Perspective - Interventional Cardiology (2015) Volume 7, Issue 3
Technical and procedural advances in percutaneous coronary intervention for chronic total occlusion
- Corresponding Author:
- Toshiya Muramatsu
Department of Cardiology, Saiseikai Yokohama-City Eastern Hospital,3-6-1 Shimosueyoshi, Tsurumi-ku,Yokohama-shi, Kanagawa-pref, Japan
Tel: +81 576 3000
Fax: +81 576 3525
E-mail: t-mura@tj8.so-net.ne.jp
Abstract
Chronic total occlusions (CTO) are the most challenging lesions to treat using coronary angioplasty. The benefits of CTO coronary intervention (PCI) are symptom relief, and improved left ventricular function and long-term prognosis of the patient. In recent years, interventional cardiologists in Japan have made a great contribution to the progress of CTO PCI. Innovative techniques, including the retrograde approach, are hot topics in the CTO field. After initial reports of retrograde cases, important changes have occurred that resulted in faster and more successful procedures. Strategies for treating CTO are continually evolving and the author’s strategy also continues to change and progress over time. In this manuscript the author describes the latest advanced techniques and strategies based on personal experience, summarizing the most important literature related to CTO PCI.
Keywords
antegrade approach,CART,chronic total occlusion,collateral channel,microchannel,parallel wire,percutaneous coronary intervention,retrograde approach,reverse CART,tapered wire
Percutaneous coronary intervention (PCI) for chronic total occlusion (CTO) is the most difficult of the percutaneous procedures to accomplish, with successful recanalization of CTO representing the ‘ultimate goal’ of PCI. However, there has been considerable progress in this field over the last decade, especially during the past few years. The primary success rate of PCI for CTO has increased by 15–20% in the past several years to reach approximately 90%. This is primarily due to various new devices, technical and strategic improvements, and refinements of procedures for tackling CTO, which still continue to improve [1–4]. This article provides an overview of the current state-of-the-art PCI techniques for CTO. The discussion throughout the manuscript is separated under the headings ‘Author perspective’ and ‘Literature review.’
Indications & short-term outcome (literature review)
Coronary CTO is defined as a total occlusion with complete interruption of antegrade flow (Thrombolysis In Myocardial Infarction TIMI flow grade 0) and a duration of occlusion more than 3 months. The definition of PCI success has not yet been established. Technical success is defined as the ability to cross the occluded segment with both a wire and a balloon, and successfully open the artery; the restoration of antegrade TIMI flow 2 or 3 and a <30% residual stenosis. Procedural success is defined as technical success with no in-hospital major adverse cardiac events (MACE). Another paper has described procedural success as the successful recanalization and dilation of at least 1 CTO/patient with or without stent implantation, residual stenosis of <50%, and TIMI flow grade >1 [5,6].
Some guidelines define the indications for performing PCI for CTO. The American Heart Association consensus documents recommend that percutaneous revascularization of CTO should only be attempted if the following three conditions are fulfilled: the occluded vessel is responsible for symptomatic ischemia or angina; the myocardial territory previously perfused by the occluded artery is viable; and the likelihood of success is greater than 60%, with a low anticipated risk of major complications [7,8]. Conversely, Thompson et al. [9] found that the outcome of percutaneous revascularization of CTO varied with the expertise of the interventionist, concluding that the indications for percutaneous intervention are dependent on the operators’ skill
Brilakis described the indications of CTO PCI. CTO PCI can provide angina relief, improve exercise tolerance and left ventricular function, improve tolerance of a future acute coronary syndrome, reduce the need for coronary artery bypass graft surgery and possibly improve survival. Moreover, CTO PCI carries similar risks to non-CTO PCI [1].
Saito described the indications of PCI for CTO. If the patients had angina symptoms caused by the CTO lesions, they were eligible for PCI treatment. CTO lesions with evidence of partially or completely reversible distal ischemia, even if not any obvious symptoms, were also considered for PCI. CTO lesions with complete necrosis of the distal myocardium were generally not eligible for PCI, because such treatment would be unlikely to make a difference [10].
There is still controversy regarding the clinical value of percutaneous revascularization of CTO. Some authors have questioned its usefulness because of the chronicity of occlusion or the lack of improvement in survival after successful revascularization; others have suggested that the procedure is questionable in the absence of documented myocardial viability. Conversely, it has been stated that PCI should be attempted for CTO if the likelihood of success is moderate or better and if the myocardial territory of the occluded vessel is viable. When considering PCI for CTO, it should be noted that differences between interventionists have a greater influence on the indications than is the case for PCI of nonoccluded coronary arteries. In other words, the indications become narrower or broader depending on the operator’s skill in the case of PCI for CTO.
Patel et al. [11] analyzed the outcomes of attempted percutaneous revascularization of CTO in a total of 18,063 patients from 65 trials, finding a high initial success rate of 77%. Successful recanalization was associated with a low rate of major complications (death, CABG and cerebral infarction), while the occurrence of major complications was reported after failed recanalization, probably due to aggressive wiring. The Multicenter CTO Registry Japan (J-CTO) trial group [12] developed the J-CTO score based on lesion length, extent of calcification, angle, retry and occlusion morphology, and concluded that the probability of primary success can be predicted by analyzing the complexity of the occlusion via the antegrade approach.
Intravascular ultrasound (IVUS) and multislice computed tomography (MSCT) are useful adjuncts to coronary angiography (CAG). When employing the antegrade approach, an IVUS probe can be introduced into a side branch to locate the entry point of a CTO with a blunt and poorly defined stump (stumpless CTO) [13]. Additionally, an IVUS probe can be introduced into the space after a guidewire has entered a subintimal space and can be used to guide another guidewire into the intimal space (IVUS-guided PCI) [14].
Performing MSCT before PCI can provide valuable information about the extent of calcification, vessel dimensions, lesion length and vascular anatomy around the lesion. Takimura et al. [15] reported several successful cases in which MSCT was used to locate previous stents and to accomplish percutaneous revascularization of CTO via the retrograde approach. Rolf et al. [16] reported that the success rate of PCI for CTO was significantly higher with preprocedural MSCT than without such assessment (90 vs 63%).
Long-term outcome (literature review)
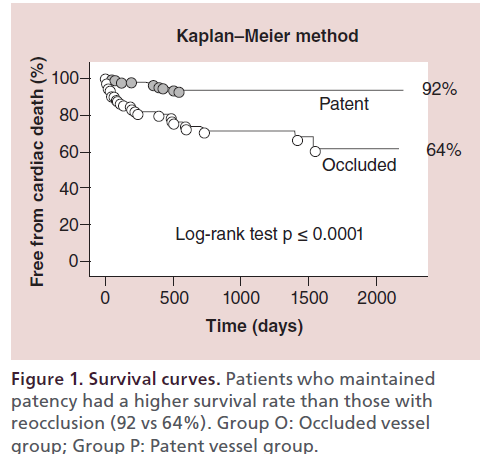
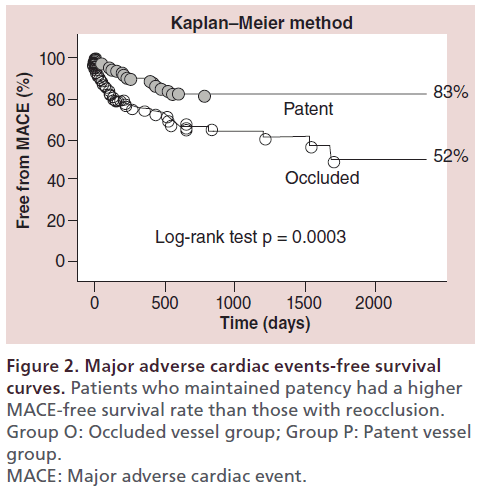
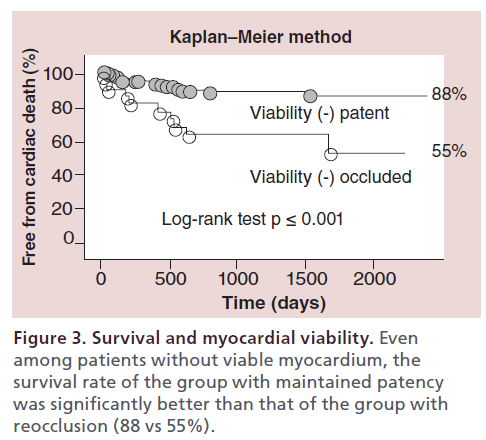
The author retrospectively analyzed the long-term outcome of 606 patients (1145 CTO lesions) treated with PCI at the author’s center between January 1996 and December 2003 [17]. The cumulative survival rate was significantly higher for patients who maintained patency than for those with reocclusion (92 vs 64%); the rate of MACE was also higher in the group with patency (Figure 1 & 2). Among patients in whom the myocardial territory previously perfused by the occluded artery showed little or no viability, the cumulative survival rate was significantly higher for those who maintained patency than for those with reocclusion (88 vs 55%) (Figure 3).
