Review Article - Interventional Cardiology (2021)
Technical description, deployment technique and potential applications of the Occlutech atrial flow regulator device
- Corresponding Author:
- Gareth Morgan
University of Colorado School of Medicine,
Anschutz Medical Campus,
Aurora,
Colorado,
USA,
Email: drgarethjmorgan@gmail.com
Received date: December 18, 2020 Accepted date: January 08, 2021 Published date: January 15, 2021
Abstract
The creation of an atrial communication with long term patency is a desirable scenario in a number of disease phenotypes affecting children and adults with both congenital and acquired cardiovascular diseases. Patients suffering from these conditions represent a small proportion of the population but bear a significant symptom and survival burden due to a lack of treatments and innovations to ameliorate their conditions.
The Occlutech™ Atrial Flow Regulator (AFR) was originally designed to treat significant systolic and diastolic left ventricular dysfunction in adults. The device has material and design properties that can potentially benefit those with congenital heart disease, left atrial hypertension as well as those with pulmonary vascular disease. The device can be implanted percutaneously via femoral venous approach using trans-esophageal echocardiographic guidance. The implantation is technically similar to the device closure of an Atrial Septal Defect (ASD) and will be familiar to those who implant ASD occlusion devices frequently.
Here we describe the potential applications, technical considerations and expanded implementation of the AFR device for children and adults with congenital heart disease and pulmonary hypertension.
Keywords
Heart defect; Congenital cardiac catheterisation; Pulmonary hypertension; Single ventricle palliation; Heart failure
Introduction
As well as the rapidly expanding morbidity and economic burden facing healthcare systems due to diastolic heart failure, there is an ever increasing population of patients with Congenital Heart Disease (CHD) who are surviving beyond their initial palliative and curative procedures to more chronic but still critical states of pathophysiology. This is mainly due to advances in treatment options for what were previously regarded as life limiting conditions [1]. Whilst these advances represent progress and success with respect to survival, it also presents clinicians with a new population of more severely symptomatic and complex patients who will require yet further advances and innovations in practice to improve their quality of life and survival.
This review focuses on a complex group of patients who may benefit from an atrial level shunt to offload a pathological right or left heart circuit whilst providing increased volume to the opposing circuit to potentially improve cardiac output. These include patients with heart failure with both preserved and reduced ejection fraction (HFpEF, HFrEF), patients with idiopathic pulmonary arterial hypertension and patients with complex congenital heart disease with associated pulmonary hypertension.
Overview
Current strategies for the treatment of the above conditions include conventional heart failure medications for patients with left sided heart disease, and pulmonary antihypertensive medications for those with pulmonary arterial hypertension [2,3]. Some patients may be considered for heart and/ or lung transplantation, though organ supplies are limited and organ transplantation does not represent a disease free endpoint. In order to maximize the chances of benefiting from a potential transplant, those under consideration should be sustained in an optimized physiological state using medical or other supportive therapies.
The benefits of LA decompression in LH diastolic dysfunction are patho-physiologically comprehensible, applicable to a widespread portion of the cardiovascular population and hence tangible from and industry and commercial view point. Conversely, congenital heart disease and pulmonary vascular disorders represent a small subset of disease within the general population, provoking limited interest from biomedical firms. Congenital and pediatric cardiologists therefore must borrow and manipulate already existent innovations and use them off label or in unusual indications to provide potential treatment and palliative strategies to patients whereby the ultimate goal is symptom reduction, survival and/or bridge to transplantation.
The creation of an atrial level shunt may be favorable in some of the above patient groups, providing symptomatic relief and potential survival benefit. Current practice is limited to surgical aorto-pulmonary [4] or atrial shunt creation [5], which is felt to be high risk in those with severe hemodynamic compromise as well as some catheter based interventions which may be better tolerated than surgery due to the less invasive approach. Current catheter based interventions include: atrial septostomy, static balloon atrial septal dilation and atrial septal stenting to achieve and maintain an interatrial communication. Whilst these procedures are technically feasible, their long term success is limited by early shunt occlusion and consequent clinical deterioration which is often poorly tolerated. Reintervention rates are high [5–8].
