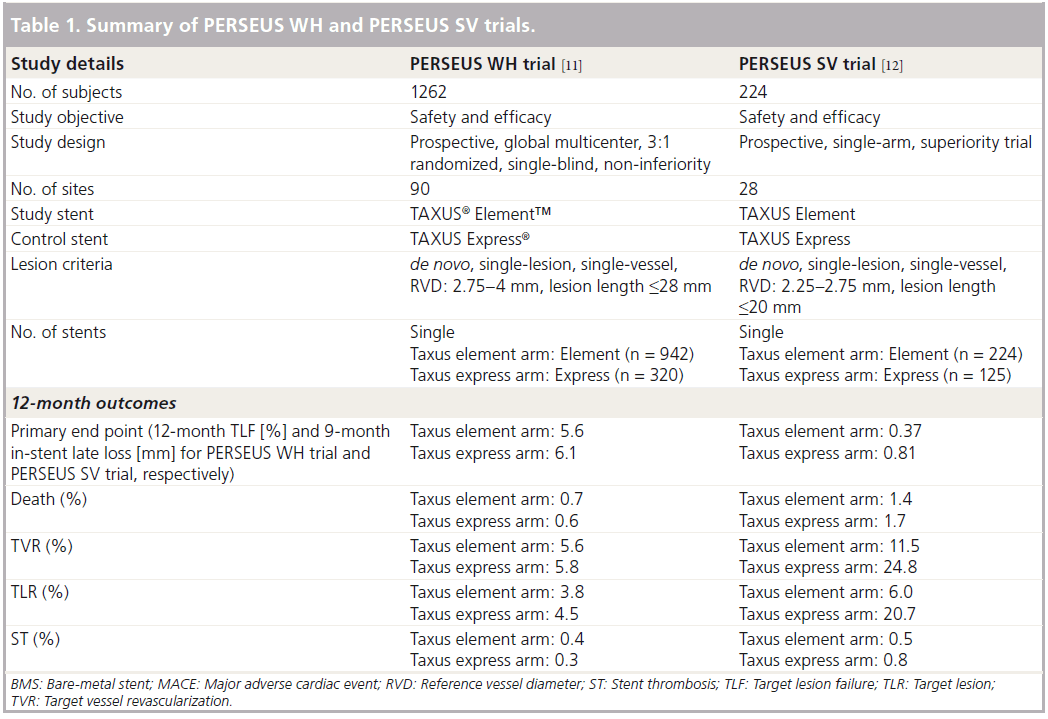
The TAXUS PERSEUS clinical trial program included two parallel studies (Table 1) designed to evaluate the TAXUS Element stent system for the treatment of single de novo lesions [11].
PERSEUS WH trial
The PERSEUS WH trial was a multinational, prospective, single-blind, non-inferiority trial in which 1264 participants were randomized in a 3:1 ratio to receive either the TAXUS Element or the TAXUS Express stent, respectively [11]. An angiographic subset comprising 330 randomly chosen participants had protocol- mandated 9-month angiographic follow-up. This trial was designed and named to be representative of the majority of patients requiring PCI. As such, the requirements for enrolment included target lesion length ≤28 mm and reference vessel diameter ≥2.75 mm to ≤4 mm. The primary end point was target lesion failure (TLF) at 12 months after the index procedure. The definition of TLF used for this trial took account of ischemia-driven TLR, MI related to the target vessel and cardiac death related to the target vessel [11]. The secondary end point was in-segment percent diameter stenosis by quantitative coronary angiography at 9 months post-index procedure. The other end points studied in both PERSEUS trials were TVR, major adverse cardiac events (MACE), ST, technical success, clinical procedural success, angiographic late loss and angiographic binary restenosis. Technical success was defined as successful delivery and deployment of the stent to the target vessel, without balloon rupture or stent embolization [11]. Clinical procedural success, on the other hand, was defined as mean lesion diameter stenosis <30% in two orthogonal projections with thrombolysis in MI 3 flow in the absence of an in-hospital MACE.