Joyal et al. [18] confirmed the effectiveness of percutaneous revascularization for CTO by performing a meta-analysis of 13 trials. Patients who underwent PCI had better outcomes than those not receiving PCI with respect to the survival rate, incidence of CABG and improvement of angina. Several studies have shown that the mortality rate is lower (by 3.8–8.4%) after successful PCI for CTO than after unsuccessful PCI [19–23]. Even in patients with left ventricular dysfunction, if available collaterals allow procedural success, PCI not only improves cardiac function but also reduces mortality [24–26].
Approaches to CTO & guiding catheters (author perspective)
Several important steps need to be taken for successful percutaneous revascularization of CTO. The first step is to perform appropriate CAG and to properly interpret the images before the procedure. At least three different views should be obtained in which the major branches of interest are visible without overlapping. Careful observation should be performed to determine the pattern and length of the CTO, the course and branches of the occluded vessel, the vascular anatomy distal to the CTO and the extent of vascular calcification. The presence of collateral channels suitable for PCI also needs to be determined. Characteristics of a collateral channel that are checked include its size, its origin and course, and the entry point of blood flow from the channel into the occluded vessel distal to the CTO. Recent increased use of the retrograde approach has made it more important to check for collaterals and to characterize these vessels before intervention. RAO caudal, RAO cranial and lateral views must be obtained to delineate septal channels, which are commonly used as collaterals for the retrograde approach. Recently, epicardial channels have also been used increasingly, including apical, atrial and ipsilateral channels. There are no rules regarding the best angiographic views to display epicardial channels, so the optimal views should be chosen on a case-by-case basis. Important tips for viewing collateral channels are to adjust the image size to cover the entire course, to avoid panning while obtaining the image and to use a sufficient dose of contrast medium to demonstrate collateral flow into the coronary artery distal to the CTO. The goal of preprocedural imaging is to determine the vessel from which the collateral arises and the location where it enters the coronary artery distal to the CTO.
The second important step towards achieving successful PCI is to select the optimal PCI system for the CTO. The guiding catheter should be at least 7 Fr to ensure sufficient back-up [27]. However, some experienced operators can perform CTO PCI using a smaller guiding catheter [28]. The 6F transradial approach is associated with a greatly reduced risk of bleeding and better patient comfort.
Consequently, the use of a bilateral femoral or radial approach is a matter of operator preference and the acknowledgment of risks/benefits of both approaches is important.
To confirm the presence of a collateral channel that enters the coronary artery distal to the CTO, bilateral artery puncture should be performed for simultaneous imaging of the left coronary artery (LCA) and the right coronary artery (RCA). Supportive guiding catheters should be used, such as those of the AL type for insertion into the RCA and those of the XB type for the LCA. However, a supportive guiding catheter tends to cause injury to the proximal coronary artery because of deep engagement of its tip; consequently, care should be taken to avoid this. The author generally employs a guiding catheter with a side hole to perform PCI for CTO in order to avoid coronary artery injury by the catheter tip and pressure damping.
Antegrade approach
Antegrade device
Guidewire selection
Tapered guidewires (author perspective)
Recent efforts to clarify the pathology of CTO have shown that frequent negative remodeling of the lesion occurs and that there are fewer microchannels than was expected [29].
For this reason, the operator should begin with a soft tipped, tapered hydrophilic wire such as Fielder XT (Asahi Intec, Japan) in an effort to traverse the CTO through the microchannels. The advantage of using a tapered guidewire is that, compared with other guidewires, a tapered guidewire is less likely to enter a subintimal space because it is a floppy wire. Another advantage is that successfully passing it through a microchannel will considerably shorten the procedural time. Approximately 10–15 min is the optimal duration for attempting to cross the CTO with a tapered guidewire. If the wire cannot pass through a microchannel within this period, it should be promptly exchanged for a different guidewire.
Intermediate guidewires (author perspective)
If the affected artery has bends, tortuous or unsuccessful penetration, the tapered guidewire should be switched to an intermediate guidewire. The Miracle 3g, 4.5g and 6g guidewires (Asahi Intec, Japan) are representative of this type. This guidewire are moderately high-gram force, nontapered 0.014-inch guidewire. While the recently introduced Gaia series of guidewires (Asahi Intec, Japan) are superior to conventional wires with regard to tractability. The torque responsiveness of the Gaia guidewire has been improved by changing the tip core to a composite with a doublecore structure. Resistance of a CTO to the guidewire tip occurs in response to the force required to advance it through the lesion tissues, resulting in the occurrence of deflection. A CTO can be penetrated by the deflection control method in which such deflection is used to control the course of the guidewire.
Owing to improved torque performance (1:1), this novel guidewire series is almost ideal for wiring the vascular anatomy of a CTO, since these wires allow active control within the intimal space while advancing through a CTO without entering a subintimal space.
Stiff guidewires (author perspective)
To reanalyze a CTO in a relatively straight or minimally tortuous artery, the author often directly exchanges a tapered guidewire for a stiff wire rather than for an intermediate one because this strategy saves time and reduces cost. The Conquest (Confienza) guidewire (Asahi Intec Co., Japan) is a representative stiff guidewire that has better penetrability than other guidewires of this class. The Conquest guidewire has the characteristics of taper tip shape (0.009 inch) and hydrophilic coating except for the 1-mm distal tip. The variety of tip weights includes 9, 12 and 20 g. However, use of the Conquest guidewire is associated with an increased risk of coronary artery injury, so its selection should be avoided if the course of the target vessel is unknown or unpredictable. If the CTO lesion is too hard to penetrate, the guidewire should be exchanged for one with a stiffer tip such as the Conquest 12 g or 20 g. Guidewires of the Miracle series are preferable for crossing a CTO in a bending or tortuous artery.
Microcatheter use & selection (author perspective)
A microcatheter is used to support the guidewire as it is advanced precisely through a CTO in the intimal space, and this catheter stabilizes the guidewire and thus reduces the risk of entering a subintimal space. A microcatheter can also be used to straighten a tortuous and/or kinked coronary artery proximal to the CTO, thus increasing the guidewire torque until it is nearly 1:1.
Microcatheter
Microcatheters have the advantage of advancing smoothly through a narrow space in a CTO to provide good support. Another advantage is greater steering control of the GW by allowing straightening of the secondary bend of the wire tip. Microcathters include Finecross (Terumo Co., Japan), Mizuki (Kaneka Co., Japan), Supercross (Vascular Solution, MN, USA) and Quick cross (Spectrancis Co., CO, USA).
Corsair
The Corsair microcatheter (Asahi Co.) was developed for use with the retrograde approach. Although it was originally employed to select a collateral channel for the retrograde approach, it is also useful as an antegrade microcatheter. The Corsair has a 2.7 Fr shaft composed of eight fine wires and two thicker wires, while its tapered tip has a hydrophilic coating on the distal 60 mm. When manipulated with rotation, it can automatically enter a CTO or collateral channel by a similar mechanism to that of the Tornus microcatheter. Owing to its low-friction coating, the Corsair is also able to dilate a vessel to its diameter size without injuring the walls. Regarding its pushability as an antegrade microcatheter, the Corsair is slightly inferior to the Tornus but better than the Finecross. The Corsair is also more flexible than the Tornus so that it is less likely to cause vascular injury even when used in very fine vessels. When used for the retrograde approach, the Corsair can pass through a collateral channel more easily than conventional microcatheters, and makes it easier to accomplish subsequent procedures. Once it has entered a channel, the Corsair can be advanced without much resistance if it is rotated with the right hand and pushed forward with the left hand. However, it should be remembered that excessive rotation of the Corsair may disrupt its shaft [30].