Left Heart Diastolic Dysfunction
The Atrial Flow Regulator (AFR) by Occlutech® was designed primarily to treat symptomatic heart failure in adult patients with and without preserved systolic Left Ventricular (LV) function [9,10]. The device is designed to maintain a long-term interatrial communication, to offload increases in left atrial pressure both during exercise and at rest. Indications for the device in this subgroup include established Heart Failure with reduced (HFrEF) or preserved (HFpEF) ejection fraction with a Pulmonary Arterial Wedge Pressure (PAWP) of 15 mmHg+ at rest or 25 mmHg+ on exertion, Right Atrial (RA) pressure of less than 20 mmHg and life expectancy of >1 year [11]. An extensive overview of patient selection, procedural considerations and relevant hemodynamics reported by the Hamburg group provides some preliminary sizing guidelines for adult patients with left heart indications for implantation [12]. The authors recommend a device height of 5 mm for patients with a septal thickness of <5 mm and a device height of 10 mm for patients with septal thicknesses of 6-10 mm. They suggest the implantation of an 8 mm diameter device in patients with a PAWP of 15 mmHg or a 10 mm diameter device in those with resting PAWP of <15 mmHg that increases to >25 mmHg on exercise. The hemodynamic and anatomical considerations as well as variability in patient size and indication for treatment in our patient group mean patient specific sizing considerations are required to ensure the device can perform to its hemodynamic intentions.
Pulmonary Arterial Hypertension
Patients with Pulmonary Arterial Hypertension (PAH) are at risk of developing Right Ventricular (RV) dysfunction, syncope and sudden death as their disease progresses [13]. Providing an interatrial shunt to these patients facilitates volume and pressure unloading of the right heart and maintenance of LV preload and left heart cardiac output at the expense of desaturation. This may provide symptomatic improvement, improve survival and provide a bridge to transplantation in patients with irreversible, treatment resistant pulmonary vascular disease. The AFR device could provide a stable long term potential shunt for these patients who have a high rate of septostomy failure in the short to medium term, often requiring repeated re-intervention to maintain shunt patency [14]. At this time, published cases in the literature report some success with AFR in PAH, both idiopathic and in association with congenital heart disease [15,16].
Fontan Failure in those with Single Ventricle Physiology
In a pathophysiological process which has many parallels with PAH, patients with a Fontan type single ventricle palliation who often have a relatively increased pulmonary vascular resistance may benefit from a communication between the Fontan conduit and the pulmonary atrial mass, effectively offloading pressure from the pulmonary arterial circuit, at the expense of systemic arterial desaturation. This can increase the systemic ventricular preload facilitating improved cardiac output and diminishing the pathophysiological complications of systemic venous (hence pulmonary arterial) hypertension such as protein losing enteropathy and plastic bronchitis. The AFR may be considered to be a suitable device to achieve this controlled hemodynamic alteration in such patients and there are some reports in the literature which detail these individual successes [14,17,18].
The Device
The Occlutech AFR device is a self-expanding double disc nitinol wire mesh device with a central fenestration (Figure 1) that is percutaneously implanted via antegrade femoral venous approach. Fenestration diameters measure 4 to 10 mm in 2 mm increments and the length of the central fenestration (known as the device height) is 2, 5 or 10 mm. The device is repositionable and retrievable. It can be placed and evaluated with the aid of transesophageal (Figure 2) or intracardiac echocardiographic guidance. Implanters familiar with the technical aspects of Atrial Septal Defect (ASD) occlusion will likely find the implantation of this device very similar.