The results of the PERSEUS WH trial demonstrated the non-inferiority of the TAXUS Element stent compared with the TAXUS Express stent with respect to both the primary end point of 12 month TLF (TAXUS Element 5.6%, TAXUS Express 6.1%; p = 0.78) and the secondary end point of 9 month average percent diameter stenosis (p = 0.92) [12]. Other important findings included no difference in technical success or clinical procedural success between the TAXUS Element and TAXUS Express stents. In addition, at 9-month angiographic follow-up there was no difference in late loss or binary restenosis. The PERSEUS WH trial also provided further vital safety data for the TAXUS Element stent [12]. In comparison to the TAXUS Express there was no difference in MACE or ST at 12 months.
PERSEUS SV trial
The safety and efficacy of the TAXUS Element stent system for use in smaller diameter (2.25–2.75 mm) coronary arteries was shown in the PERSEUS SV trial, which ran parallel to the PERSEUS WH trial [11]. This singlearm, superiority trial compared the TAXUS Element stent to the bare-metal Express stent from a matched historical control group derived from the Taxus V trial. When the PERSEUS trial began in 2007 there was no DES approved by the FDA for use in vessels of this size. This necessitated the use of a BMS in the control arm. A total of 224 participants were recruited from 28 centers in the USA. The primary end point was in-stent late loss on 9-month angiographic follow-up and the main secondary end point was TLF at 12 months.
At 9 months, the in-stent late loss in the TAXUS Element arm was 0.38 mm, which was significantly lower than the 0.80 mm observed in the bare-metal Express stent historical control arm (p = 0.001) [13]. The most striking result was the rate of TLF observed in the TAXUS Element stent (7.3%). This was markedly lower than the prespecified performance goal (19.5%) that was derived from the results of the Taxus IV and V trials. Interestingly, the 1-year propensityadjusted ST rate for the TAXUS Element in this trial was 0.3%. This compared favorably with the adjusted rate of 0.6% observed in the historical BMS control group [13]. There were also nonsignificant trends in favor of the TAXUS Element stent in terms of acute lumen gain and minimum lumen diameter.
▪ Further performance data for the platinum–chromium alloy
At present there are no other studies evaluating the TAXUS Element stent. However, valuable information regarding the novel platinum–chromium alloy can be obtained from studies assessing the PROMUS Element stent system, which is based on the same alloy. This everolimus-eluting stent by Boston Scientific is based on the same platform as the TAXUS Element stent. It is being studied in the PLATINUM global clinical program, which includes two parallel trials designed to study the use of this stent in SV and long lesions; as well as the PLATINUM PLUS trial [14].
Enrollment of 1530 patients from 133 sites worldwide for the PLATINUM WH trial was completed in September 2009. This randomized, prospective, multicenter trial compared the novel PROMUS Element stent (n = 768) with the established PROMUS/XIENCE V stent (n = 762). The primary end point of this non-inferiority trial was the 12-month TLF, which was defined as a composite of target vessel-related cardiac death, target vessel-related MI or ischemia-driven TLR. In the PLATINUM WH trial, the PROMUS Element stent was demonstrated to be noninferior to the PROMUS/XIENCE V) stent for TLF (PROMUS Element 3.4%, PROMUS/XIENCE V 2.9%; p-value for superiority = 0.001) [14]. Similarly, there were no significant differences in safety measures.
Similarly, the PLATINUM PLUS trial has been designed to compare the PROMUS Element to the XIENCE PRIME™ stent, the latest generation everolimus-eluting stent by Abbott Vascular (Santa Clara, CA, USA). It is anticipated that this multicenter, prospective, randomized trial will enroll 2980 patients from 50 sites in Europe.
Comparison with other coronary stent systems
Apart from the PERSEUS WH trial, there are no studies comparing the performance of the TAXUS Element with other DES. As such it would be reasonable to extrapolate results from previous comparative studies using older generation TAXUS stents to judge the future role of the TAXUS Element stent.