Tornus
The Tornus (Asahi Co.) is a microcatheter with improved crossability. It has a 2.1 Fr or 2.6 Fr shaft composed of eight stainless steel wires. Its spiral structure ensures automatic entry into a CTO when rotational manipulation is performed. The Tornus has the advantage of being able to dilate a channel leading to a CTO that is too solid for even a balloon catheter to enter. Like the Corsair, the Tornus can be disrupted and become stuck if subjected to excessive rotation.
Over-the-wire balloon
Several over-the-wire (OTW) balloon catheters with a small diameter and improved performance are currently available. These catheters can be used as a substitute for a microcatheter. However, the less flexible solid shaft of the balloon part limits guidewire manipulation in a tortuous artery when an OTW balloon catheter is used as a substitute for a microcatheter. When the CTO is in a severely tortuous artery, care should be taken to maintain the OTW balloon catheter at its optimal position.
Antegrade technique
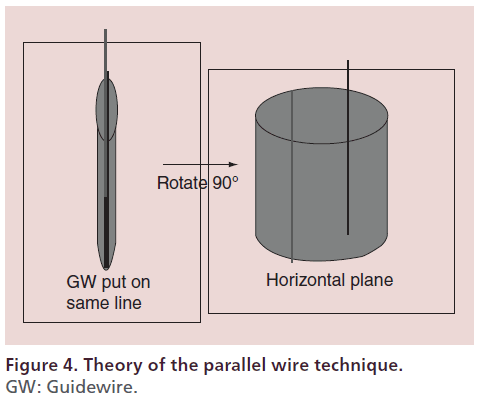
Parallel wire technique (author perspective)
The parallel wire technique involves antegrade advancement of a second wire toward the intimal space parallel with the first antegrade guidewire that has entered a subintimal space (Figure 4) [31]. The key points for successful application of this technique are to visualize the site at which the first wire appears to be most parallel with the intimal space and to manipulate the second wire at the place where the intimal space is clearly visible on a distal image. Aggressive wiring will enlarge the subintimal space and lead to compression of the intimal space so that it becomes harder to visualize, which will in turn promote distal enlargement of the subintimal space. To prevent this undesirable outcome, the site of entry of the first guidewire into the subintimal space should be identified at the most proximal position possible (e.g., proximal to the midpoint of the CTO) and the parallel wire technique should be initiated from this site. This technique is useful for the antegrade approach if executed properly. In fact, a technical success rate of 90% was obtained in the Conquest registry trial [32].
Antegrade re-entry (literature review)
Antegrade re-entry has mostly been attempted in western countries. Antegrade re-entry is a procedure that involves intentional creation of RCA dissection and subsequent returning of the wire to the true lumen from a distal branch; it was initially reported as the ‘subintimal tracking and re-entry (STAR)’ technique [33,34]. This has now become unpopular because subsequent trials showed that intentional dissection was occasionally associated with complications including coronary artery injury and perforation, as well as a relatively poor long-term outcome and patency rate [35].
Subsequently, a similar theory was applied to develop the CrossBoss system (Bridgepoint Co., MN, USA) [36], which is a metal OTW microcatheter with a round tip to prevent vessel exit. This device is rotated rapidly using a ‘fast spin’ technique and can advance into CTO. If a subintimal/subadventital position is required, Stingray balloon and guidewire is performed. The Stingray balloon is a 1-mm flat balloon with three exit ports connected to the same guidewire lumen. When the balloon inflated, one port is oriented to the lumen and the other toward the adventitia. The stingray guidewire is then used to penetrate to the distal true lumen from subintimal space.
Studies have reported achieving a success rate of 87% by Wilson et al. [37]. Outcomes of the CrossBoss system treating CTO have shown similar vascular patency rates for PCI employing this system compared with conventional methods [38]. CTO revascularization with this system, which is termed the antegrade re-entry technique, is currently primarily performed in the USA. This technique can also be thought of as the ‘last resort’ because it does not conform to the fundamental PCI concept that the wire should remain in the true lumen as much as possible.
Retrograde approach
The retrograde approach to CTO was initially developed by Japanese interventional cardiologists [39,40]. This approach is principally employed because of previous or anticipated failure of the antegrade approach due to difficulty in locating the entrance of the CTO or due to the presence of severe calcification.