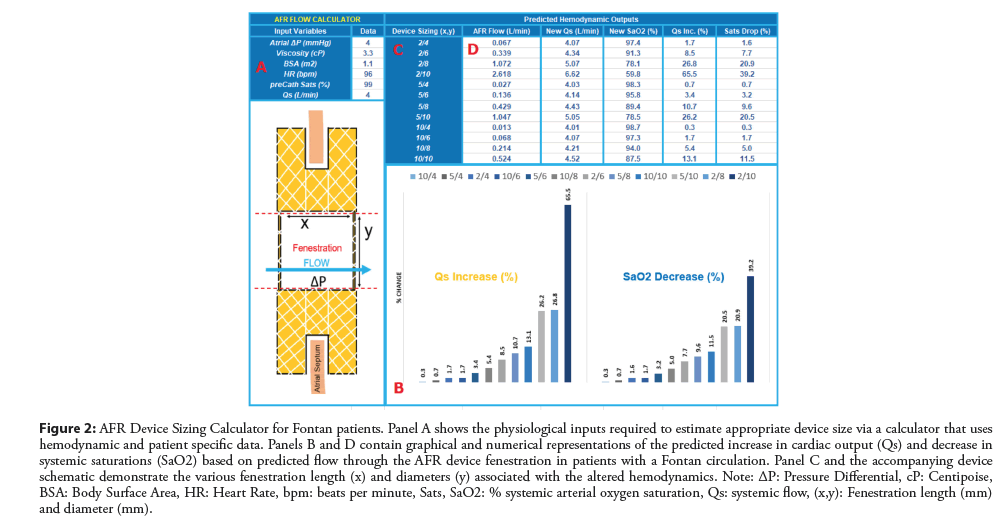
Figure 2: AFR Device Sizing Calculator for Fontan patients. Panel A shows the physiological inputs required to estimate appropriate device size via a calculator that uses hemodynamic and patient specific data. Panels B and D contain graphical and numerical representations of the predicted increase in cardiac output (Qs) and decrease in systemic saturations (SaO2) based on predicted flow through the AFR device fenestration in patients with a Fontan circulation. Panel C and the accompanying device schematic demonstrate the various fenestration length (x) and diameters (y) associated with the altered hemodynamics. Note: ΔP: Pressure Differential, cP: Centipoise, BSA: Body Surface Area, HR: Heart Rate, bpm: beats per minute, Sats, SaO2: % systemic arterial oxygen saturation, Qs: systemic flow, (x,y): Fenestration length (mm) and diameter (mm).
Current sizing recommendations are based on adult patients with HRpEF or HRrEF. For patients with congenital heart disease; particularly those in whom a physiological R-L shunt is required, we have devised a calculator (Figure 2) to assist with decision making around device sizing based on both patient size, anatomical landscape and hemodynamic profile. The calculator and formulas are based on Poiseuille’s Law governing blood flow and account for device size, height, pressure differentials and a variety of other relevant variables that play into the flow parameters across the device.
Standard Procedural Technique
For those patients who require an atrial septal or cavoatrial (in Fontan patients) puncture; this should be performed as per standard practice via a femoral venous approach. After confirming access to the left atrium, heparinize the patient and proceed. Place a stiff 0.035 guide wire in the Left Upper Pulmonary Vein (LUPV) using a Multipurpose (MP) or similar catheter. Using Trans Esophageal (TEE) guidance, perform static balloon dilation of the atrial septum using a non-compliant balloon in order to expand the atrial septostomy to accommodate the waist diameter of the chosen AFR. Select a device based on consideration of patient hemodynamics and using a calculator or table similar to Figure 2. Ensure that the atrial septal thickness (measured on TEE) (see Figure 3) can accommodate the selected device height. From this point, as shown in Figure 4, the steps to deployment are very similar to ASD device closure. After documenting the hemodynamic changes and resultant status of the patient including the arterial saturations, the device can be released. If the fenestration appears constrained or compressed below its nominal diameter, balloon dilation can be attempted.