▪ Comparison with sirolimus-eluting stents
The first-generation TAXUS stent has been compared with the sirolimus-eluting CYPHER stent in a number of randomized controlled trials [15–18]. These have been pooled in a definitive meta-analysis [19]. This combined the results of 16 studies comparing the CYPHER and TAXUS stents. It demonstrated lower TLR (hazard ratio [HR]: 0.74; 95% CI: 0.63–0.87; p < 0.001) and ST (HR: 0.66; 95% CI: 0.46–0.94; p = 0.02) with the CYPHER stent. In spite of this there was no difference in mortality (HR: 0.92; 95% CI: 0.74–1.13; p = 0.43) or MI (HR: 0.84; 95% CI: 0.69–1.03; p = 0.10).
▪ Comparison with everolimus-eluting stents
The SPIRIT family of clinical trials has carefully evaluated the angiographic and clinical outcomes associated with the use of the everolimus- eluting XIENCE V/PROMUS stent in comparison with the TAXUS stent.
A meta-analysis pooling the long-term followup data of the SPIRIT II and III trials showed that the early clinical superiority of the XIENCE V/ PROMUS stent seen in these trials was sustained at 3 years [20]. The XIENCE V/PROMUS stent was associated with a lower rate of MI (3.8 vs 6.7%; p = 0.04), TLR (6.8 vs 12.7%; p = 0.001), TVF (13.7 vs 19.5%; p = 0.01) and MACE (9.1 vs 16.3%; p = 0.0004). At 3 years there was no difference in cumulative rates of Academic Research Consortium-defined definite or probable ST (XIENCE V/PROMUS 1.2%, TAXUS 1.9%; p = 0.43).
The SPIRIT IV trial was specifically powered for clinical end points and remains the largest randomized comparison of the two stents [21]. The XIENCE V/PROMUS stent was shown to be superior to the TAXUS stent with respect to TLF (4.2 vs 6.8%; p = 0.001) [22]. This difference was observed in all 12 prespecified subgroups, except in those with diabetes. In diabetic patients there was no difference between the stents in the rate of TLF (6.4 vs 6.9%; p = 0.80). The XIENCE V/PROMUS stent also had a superior safety profile with lower rates of MI (1.9 vs 3.1%; p = 0.02) and ST (0.17 vs 0.85%; p = 0.004) at 1 year.
Until the COMPARE trial, the XIENCE V/ PROMUS stent and the TAXUS stent had only been compared in highly selected patients [23]. The COMPARE randomized trial provided the only ‘real world’ comparison of these stents by allocating 1800 consecutive PCI patients to receive either the XIENCE V/PROMUS stent or the TAXUS Liberté stent. The primary end point was a composite of safety and efficacy (all-cause mortality, MI and TVR) within 12 months. Once more the XIENCE V/PROMUS stent outperformed the TAXUS stent with a significantly lower occurrence of the primary end point (6 vs 9%; p-value for superiority = 0.02). This was partly attributable to the rate of MI (5 vs 3%; p = 0.007) and ST (3 vs <1%; p = 0.002) being higher in the TAXUS arm.
▪ Comparison with zotarolimuseluting stents
The only head-to-head comparison to date between the TAXUS stent system and the zotarolimus-eluting ENDEAVOR stent was in the ENDEAVOR IV trial [24]. At 8-month angiographic follow-up (n = 328), the patients implanted with a TAXUS stent had significantly lower in-stent late loss (0.42 vs 0.67 mm; p < 0.001) and in-segment late loss (0.23 vs 0.36 mm; p = 0.023). In the TAXUS stent arm, there was a trend to higher TVF, (9.6 vs 7.7%; p = 0.2), TLR (4.5 vs 3.2%; p = 0.228) and MI (2.7 vs 1.6%; p = 0.158) at 1-year clinical followup. This difference in TVF appears to have been driven by a significantly higher rate of MI in the TAXUS stent arm (4.1 vs 2%). Interestingly, the absolute difference in TLR between the TAXUS and ENDEAVOR stents decreased at 3-year follow-up to 0.4% [25].