My experience with the retrograde approach
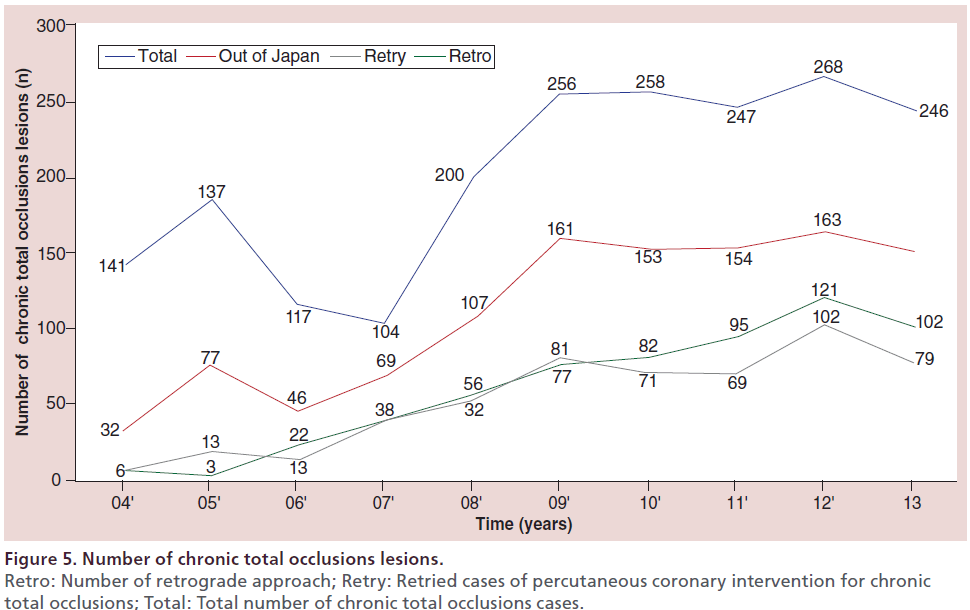
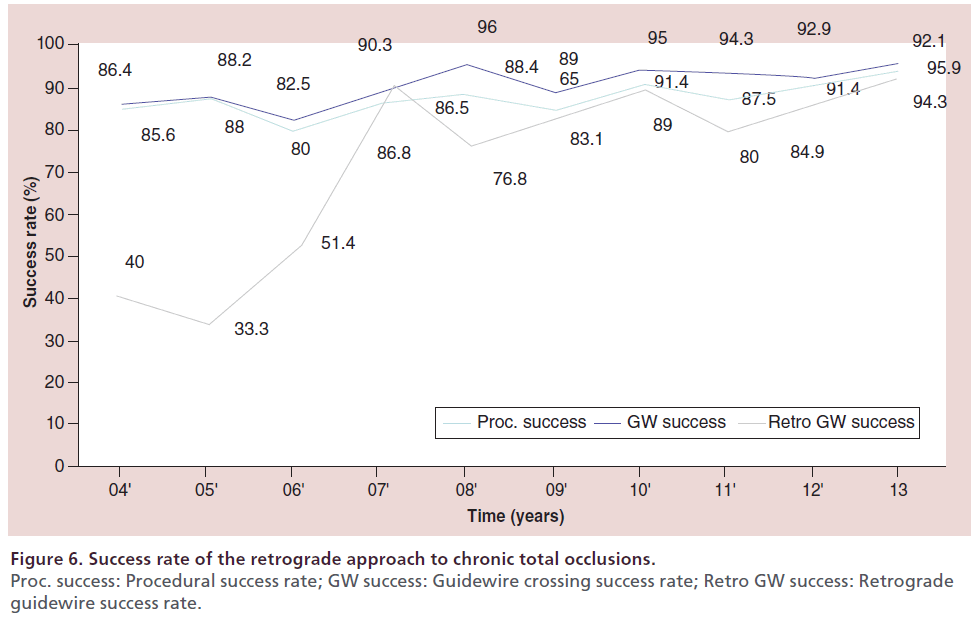
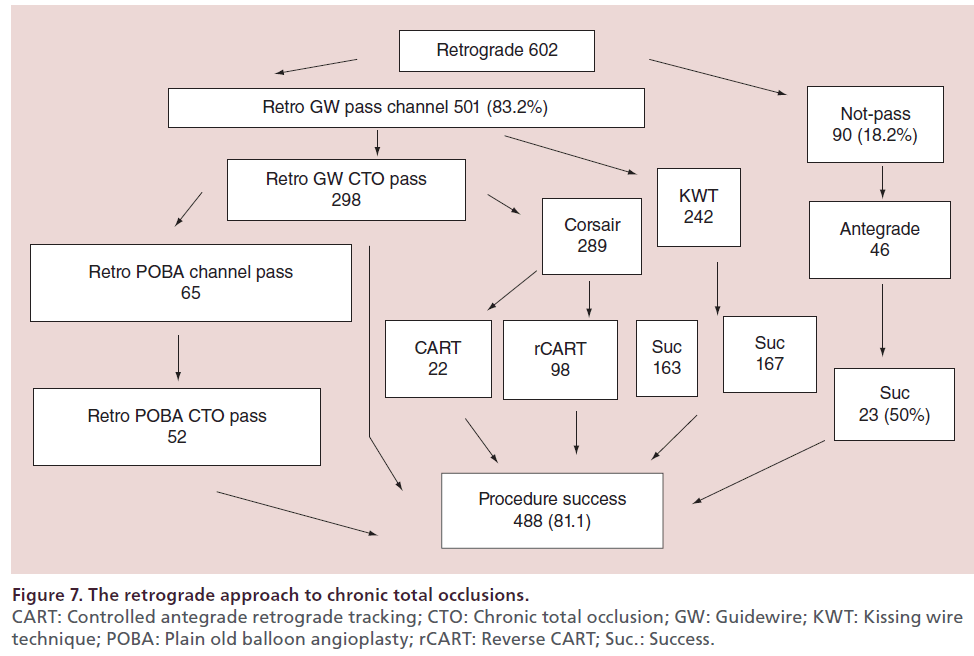
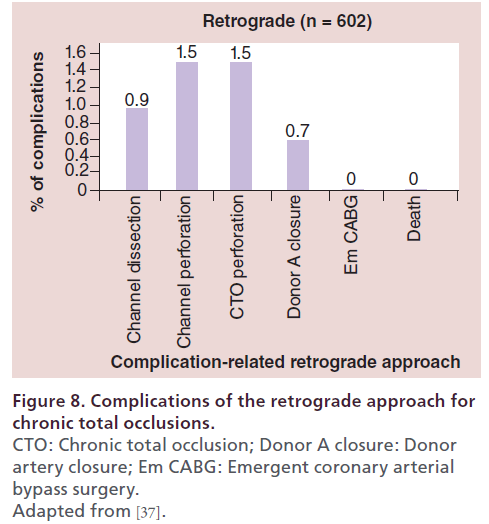
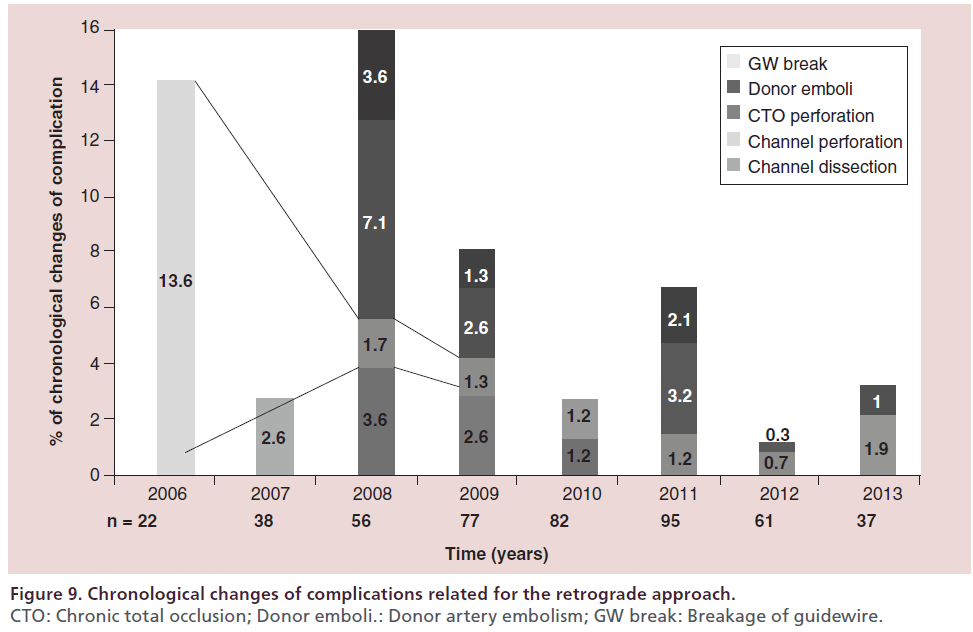
At the author’s center, the retrograde approach was first adopted in 2004, and the devices and techniques for this approach have been refined considerably since then. The author recently reviewed the outcomes of retrograde PCI and the procedural changes at their center over the past 10 years. During the period from January 2004 (when the retrograde approach was introduced) to December 2013, 2024 patients underwent PCI for CTO at this center, with the number of patients increasing every year. In recent years, approximately 200–250 patients have been treated annually, 25–30% of whom have a history of prior PCI. With the increase of re-try cases, the percentage of patients treated by the retrograde approach has increased to approximately 30% of all CTO cases (Figure 5). Both the procedural and guidewire success rates reached 85–90% over the review period, with both success rates exceeding 90% since 2010 (Figure 6). The most common reason for selecting the retrograde approach was ‘re-try’ (n = 444), and the major collateral channel used for this approach was a septal branch (n = 410) (Table 1). The author also analyzed the procedural details in 602 retrograde patients. Retrograde channel tracking was successful in 83.2%, of cases with successful retrograde CTO wire crossing in 298 patients. The kissing wire technique (KWT) was attempted in 242 patients and CART technique was combined in 22 patients, while the reverse CART technique was combined in 98 patients. The Corsair microcatheter was used in 298 patients and total procedural success was achieved in 488 patients (81.1%) (Figure 7). Complications related to the retrograde approach included collateral channel dissection (0.9%), channel perforation (1.5%), CTO perforation (1.5%) and donor artery closure (0.7%). However, none of the patients required surgical intervention (CABG) and there were no cardiac deaths (Figure 8). The incidence of channel perforation has been reduced recently, but other complications such as CTO perforation and donor artery thrombosis still occur occasionally (Figure 9) [41].
| Types of chronic total occlusions | n |
|---|---|
| Total number | 602 |
| Retry | 444 |
| Unknown entry | 145 |
| Abrupt | 22 |
| Diffuse | 12 |
| Septal channel | 410 |
| Epicardial channel | 192 |
Retry: Retried percutaneous coronary intervention for chronic total occlusions.
Table 1. Background of retrograde approach.
Figure 8: Complications of the retrograde approach for chronic total occlusions. CTO: Chronic total occlusion; Donor A closure: Donor artery closure; Em CABG: Emergent coronary arterial bypass surgery. Adapted from [37].
Retrograde approach (literature review)
By comparison, the above-mentioned US study [9] showed that the initial success rate differed between operators using the retrograde and non-retrograde approaches, with a high success rate (sometimes >90%) for retrograde procedures. A recent US registry study [42] of 1361 CTO patients treated by retrograde PCI also showed favorable outcomes, with a technical success rate of 85.5%, a procedural success rate of 84.2%, and a major complication rate of 1.8%. Similarly, the Euro CTO Club [43] reported successful channel crossing in 80.6% and a procedural success rate of 83.6% of cases. In Japan, a retrograde PCI registry study [44] of 801 patients revealed that technical and procedural success was achieved in 84.8 and 83.8% of cases, respectively, with the CART technique being used in 2/3 of successful cases. Another Japanese retrograde PCI registry study [45] of 378 patients treated at 27 centers also showed favorable outcomes of retrograde PCI, with successful channel crossing in 81.7% and a procedural success rate of 83.6% cases (Table 2).
| Author | n | Retro | Success (%) | MACE (%) | Ref. |
|---|---|---|---|---|---|
| Morino | 498 | 156 | 87.70 | 2.70 | [12] |
| Yamane | 378 | 378 | 83.60 | 0.60 | [45] |
| Kimura | 224 | 224 | 92.40 | 1.80 | [46] |
| Sianos | 175 | 175 | 83.40 | 4.60 | [43] |
| Galassi | 1983 | 234 | 82.90 | 1.60 | [47] |
| Thompson | 636 | 122 | 69.00 | 3.45 | [9] |
| Karmpaliotis | 462 | 462 | 81.40 | 2.60 | [48] |
Table 2. Comparison of results for retrograde approach.