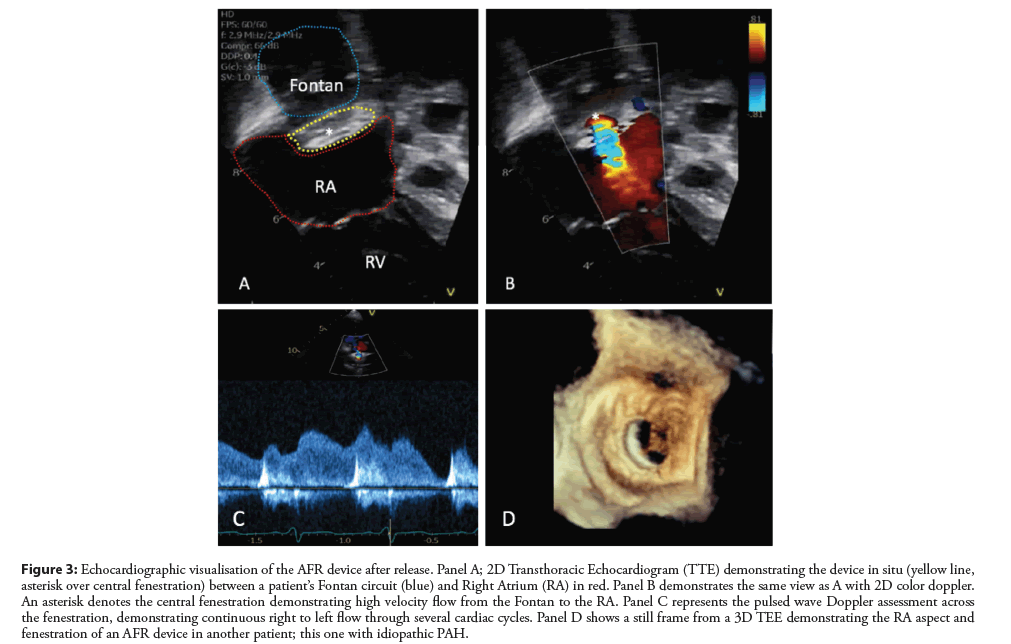
Figure 3: Echocardiographic visualisation of the AFR device after release. Panel A; 2D Transthoracic Echocardiogram (TTE) demonstrating the device in situ (yellow line, asterisk over central fenestration) between a patient’s Fontan circuit (blue) and Right Atrium (RA) in red. Panel B demonstrates the same view as A with 2D color doppler. An asterisk denotes the central fenestration demonstrating high velocity flow from the Fontan to the RA. Panel C represents the pulsed wave Doppler assessment across the fenestration, demonstrating continuous right to left flow through several cardiac cycles. Panel D shows a still frame from a 3D TEE demonstrating the RA aspect and fenestration of an AFR device in another patient; this one with idiopathic PAH.