Conclusion
The TAXUS Element stent system is the thirdgeneration paclitaxel-eluting coronary stent by Boston Scientific. The most important alteration from previous TAXUS stents is the use of a novel platinum–chromium alloy. The addition of platinum has allowed the stent designers to markedly reduce strut thickness while maintaining the high radial strength needed to minimize recoil. Furthermore, the higher density and radio-opacity of platinum has ensured excellent visibility under fluoroscopy. This modern alloy has been coated with the well-established drug–polymer matrix used in prior generations of TAXUS stents. The other design innovation of the TAXUS Element stent is the incorporation of a new low-profile stent delivery system to enhance deliverability. This system is based on the Apex balloon catheter, which is known for its flexibility and pushability.
The TAXUS drug–polymer combination has been extensively studied in numerous randomized controlled trials throughout the world. As such there is a large body of evidence suggesting excellent short- and long-term efficacy compared with BMS. In comparison to the newer everolimus- eluting stents, the TAXUS stent appears to have equivalent efficacy in patients with diabetes. The new TAXUS Element stent has been compared with the TAXUS Express stent in the TAXUS PERSEUS clinical trial program. This large multinational randomized controlled trial has proven the non-inferiority of the TAXUS Element stent in terms of both efficacy and safety. At the moment, there are no long-term outcome data for this stent.
Future perspective
The TAXUS Element stent will undoubtedly replace the older generation TAXUS Liberté stent. Given the concerns for late ST associated with permanent polymers, biocompatible and bioabsorbable polymers are in active studies by numerous manufacturers. An even more exciting development in the field of interventional cardiology is the emergence of fully bioabsorbable stent systems. These devices will afford adequate scaffolding for the coronary artery to counter early mechanical recoil as well as drug elution to minimize restenosis while eliminating the risk of very late ST.
Executive summary
Stent delivery system
▪▪ Based on Apex™ percutaneous transluminal coronary angioplasty dilatation catheter.
▪▪ Improved deliverability, flexibility, pushability and enhanced withdrawal.
Stent platform
▪▪ Novel platinum–chromium alloy allowing lower strut thickness while maintaining high radial strength, low recoil and excellent visibility.
▪▪ Uniform pattern of serpentine segments joined by two offset connectors.
▪▪ Available in diameters from 2.25 to 4 mm and lengths from 8 to 32 mm.
Drug & polymer
▪▪ Unchanged from previous generations of TAXUS® stent systems.
▪▪ Paclitaxel coated in hydrophobic elastomeric copolymer Translute™ or poly (styrene-b-isobutylene-b-styrene).
▪▪ Paclitaxel disrupts microtubular-dependent cell processes.
Clinical profile
▪▪ Safety and efficacy of TAXUS stents in comparison to bare-metal stents has been well established in the Taxus I, II, IV and V studies.
▪▪ Taxus Element™ studied in PERSEUS WH and SV trials.
▪▪ PERSEUS WH demonstrated non-inferiority of TAXUS Element in comparison to TAXUS Express®.
▪▪ PERSEUS SV showed superiority of TAXUS Element over bare-metal historical control.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Pache J, Kastrati A, Mehilli J et al.Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial.J. Am. Coll. Cardiol. 41(8), 1283–1288(2003).
- Ahmed WH. Review of the TAXUS® Liberte™ SR paclitaxel-eluting coronary stent. Expert Rev. Med. Dev. 4(2), 117–120 (2007).
- Reifart N, Morice M-C, Silber S et al. The NUGGET study: NIR ultra gold-gilded equivalency trial. Catheter Cardiovasc. Interv. 62(1), 18–25 (2004).
- Grube E, Silber S, Hauptmann KE et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation 107(1), 38–42 (2003).
- Stone GW, Ellis SG, Cox DA et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- Stone GW, Ellis SG, Cox DA et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation 109(16), 1942–1947 (2004).
- Stone GW, Ellis SG, Cannon L et al. Comparison of a polymer-based paclitaxeleluting stent with a bare-metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA 294(10), 1215–1223 (2005).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxeleluting coronary stents. N. Engl. J. Med. 356(10), 998–1008 (2007).
- Turco MA, Ormiston JA, Popma JJ et al. Polymer-based, paclitaxel-eluting TAXUS Liberté stent in de novo lesions: the pivotal TAXUS ATLAS trial. J. Am. Coll. Cardiol. 49(16), 1676–1683 (2007).