Brilakis et al. [1] systematized PCI for CTO by proposing the following hybrid algorithm: antegrade wire escalation, antegrade dissection/re-entry, and finally the retrograde approach. Michael et al. [49] analyzed the outcome of intervention for CTO by this hybrid approach in 73 patients and reported a high technical success rate of 90.4%. They found that the strategy leading to success was antegrade wire escalation in 50% of the patients, antegrade dissection/re-entry in 24.2% and the retrograde approach in 25.8%.
For the long-term patency, Muramatsu et al. [50] analyzed 12 mon TLR of subintimal stenting is similar in that of intimal stenting. It suggested subintimal tracking of reverse CART is acceptable long term patency.
Collateral channel wiring (author perspective)
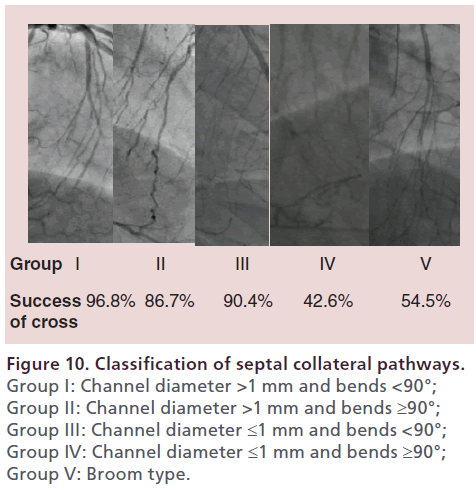
The first step towards success with the retrograde approach is to find a suitable collateral channel. CAG should be performed to obtain at least two fixed views that are focused on the available collaterals. A wide and straight septal channel is most desirable. Some channels may initially seem to be ideal, but an unexpected bend or tortuosity can make it difficult for the guidewire to cross. Relatively straight collateral channels are easier to traverse, even if the diameter is not large. With reference to the classification of collaterals proposed by Werner et al. [51], the author classifies septal channels into the following five groups in the order of increasing difficulty for tracking: Group I is channels with a diameter >1 mm and bends <90° 96.8% success; Group II is channels with a diameter >1 mm and bends ≥90° 86.7% success; Group III is channels with a diameter ≤1 mm and bends <90° 90.4% success; Group IV is channels with a diameter ≤1 mm and bends ≥90° 42.6% success; and Group V is broom-type channels 54.5% success. A broom-type septal channel has multiple subchannels, each of which is very fine and difficult to cross, so careful consideration is needed before trying the retrograde approach with this type of septal channel (Figure 10). Epicardial channels usually have a larger diameter, but are often tortuous. Some collateral channels have an unexpected origin, which means that angiograms need to be interpreted carefully. After isolating a collateral channel with a guidewire, the Finecross or Corsair microcatheter should be used to track the channel while tip injection with a 3-ml syringe is performed to confirm the course. Before injecting contrast medium, it is safer to confirm backflow of blood into the syringe under negative pressure. For selecting a collateral channel, the Sion guidewire (Asahi Intecc Co., Japan) should be the first choice. This guidewire can be advanced smoothly and safely because of its coated tip and 0.7-g tip load. The tip of guidewire is coated with a hydrophilic coating and rope coil technology. It facilitates good guidewire retention, advanced torque performance and flexible atraumatic tip.
The Sion Black guidewire (Asahi Intecc Co.) is a recent addition to the PCI armamentarium that can be advanced more smoothly and, consequently, it has become a useful choice for fine channels. This guidewire features polymer jacket and slip coating with rope coil structure of tip make better sliding in the small channels.
Even fine collaterals can be effectively selected by using the Fielder XTR guidewire (Asahi Intecc Co.) which has a polymer jacket coating with a 0.010 inch tapered tip. While the Suoh guidewire (Asahi Co.) is used for curved but nonbranching collaterals. The Suoh guidewire has a light weight of wire tip, 0.5 g, resulting in less resistance cross for bent point channel.
The tip of the Sion guidewire can be shaped into a small curve that may be compatible with the course of the collateral. If the collateral channel shows marked curvature, the curve of the guidewire tip should be altered accordingly.
When tracking a collateral channel, the guidewire is manipulated in a completely different manner from that employed to cross a CTO. In brief, the guidewire should be rotated gently to straighten the artery while minimizing forward/backward movement so that it can be advanced smoothly through the channel without much friction. Since a single CAG view is often insufficient to understand the detailed anatomy of a tortuous collateral channel, multiple views (e.g., RAO cranial, RAO caudal and lateral) should be obtained in combination during collateral channel tracking to provide precise information about the vascular anatomy. If resistance to the guidewire is felt, the anatomy of the target channel should be confirmed by obtaining additional angiographic views to avoid blind wiring and to ensure safe crossing of the channel. When performing additional angiography for this purpose, it is important to withdraw the microcatheter back to the main trunk in order to enhance the contrast effect because tip injection alone often fails to provide sufficient contrast and the guidewire limits adequate performance of CAG. If the collateral is a fine channel and the precise anatomy has not been confirmed, wiring by blind septal surfing is sometimes acceptable [52] but this should not be attempted by beginners and should not be performed for an epicardial channel.
After a guidewire has passed through the collateral channel, advancing a microcatheter should be attempted.
The Corsair microcatheter is often the first choice for this purpose, while the Finecross may be selected initially for a very tortuous epicardial channel. The Corsair is sometimes blocked by severe tortuosity of a collateral and it should be exchanged for the Finecross in such cases. An alternative method that may also be successful is to exchange it for another Corsair. This is because the tip of the Corsair is delicate and is quite likely to be damaged during the procedure or by vascular tortuosity. Insertion and exchange of microcatheters requires strong support from a retrograde guiding catheter, but the guiding catheter sometimes deviates from the coronary artery, resulting in destruction of the entire PCI system. Such problems during microcatheter exchange can be avoided by using the balloon anchor technique, in other words, anchoring the guidewire by inflating a balloon within the guiding catheter.
If the channel cannot be crossed by a microcatheter after trying all of these strategies, many difficulties will be encountered subsequently; consequently, it is better to attempt another channel instead.
Handling the retrograde guidewire after crossing a collateral channel (literature review)
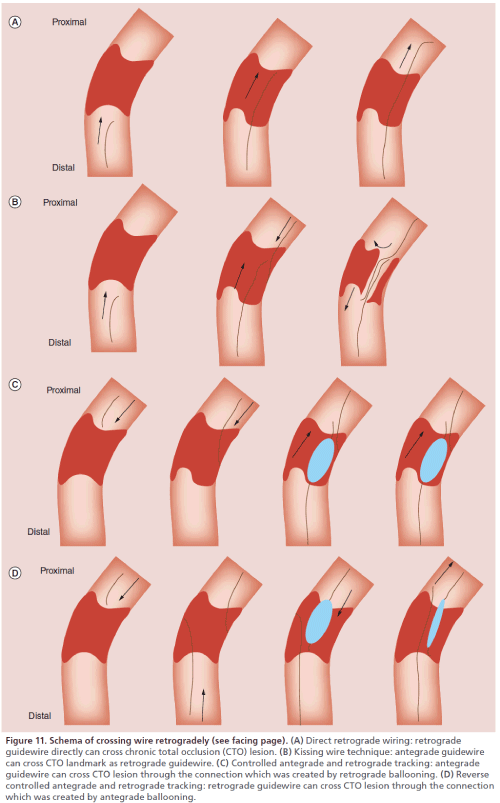
After a retrograde guidewire has crossed the collateral channel and reached the lumen of the coronary artery distal to the CTO, the following four strategies [53] are available to achieve CTO wire crossing: direct retrograde wiring; the kissing wire technique (KWT); the controlled antegrade and retrograde tracking (CART) technique; and the reverse CART technique.