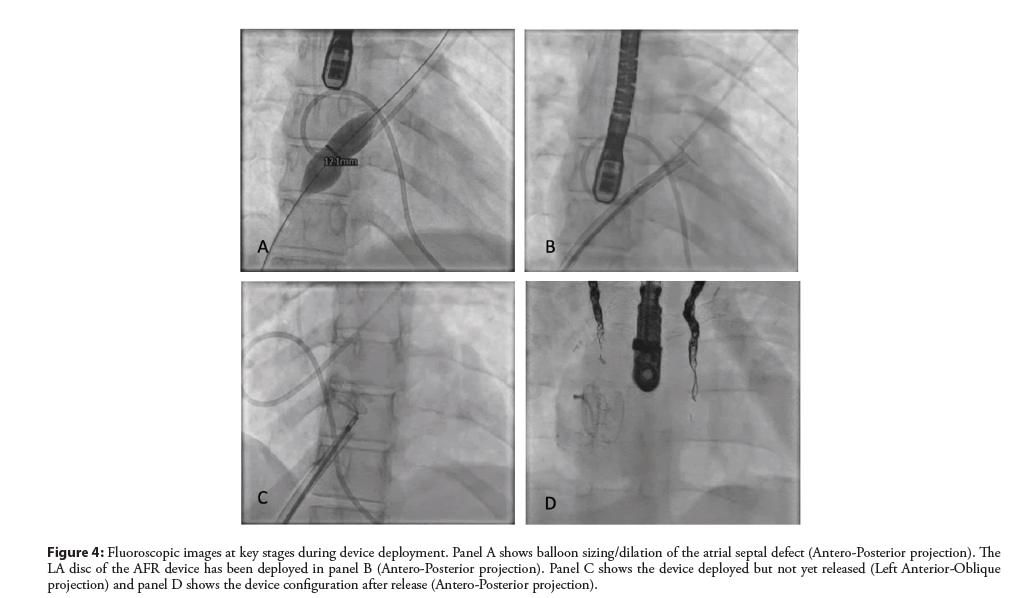
Figure 4: Fluoroscopic images at key stages during device deployment. Panel A shows balloon sizing/dilation of the atrial septal defect (Antero-Posterior projection). The LA disc of the AFR device has been deployed in panel B (Antero-Posterior projection). Panel C shows the device deployed but not yet released (Left Anterior-Oblique projection) and panel D shows the device configuration after release (Antero-Posterior projection).
Current Status and Future Prospects
We have recently published an animal study which details the technical details and potential clinical applications of the Occlutech AFR device in human patients with a variety of indications [19]. We have proposed the potential safety of its use by evaluating for both intracardiac and extra cardiac complications of implantation both clinically and on histological/pathological animal evaluation post mortem. There appears to be an acceptable endothelialization profile to the device without any evidence of exaggerated or excessive thrombosis within the chambers of the heart and its related vessels. We saw acceptable patency rates of the fenestration in this study despite the absence of a significant atrial pressure differential. We anticipate similar if not improved patency rates in human subjects where there is a significant atrial pressure differential, particularly during activity; promoting flow across the fenestration. The preliminary practice with perioperative antiplatelet and anticoagulation management mirrors the current practice used in device implantation of devices similar to this like ASD occluders and also accommodates for the additional coagulation status of the patient based on their underlying disease and risk profile.
This animal study has since led to the compassionate use of this device in children and adults with congenital heart and/or pulmonary vascular disease here in the United States (US) whereby the authors in this editorial have assisted operators at 5 different centers in the US to implant this device in 15 patients for a variety of indications. We are currently in the process of reviewing intermediate data on these compassionate use cases to improve our knowledge of important hemodynamic and echocardiographic markers to assist with decision making and resolve success. To date all patients who have undergone the rigorous process of compassionate use applications with the Food & Drug Administration (FDA) have gone on to have technically successful implantation with acceptable parameters of device function on short to medium term follow up. Establishing FDA approval is challenging. This device is currently classified as a ‘Humanitarian Use Device’ (HUD) in the US. This is due to the fact that the diseases it is currently being used to treat affect less than 8000 individuals per year in the US. Therefore each practitioner must go through a rigorous ‘Humanitarian Device Exemption’ (HDE) protocol on every occasion where implantation is indicated. We hope that data from these cases will facilitate FDA approval and allow an expanded use profile for the AFR device. This should be assisted by the establishment of a data registry of patients who have undergone implantation and the continued publication of experience with the AFR device.
Conclusion
Use of the Occlutech AFR device in children and adults with complex heart disease may produce beneficial results. Widespread uptake of this technique and treatment at specialist centres is very likely once the device is approved beyond compassionate use exemptions. The AFR has the potential to provide symptomatic and survival benefit to a variety of patients with limited treatment options and indeterminate prognosis.