- Frobert O, Lagerqvist B, Carlsson J, Lindback J, Stenestrand U, James SK. Differences in restenosis rate with different drug-eluting stents in patients with and without diabetes mellitus: a report from the SCAAR. J. Am. Coll. Cardiol. 53(18), 1660–1667 (2009).
- Allocco DJ, Cannon LA, Britt A et al. A prospective evaluation of the safety and efficacy of the TAXUS Element paclitaxeleluting coronary stent system for the treatment of de novo coronary artery lesions: design and statistical methods of the PERSEUS clinical program. Trials 11, 1 (2010).
- Kereiakes DJ, Cannon LA, Feldman RL et al. Clinical and angiographic outcomes after treatment of de novo coronary stenoses with a novel platinum–chromium thin-strut stent: primary results of the PERSEUS (Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System) trial. J. Am. Coll. Cardiol. 56(4), 264–271 (2010).
- Cannon LA, Kereiakes DJ, Mann T et al. A prospective evaluation of the safety and efficacy of TAXUS element paclitaxeleluting coronary stent implantation for the treatment of de novo coronary artery lesions in small vessels: the PERSEUS SV trial. EuroIntervention 6(8), 920–927 (2011).
- Stone GW, Teirstein PS, Meredith IT et al. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a prospective, randomized, multicenter trial to assess an everolimuseluting coronary stent system for the treatment of up to two de novo coronary artery lesions) trial. J. Am. Coll. Cardiol. 57(16), 1700–1708 (2011).
- Windecker S, Remondino A, Eberli FR et al. Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N. Engl. J. Med. 353(7), 653–662 (2005).
- Goy J-J, Stauffer J-C, Siegenthaler M, Benoit A, Seydoux C. A prospective randomized comparison between paclitaxel and sirolimus stents in the real world of interventional cardiology: the TAXi trial. J. Am. Coll. Cardiol. 45(2), 308–311 (2005).
- Morice M-C, Colombo A, Meier B et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA 295(8), 895–904 (2006).
- Galloe AM, Thuesen L, Kelbaek H et al. Comparison of paclitaxel- and sirolimuseluting stents in everyday clinical practice: the SORT OUT II randomized trial. JAMA 299(4), 409–416 (2008).
- Schomig A, Dibra A, Windecker S et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J. Am. Coll. Cardiol. 50(14), 1373–1380(2007).
- Caixeta A, Lansky AJ, Serruys PW et al. Clinical follow-up 3 years after everolimus- and paclitaxel-eluting stents: a pooled analysis from the SPIRIT II (a clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions) and SPIRIT III (a clinical evaluation of the investigational device XIENCE V everolimus eluting coronary stent system [EECSS] in the treatment of subjects with de novo native coronary artery lesions) randomized trials. JACC Cardiovasc. Interv. 3(12), 1220–1228 (2010).
- Nikolsky E, Lansky AJ, Sudhir K et al. SPIRIT IV trial design: a large-scale randomized comparison of everolimus-eluting stents and paclitaxel-eluting stents in patients with coronary artery disease. Am. Heart J. 158(4), 520–526.e2 (2009).
- Stone GW, Rizvi A, Newman W et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N. Engl. J. Med. 362(18), 1663–1674 (2010).
- Kedhi E, Joesoef KS, McFadden E et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet 375(9710), 201–209 (2010).
- Leon MB, Mauri L, Popma JJ et al. A randomized comparison of the endeavor zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions: 12-month outcomes from the ENDEAVOR IV trial. J. Am. Coll. Cardiol. 55(6), 543–554 (2010).
- Leon MB, Nikolsky E, Cutlip DE et al. Improved late clinical safety with zotarolimus-eluting stents compared with paclitaxel-eluting stents in patients with de novo coronary lesions: 3-year follow-upfrom the ENDEAVOR IV (randomized comparison of zotarolimus- and paclitaxel-eluting stents in patients with coronary artery disease) trial. JACC Cardiovasc. Interv. 3(10), 1043–1050 (2010).