Direct retrograde wiring (author perspective)
The retrograde wire was used to completely cross the CTO, being advanced upstream from the distal true lumen, through the CTO and into the proximal true lumen (Figure 11A).After the coronary artery lumen distal to the CTO is reached, a soft guidewire such as the Fielder XT can be used to attempt direct retrograde crossing of the CTO. The guidewire can enter the CTO more easily when it is manipulated retrogradely and may often cross the lesion directly because the distal fibrous cap of a CTO is softer than its proximal fibrous cap. If the Fielder XT guidewire cannot cross the CTO, it should be exchanged for the Ultimate bros 3 (Asahi Intecc Co.) or for a Gaia 2nd guidewire. The Ultimate bros 3 guidewire has a long hydrophilic coating except for the wire tip. It provides better torque response in comparison with the Miracle 3g guidewire. If the CTO is severely calcified, the guidewire is appropriately exchanged for a Conquest Pro or a Pilot 200 (Abbott Co.) which are high gram force, nontapered and polymer jacket guidewires. As with the antegrade approach, active wire control has become the mainstream procedure, which means that the interventionist aims to follow the true lumen through the lesion by using a guidewire with good trackability (e.g., one from the Gaia series). Crossing the CTO without entering a subintimal space is important for achieving long-term patency and for improving the safety of the procedure. If direct retrograde wiring is successful, the guidewire should be led into the antegrade guiding catheter. If a microcatheter can also be led into the antegrade guiding catheter, the guidewire can then be exchanged for a 3-m guidewire RG3 (Asahi Co., Japan) that is withdrawn from the contralateral femoral sheath to allow antegrade passage of a balloon catheter (externalization). If the guidewire is anchored by inflating a 2.5-mm balloon in the guiding catheter, the microcatheter can easily be advanced into the guiding catheter. Caution should be exercised when navigating the microcatheter (e.g., a Corsair) into the antegrade guiding catheter as this pulls the antegrade guiding catheter deeper into the coronary artery. Conversely, advancing an antegrade device into a CTO or transient withdrawal of a retrograde microcatheter (e.g., a Corsair) pulls the retrograde guiding catheter deeper into the coronary artery.
Kissing wire technique (author perspective)
If direct retrograde wiring is impossible or the microcatheter fails to cross the collateral channel to reach the coronary artery lumen distal to the CTO, the antegrade guidewire should be manipulated while using the retrograde wire as a landmark. This is known as the kissing wire technique (KWT; Figure 11B). The retrograde guidewire can serve as a good landmark that allows much better manipulation of the antegrade wire. An important tip for success with this technique is to leave the retrograde guidewire in the intimal space so that it acts as a landmark. Advancing the antegrade wire toward the retrograde guidewire can ensure that the antegrade wire will cross the CTO almost completely through the intimal space. While attempting to cross the CTO, two different angiographic views should be obtained to check the position of the retrograde guidewire and to confirm that it meets the antegrade guidewire at a single point. If the two guidewires fail to overlap precisely, one of them may have entered a subintimal space. Antegrade wiring should be initiated from a site proximal to the retrograde wire so that the tips of the two guidewires can meet at a single point. If the two guidewires are not coaxial, one of them has probably entered a subintimal space.
Figure 11: Schema of crossing wire retrogradely (see facing page). (A) Direct retrograde wiring: retrograde guidewire directly can cross chronic total occlusion (CTO) lesion. (B) Kissing wire technique: antegrade guidewire can cross CTO landmark as retrograde guidewire. (C) Controlled antegrade and retrograde tracking: antegrade guidewire can cross CTO lesion through the connection which was created by retrograde ballooning. (D) Reverse controlled antegrade and retrograde tracking: retrograde guidewire can cross CTO lesion through the connection which was created by antegrade ballooning.
Coping with failure of retrograde wiring The CART technique (literature review)
The traditional CART technique defined as creation of retrograde subintimal dissection and connects this with controlled antegrade dissection to enable the passage of a guidewire into the distal true lumen (Figure 11C). Since the introduction of the Corsair microcatheter, the CART technique has become relatively unpopular and the reverse CART technique has gained wider acceptance instead. If the retrograde guidewire is blocked by the CTO, an antegrade guidewire should be manipulated while using the retrograde wire as a landmark. If the two guidewires come to overlap within the subintimal space, the CART technique can be tried. Kimura et al. [46] analyzed a CART registry and reported a favorable outcome of treating CTO by this technique, with a wire success rate of 87.9%, procedural success rate of 92.4% and MACE rate of 1.8%.
The reverse CART technique (literature review)
If direct retrograde wiring and the KWT are impracticable, the reverse CART technique should be considered.
Reverse CART is defined as the antegrade guidewire being introduced into the CTO segment. The antegrade balloon dilation is performed either inside the vessel or in the subintimal space to create intimal and medial disruption. Following this, the antegrade subintimal space can be automatically connected with the retrograde subintimal space if the retrograde wire has already created the subintimal dissection (Figure 11D).
Thanks to the Corsair microcatheter, the reverse CART technique has recently become more widely accepted than the CART technique because of its technical simplicity and safety [54]. The first step is antegrade insertion of an IVUS catheter, which is used to determine whether the antegrade and retrograde guidewires have entered the intimal or subintimal space [55]. If both guidewires are obstructed in the intimal space or within the subintimal space, the reverse CART technique can be accomplished easily. However, if one of the guidewires lies in the intimal space and the other is located in the subintimal space, the two wires are separated by the vessel wall and it is often hard to appose them. Tsujita et al. [56] reported that subintimal tracking was significantly more frequent for retrograde guidewires than antegrade guidewires (40% vs 9%). To avoid subintimal tracking as far as possible, following the intimal space with a retrograde guidewire that has superior trackability (i.e., active wire control) has recently become the preferred technique. Even with this strategy, careful and subtle wire control is recommended, as is required for the KWT.
If IVUS reveals that one guidewire is within the intimal space and the other lies in a subintimal space, a balloon catheter that is slightly larger than the estimated vessel diameter obtained by the IVUS should be inserted antegradely and inflated in an attempt to create a connection between the two spaces. Special techniques can also be tried, such as the balloon slipping technique (an attempt to advance the wire immediately after balloon deflation) and the knuckle wire technique. The balloon slipping technique is the retrograde guidewire puncture to the inflated antegrade balloon; consequently, the wire is inside the balloon chamber. After successfully puncturing the antegrade balloon and deflating the balloon, it is possible to pull back the balloon simultaneously while advancing the retrograde wire.
The advantages of this technique include maintained enough subintimal space by balloon inflation, allowing the retrograde wire to push against it and to puncture it. Additionally, its ability to protect the proximal vessel from retrograde wire damage by using balloon inflation is an advantage [57]. The modified reverse CART technique is also useful. Sometimes, it is difficult to perform reverse CART at CTO body, in which the case is to form a connection by inflating the balloon at the proximal optimal position [58].
If the retrograde guidewire crosses the CTO but fails to reach the entrance of the lesion, the ‘capture’ technique with an antegrade Guideliner (Vascular Solution Co.) or a child catheter such as a Kokate (Asahi Co.) may be useful. The use of inner catheter with the mother in child method inserts into the space to help to connect the retrograde wire to the antegrade guidingcatheter.
The stent reverse CART technique (which combines reverse CART with stenting) involves deploying a stent withing the antegrade subintimal space to create an open target for retrograde wire crossing [59].
The knuckle wire technique (literature review) The knuckle wire technique has the same indications as the reverse CART technique and involves advancing a guidewire with a knuckled tip into a subintimal space. This technique is primarily effective when the retrograde guidewire has become blocked within a subintimal space. In some cases, knuckled wires are advanced both antegradely and retrogradely. Polymer jacket guidewires such as the Fielder XT and Fielder FC are suitable for the knuckle wire technique. Combining it with the reverse CART technique ensures adequate enlargement of the subintimal space [60].
Complications of the retrograde approach (author perspective)
In general, the retrograde approach is associated with a higher rate of complications than the antegrade approach [47]. The most common complications of the retrograde approach are collateral channel dissection and perforation. However, if a septal channel is used for this approach, severe complications are uncommon in the case of channel perforation since it usually results in bleeding into the ventricle. If channel perforation causes hemorrhage within the myocardium rather than the ventricle, a hematoma will gradually enlarge and it will need to be treated by hemostasis. If an epicardial channel is used for the retrograde approach, perforation of the channel leads to a risk of pericardial tamponade and must be treated by hemostasis. A more serious complication is acute obstruction of the donor artery due to thrombosis or dissection. Acute donor artery obstruction is a critical situation because the target artery is already occluded by the CTO. To prevent this serious complication, the position of the guiding catheter should be checked frequently to avoid deep engagement, an 8-Fr guiding catheter should be selected to accommodate more than one microcatheter, and the activated clotting time should be monitored hourly and maintained at ≥300 s. If the collateral channel is too fine and/or tortuous, the guidewire or catheter may become kinked or may be difficult to withdraw from the channel. Therefore, it is better to avoid using channels that are too fine or tortuous.