References
- Best KE, Rankin J. Long‐Term Survival of Individuals Born With Congenital Heart Disease: A Systematic Review and Meta‐Analysis. J Am Heart Assoc. 5(6): 1-15 (2016).
- Abman SH, Hansmann G, Archer SL. Pediatric pulmonary hypertension: Guidelines from the American heart association and American thoracic society. Circulation. 132(21): 2037‐2099 (2015).
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 134(13): e282–93 (2016).
- Baruteau AE, Serraf A, Lévy M. Potts shunt in children with idiopathic pulmonary arterial hypertension: long-term results. Ann Thorac Surg. 94(3): 817–824 (2012).
- Bauer A, Khalil M, Schmidt D, et al. Creation of a restrictive atrial communication in pulmonary arterial hypertension (PAH): effective palliation of syncope and end-stage heart failure. Pulm Circ. 8(2) (2018).
- Kerstein D, Levy PS, Hsu DT, et al. Blade balloon atrial septostomy in patients with severe primary pulmonary hypertension. Circulation. 91(7): 2028–2035 (1995).
- Kuhn BT, Javed U, Armstrong EJ. Balloon dilation atrial septostomy for advanced pulmonary hypertension in patients on prostanoid therapy. Catheter Cardiovasc Interv. 85(6): 1066–1072 (2015).
- Sandoval J, Gaspar J, Pulido T. Graded balloon dilation atrial septostomy in severe primary pulmonary hypertension. A therapeutic alternative for patients nonresponsive to vasodilator treatment. J Am Coll Cardiol. 32(2): 297–304 (1998).
- Guimarães L, Del Val D, Rodés-Cabau J. The Atrial Flow Regulator device: Expanding the field of interatrial shunting for treating heart failure patients. EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 15(5): 398–400 (2019).
- Paitazoglou C, Özdemir R, Pfister R, et al. The AFR-PRELIEVE trial: A prospective, non-randomised, pilot study to assess the Atrial Flow Regulator (AFR) in heart failure patients with either preserved or reduced ejection fraction. EuroIntervention. 15(5): 403-410 (2019).
- Gupta A, Bailey SR. Update on devices for diastolic dysfunction: Options for a no option condition? Curr Cardiol Rep. 20(10): 85 (2018).
- Paitazoglou C, Bergmann MW. The atrial flow regulator: Current overview on technique and first experience. Ther Adv Cardiovasc Dis. (2020)
- Frank DB, Hanna BD. Pulmonary arterial hypertension associated with congenital heart disease and eisenmenger syndrome: Current practice in pediatrics. Minerva Pediatr. 67(2): 169–85 (2015).
- Lehner A, Schulze-Neick I, Fischer M, et al. The Creation of an interatrial right-To-Left shunt in patients with severe, irreversible pulmonary hypertension: Rationale, devices, outcomes. Curr Cardiol Rep. 21(5) (2019).
- Patel MB, Samuel BP, Girgis RE, et al. Implantable atrial flow regulator for severe, irreversible pulmonary arterial hypertension. EuroIntervention. 11(6): 706–9 (2015).
- Dąbrowska-Kugacka A, Ciećwierz D, Żuk G, et al. Atrial flow regulator for severe drug resistant pulmonary arterial hypertension after congenital heart defect correction. Cardiol J. 26(1): 102–4 (2019).
- Manuri L, Calaciura RE, De Zorzi A, et al. Atrial flow regulator for failing Fontan circulation: An initial European experience. Interact Cardiovasc Thorac Surg. 27(5): 761–4 (2018).
- Zablah JE, Morgan GJ. Use of Occlutech® atrial flow regulator in a single ventricle patient: A 3D view of a successful intervention. Eur Heart J Cardiovasc Imaging. 21(10): 1151–1151 (2020).
- McLennan D, Ivy D, Morgan GJ. Transvenous implantation of the Occlutech Atrial Flow Regulator: Preliminary results from swine models. Congenit Heart Dis. 14(5): 819–31 (2019).