Complications of the retrograde approach (literature review)
In general, complications are more frequent with the retrograde approach than the antegrade approach. Lo et al. [61] reported a myocardial injury rate of 13.8% with the retrograde approach versus 6.7% with the antegrade approach. Karmpaliotis et al. [48] studied 462 patients and reported that complications of PCI for CTO were relatively infrequent, with the rate of major complications being 2.6% and the coronary perforation rate being 1.3%. However, one patient treated via the retrograde approach developed a coronary-ventricular fistula that required embolization. The Euro CTO Club [43] reported complications of the retrograde approach in 8.6% of patients, including channel perforation and hematoma (6.9%). A Japan retrograde PCI registry study [44] showed that the rates of major collateral injury and perforation were similar after successful and unsuccessful intervention for CTO, but there was a significantly higher rate of minor collateral injury after unsuccessful intervention (17.0 vs 7.5%).
Take home messages
As the retrograde approach has become common, new PCI strategies for CTO have been developed. With advances in the retrograde approach, achieving the proper balance between the antegrade and retrograde approaches has become a key factor in treatment success. Crossing a CTO using the antegrade approach should be attempted appropriately for a limited time and then efforts should be shifted to the retrograde approach if there is a possibility of success. To achieve the correct balance, preoperative images need to be interpreted accurately and a detailed strategy should be developed by employing all available means to gain information, including CT. The key points requiring improvement for the retrograde approach include the method of appropriately selecting a collateral vessel and procedures for retrograde penetration of the CTO. It is desirable to establish comprehensive plans for managing CTO appropriately by efficient time-saving procedures.
Conclusion
Thanks to the enormous efforts of pioneering interventional cardiologists, PCI for CTO has become one of the most advanced medical treatments and has been shown to improve the long-term survival of patients with CTO. To help young interventionists acquire the high level of skill needed for CTO procedures, a systematic curriculum should be developed that includes education/training programs as well as instructions and demonstrations by experienced interventionists.
Future perspective
It is expected that more generalized and standardized strategies for CTO that can even be handled by nonspecialists will be developed over the next 10 years. To achieve this, there must be further improvements in the performance of imaging systems, guidewires, and catheter devices. It will also be necessary to develop new preoperative CTO imaging diagnosis systems and guidewire autonavigation devices that can be employed to assess the nature of plaques in detail, as well as devices for catheter treatment that can penetrate hard CTO plaques including calcified plaques.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Long-term outcome of percutaneous coronary intervention for chronic total occlusions
• Among patients in whom the myocardial territory has been previously perfused by the occluded artery, the cumulative survival rate was significantly higher in those who maintained patency than in those with reocclusion.
Antegrade approach
• Starting of antegrade approach is tapering guidwire which can insert into micro-channels in the chronic total occlusion (CTO) lesion. A second option is the wire escalation technique. The parallel wire technique and re-entry technique is the second strategy after failed wire escalation.
Retrograde approach
• Channel tracking and CTO recanalization from retrogradely is a key issue of the retrograde approach.
Conclusion
• CTO are most challenging lesion to treat by coronary angioplasty. The latest advanced techniques and strategies of percutaneous coronary intervention for CTO are described, regarding antegrade and retrograde approaches.
References
Papers of special note have been highlighted as:
• of interest
- Brilakis E, Grantham JA, Rinfret S et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. J. Am. Coll. Cardiol. Interv. 5, 467–479 (2012).
- Grantham JA, Marso SP, Spertus J et al. Chronic total occlusion angioplasty in the United States. J. Am. Coll. Cardiol. Interv. 2, 479–486 (2009).
- Brott BC. The safety and outcomes of chronic total occlusion interventions. J. Am. Coll. Cardiol. Interv. 6, 128–129 (2013).
- Brilakis ES, Kampalotis DK, Werner GS et al. Developments in coronary chronic total occlusion percutaneous coronary interventions; 2014 state-of-the-art update. J. Invas. Cardiol. 26, 261–266 (2014).
- Hsu JT, Tamai H, Kyo E et al. Traditional antegrade approach versus combined antegrade and retrograde approach in the percutaneous treatment of coronary chronic total occlusions. Catheter Cardiovasc. Interv. 74, 555–563 (2009).
- Mehran R, Classen BE, Godino C et al. long-term outcome of percutaneous coronary intervention for chronic total occlusions. JACC Interv. 4, 952–961 (2011).
- Stone GW, Kandzari DE, Mehran R et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part I. Circulation 112, 2364–2372 (2005).
- Stone GW, Reifart NJ, Moussa I et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part II. Circulation 112, 2530–2537 (2005).
- Thompson CA, Jayne JE, Robb JF et al. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions and early US experience. J. Am. Coll. Cardiol. Interv. 2, 834–842 (2009).
- Saito S. Different strategies of retrograde approach in coronary angioplasty for chronic total occlusion. Catheter Cardiovasc. Interv. 71, 8–19 (2008).
- Patel V, Brayton K, Tamayo A et al. Incidence of angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions. A weighted meta-analysis of 18061 patients from 65 studies. J. Am. Coll. Cardiol. Interv. 6, 128–136 (2013).
- Morino Y, Abe M, Morimoto T et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry Japan) score as a difficulty grading and time assessment tool. J. Am. Coll. Cardiol. Interv. 4, 213–221 (2011).
- Park Y, Park HS, Jang GL et al. Intravascular ultrasound-guided recanalization of stumpless chronic total occlusion. Int. J. Cardiol. 148, 174–178 (2011).
- Ito S, Suzuki T, Ito T et al. Novel technique using intravascular ultrasound-guided guide wire cross in coronary intervention for uncrossable chronic total occlusions. Circ. J. 68, 1088–1092 (2004).
- Takimura H, Muramatsu T, Tsukahara R. CT coronary angiography-guided percutaneous coronary intervention for chronic total occlusion combined with retrograde approach. >J. Invas. Cardiol. 24, E5–E9 (2012).
- Rolt A, Werner GS, Schunback A et al. Percutaneous coronary CT angiography significantly improves success rate of PCI for chronic total occlusion. Int. J. Cardiovasc. Imaging 29, 1819–1827 (2013).
- Muramatsu T, Hirano K, Tsukahara R et al. Long-term outcome of percutaneous transluminal coronary intervention for chronic total occlusion in BMS era in Japan. Cardiovasc. Interv. Ther. 25, 78–83 (2010).
- Joyal D, Afilalo J, Rinfret S. Effectiveness of revascularization of chronic total occlusions; a systematic review and meta-analysis. Am. Heart J. 160, 179–187 (2010).
- Suero JA, Marso SP, Jones PG et al. Procedural success and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries; a 20-year experience. J. Am. Coll. Cardiol. 38, 409–414 (2001).
- Olivari Z, Rubartelli P, Piscione F et al. Immediate results and one-year clinical outcome after percutaneous coronary intervention in chronic total occlusions: data from a multicenter, prospective, observational study (TOAST-GISE). J. Am. Coll. Cardiol. 41, 1672–1678 (2003).
- Hoye A, van Domburg RT, Sonnenschein K et al. Percutaneous coronary intervention for chronic total occlusions. The Thorax Center Experience 1992–2002. Eur. Heart J. 26, 2630–2636 (2005).
- Aziz S, Stables RH, Grayson AD et al. Percutaneous coronary intervention for chronic total occlusions; improved survival for patients with successful revascularization compared with a failed procedure. Catheter Cardiovasc. Interv. 70, 15–20 (2007).
- Valenti R, Migliorini A, Signorini U et al. Impact of complete revascularization with percutaneous coronary intervention on survival in patients with at least one chronic total occlusion. Eur. Heart J. 29, 1336–1342 (2008).
- Kirschbaum SW, Baks T, van den Ent M et al. Evaluation of left ventricular function three years after percutaneous recanalization of chronic total coronary occlusions. Am. J. Cardiol. 101, 179–185 (2008).
- Chung CM, Nakamura S, Tanaka K et al. Effect of recanalization of chronic total occlusions on global and regional left ventricular function in patients with or without previous myocardial infarction. Catheter Cardiovasc. Interv. 60, 368–374 (2003).
- Werner GS, Surber R, Ferrari M et al. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur. Heart J. 27, 2406–2412 (2006).
- Shigh M, Bell MR, Berger PR et al. Utility of bilateral coronary injections during complex coronary angioplasty. Invas. Cardiol. 11, 70–74 (1999).
- Rinfret N, Joyal D, Nguyen CM et al. Retrograde recanalization of chronic total occlusions from the transradial approach; early Canadian experience. Catheter Cardiovasc. Interv. 78, 366–374 (2011).
- Sasakura K, Nakano M, Otsuka F et al. Comparison of pathology of chronic total occlusion with and without coronary artery bypass graft. Eur. Heart J. 35(25), 1683–1693 (2013).
- Tsuchikane E, Katoh O, Kimura M et al. The first clinical experience with a novel catheter for collateral channel tracking in retrograde approach for chronic total occlusions.Am. Coll. Cardiol. Interv. 3, 165–171 (2010).
- Ochiai M, Ashida K, Araki H et al. The latest wire technique for chronic total occlusion. Ital. Heart J. 6, 489–493 (2005).
- Mitsudo K, Yamashita T, Asakura Y et al. Recanalization strategy for chronic total occlusions with tapered and stiff-tip guidewire. The results of CTO new techniQUE for Standard Procedure (CONQUEST) Trial. J. Invas. Cardiol. 20, 571–577 (2008).
- Colombo A, Mikhail GW, Michev I et al. Treating chronic total occlusions using subintimal tracking and reentry: the STAR technique. Catheter Cardiovasc. Interv. 64, 407–411 (2005).
- Micheal TT, Papayannis AC, Banerjee S et al. Subintimal dissection /reentry strategies in coronary chronic total occlusion interventions. Circ. Cardiovasc. Interv. 5, 729–738 (2012).
- Godino C, Viani GM, Spagnoio P et al. Management of large coronary dissection after STAR. Cardiovasc. Revasc. Med. 15, 58–60 (2014).
- Werner GS. The BridgePoint devices to facilitate recanalization of chronic total occlusions through controlled subintimal reentry. Expert Rev. Med. Dev. 8, 23–29 (2011).
- Wilson W, Walsh S, Hanraty C et al. A novel approach to the management of occlusive in-stent restenosis (ISR). Euro. Interv. 9, 1285–1293 (2014).
- Mogabgab O, Patel VG, Micheal TT et al. Long-term outcomes with use of the CrossBoss and Stingray coronary CTO crossing and reentry devices. J. Invas. Cardiol. 25, 579–585 (2013).
- Surmely JF, Tsuchikane E, Katoh O et al. New concept for CTO recanalization using controlled antegrade and retrograde subintimal tracking: the CART technique.Invas. Cardiol. 18, 334–338 (2006).
- Surmely JF, Katoh O, Tsuchikane E et al. Coronary septal collaterals as an access for the retrograde approach in the percutaneous treatment of coronary chronic total occlusions. Catheter Cardiovasc. Interv. 69, 826–832 (2007).
- Muramatsu T, Tsukahara R, Ito Y et al. Changing strategies of the retrograde approach for chronic total occlusion during past the 7 years. Catheter Cardiovasc. Interv. 81, E178–E185 (2013).
- Michael TT, Kampaliotis D, Brilakis ES et al. Procedural outcomes of revascularization of chronic total occlusion of native coronary arteries (from a multicenter United States registry). Am. J. Cardiol. 112, 488–492 (2013).
- Sianos G, Barlis P, Mario Di et al. European experience with the retrograde approach for the recanalization of coronary artery chronic total occlusions. A report on behalf of the Euro CTO Club. Euro. Interv. 4, 84–92 (2008).
- Tsuchikane E, Yamane M, Mutoh M et al. Japanese multicenter registry evaluating the retrograde approach for chronic total occlusion. Catheter Cardiovasc. Interv. 82, E654–E661 (2013).
- Yamane M, Muto M, Matsubara T et al. Contemporary retrograde approach for the recanalization of coronary chronic total occlusion: on behalf of the Japanese Retrograde Summit Group. Euro. Interv. 102–109 (2013).
- Kimura M, Katoh O, Tsuchikane E et al. The efficacy of a bilateral approach for treating lesions with chronic total occlusions. J. Am. Coll. Cardiol. Interv. 2, 1135–1141 (2009).
- Galassi AR, Tomasello SD, Reifart N et al. In-hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: insights from the EUROCTO registry. Euro. Interv. 7, 472–479 (2011).
- Karmpaliotis D, Michael TT, Brilakis ES et al. Retrograde coronary chronic total occlusion revascularization. J. Am. Coll. Cardiol. Interv. 5, 1273–1279 (2012).
- Michael TT, Mogabgab O, Fuh E et al. Application of the ‘Hybrid Approach’ to chronic total occlusion interventions; a detailed procedural analysis. J. Interv. Cardiol. 27, 36–43 (2014).
- Muramatsu T, Tsuchikane E, Oikawa Y et al. Long term clinical outcome of intimal versus subintimal tracking in successful recanalization of chronic total occlusion-J-PRCTOR REGISTRY. Euro. Interv. 10, 681–688 (2014).
- Werner GS, Ferrati M, Heinke S et al. Angiographic assessment of collateral connections in comparison with invasively determined collateral function in chronic total occlusions. Circulation 107, 1972–1977 (2003).
- Joyal D, Thompson CA, Grantham JA et al. The retrograde technique for recanalization of chronic total occlusions.J. Am. Coll. Cardiol. Interv. 5(1), 1–11 (2012).
- Sumitsuji S, Inoue K, Ochiai M et al. Fundamental wire technique and current standard strategy of percutaneous intervention for chronic total occlusion with histopathological insights. J. Am. Coll. Cardiol. Interv. 4, 941–951 (2011).
- Matsumi J, Saito S. Progress in the retrograde approach for chronic total coronary artery occlusion. Catheter Cardiovasc. Interv. 71, 810–814 (2008).
- Rathore S, Katoh O, Tsuchikane E et al. A novel modification of the retrograde approach for the recanalization of chronic total occlusion of the coronary arteries: intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking. J. Am. Coll. Cardiol. Interv. 2, 155–164 (2010).
- Tsujita K, Maehara A, Mintz GS et al. Intravascular ultrasound comparison of the retrograde versus antegrade approach to percutaneous intervention for chronic total occlusions. J. Am. Coll. Interv. 2, 846–854 (2009).
- Wu EB, Chan WW, Yu CM. Antegrade transit of retrograde wire to bail out dissected left main during retrograde chronic total occlusion intervention. J. Invas. Cardiol. 21, e113–e118 (2009).
- Christ G, Glogar D. Successful recanalization of a chronic occluded left anterior descending coronary artery with a modification of the retrograde proximal true lumen puncture technique. Catheter Cardiovasc. Interv. 73, 272–275 (2009).
- Mozid AM, Davies JR, Spratt JC. The utility of a guideliner catheter in retrograde percutaneous coronary intervention of a chronic total occlusion with reverse cart-the ‘capture’ technique. Catheter Cardiovasc. Interv. 83, 929–932 (2014).
- Brilakis ES, Badhey N, Banerjee S. ‘Bilateral knuckle’ technique and Stingray re-entry system for retrograde chronic total occlusion intervention. J. Invasive Cardiol. 23, E37–E39 (2011).
- Lo N, Micheal TT, Moin D et al. Periprocedural myocardial injury in chronic total occlusion percutaneous interventions: a systematic cardiac biomarker evaluation study. J. Am. Coll. Cardiol. Interv. 7, 47–54 (2014).
• This manuscript report interest of algorithm for crossing strategy.
• This manuscript reports the interest of chronic total occlusion (CTO) strategy up-date 2014.
• This manuscript reports interest of J-CTO score for antegrade approach for CTO.
• This manuscript reports the interest of first case of controlled antegrade and retrograde tracking technique.
• This manuscript reports the interest of latest data of Japan retrograde summit registry.
• This manuscript reports the interest of histopthological aspect and CTO strategy.