Review Article - Interventional Cardiology (2012) Volume 4, Issue 4
Techniques for the diagnosis of vertebral artery origin stenosis and considerations for determining treatment and timing
- Corresponding Author:
- Guilherme Dabus
Division of Neurointerventional Surgery
Baptist Cardiac and Vascular Institute and Baptist Neuroscience Center
8900 N. Kendall Drive, Miami, FL, USA
Tel: +1 786 596 5990
Fax: +1 786 596 2999
E-mail: gdabus@gmail.com
Abstract
Keywords
balloon-mounted stent, percutaneous transluminal angioplasty, posterior circulation, stenosis, stent, stroke, transient ischemic attack, vertebral artery
Patients with cerebrovascular disease have a 20–40% incidence of atherosclerotic lesions of the vertebral artery origin [1,2]. The diagnosis of vertebral artery origin stenosis (VAOS) is facilitated by the increased use of magnetic resonance (MR) and computed tomography angiography (CTA) following a transient ischemic attack (TIA) or stroke. Patients with symptomatic VAOS have an increased risk of stroke [3] but the risk of stroke in asymptomatic VAOS patients is unknown.
Anatomy
The vertebral artery (VA) is usually the first and largest branch of the subclavian artery, originating from its superior-posterior wall. However, in 2–5% of cases, the left VA arises directly from the aortic arch between the left common carotid artery and the left subclavian artery, rather than from the subclavian artery. Beyond its origin, the VA runs behind the anterior scalenus muscle, entering the foramina transversarium of C6 in 88% of people, C5 or C7 in 6% of people, and C4 in 4% of people [4].
The VA is divided into four segments. The first segment (V1) extends from the subclavian artery to the entrance into the foramina transversarium, and the second segment (V2) extends from its entrance into the foramina transversarium to C2, and ascends almost straight, passing through the foramina transversarium of each vertebra. As patients age, this segment can become tortuous and can be impinged on by bony spurs arising from the cervical spine. The third segment (V3) extends from C2 to the point at which the VA pierces the dura mater. Within this segment, the VA leaves the foramina transversarium of C2, forming a lateral convex curve, and reaches the foramen of C1. The fourth segment (V4), also known as the intradural segment, extends from the point at which the VA enters the dura mater to the vertebrobasilar junction [5]. Arterial supply to the cervical spinal cord comes predominantly from the V2 and V4 segments. Muscular branches originate from the second and third segments of the VA and supply the cervical muscles with rich anastomoses leading to the thyrocervical and costocervical trunks and the external carotid artery. Knowledge regarding these anastomoses is extremely important since they can provide important collateral flow to the distal VA in instances of severe proximal atherosclerotic disease [4].
Incidence and natural history
The overall prevalence of asymptomatic VAOS, as detected by duplex ultrasound, is approximately 7% in patients with clinically manifest atherosclerotic arterial disease [6,7]. The prevalence of VAOS as determined in routine catheter angiography is low (5.4%), increases with patient age and correlates with factors such as anterior circulation infarct (18.4%), posterior circulation infarct (33.3%), carotid atherosclerosis (30.8%) and vertebrobasilar insufficiency (33%) [8,9]. Moreover, the New England Posterior Circulation Stroke Registry suggest that 9–20% of posterior circulation strokes are caused by VAOS [2].
A recent analysis of the Second Manifestation of Arterial disease (SMART) study reported a higher incidence of posterior circulation stroke (0.4% annual stroke rate) in patients with asymptomatic VAOS (>50%) than in patients without stenosis [6].
There is scarce data regarding the natural history of VAOS. The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) included 16 patients with VA ostial stenosis, eight of which were assigned to medical treatment. After more than 4 years of follow-up, two patients in the medical treatment arm had transient ischemic attacks and none had a stroke [10].
Pathophysiology
VAOS is mainly related to two stroke mechanisms: artery–artery thromboembolism and flow-related hemodynamic hypoperfusion. The New England Posterior Circulation Registry attributed 49% of VAOS strokes to artery– artery embolism and only 16% was thought to be ‘hemodynamic’ in origin [2]. Moreover, the most common occlusive sites in this registry were extracranial VAs (52 of 407 patients, 15 bilateral), intracranial VAs (40 patients, 12 bilateral) and basilar arteries (BAs; 46 patients) [1].
In the New England Posterior Circulation Registry, 59% patients had strokes without TIAs, 24% had TIAs followed by strokes and 16% had only TIAs [1]. Infarcts most often included the distal posterior circulation territory (rostral brainstem, superior cerebellum and occipital lobes); the proximal (medulla and posterior inferior cerebellum) and middle (pons and anterior inferior cerebellum) territories were equally involved. Embolic mechanism, distal territory location and BA occlusive disease carried the poorest prognosis.
Embolism usually affects distal branches and may manifest with TIAs or strokes. An infarct of an embolic source is more likely to transform into a hemorrhagic infarct than an infarct with a hemodynamic cause [5]. Some investigators state that hemodynamic impairment is the most important mechanism of infarction when cardioembolic sources are excluded [11,12]. Furthermore, the plaque of VAOS is generally smooth, firm and nonulcerated [13], carrying a lower risk of embolism than carotid bifurcation plaques [14,15]. Hemodynamic symptoms usually result from VAOS when associated with contralateral VA absence, occlusion, stenosis or hypoplasia. Patients usually experience symptoms when the arterial blood pressure drops, become dehydrated or experience orthostatic events [5].
From their autopsy study, Fisher and colleagues concluded that VAOS are seldom symptomatic [16]. The benign course of these lesions, according to Fisher, is attributed to the capacity to develop collateral reconstitution of the VAOS – the usual presence of two viable arteries that join together intracranially, so that if one is compromised, the contralateral artery could compensate – and the slow development of luminal VAs compromised by atherosclerotic plaques allows the development of collateral flow [17].
Posterior circulation signs and symptoms suggestive of vertebrobasilar insufficiency include: dizziness, vertigo, impaired balance, dysarthria, syncope, diplopia, ataxia, nausea, vomiting, tinnitus, cortical blindness, nystagmus, motor and sensory deficits, dysphagia and decreased levels of consciousness [1,5,9]. Symptoms are more severe with contralateral VA occlusion [9]. A recent report of the New England Medical Center Posterior Circulation Registry reported dizziness (47%), unilateral limb weakness (41%), dysarthria (31%), headache (28%), and nausea or vomiting (27%) as the most frequent posterior circulation symptoms [18].
Diagnosis
The gold standard for the diagnosis of VAOS is catheter angiography. The subclavian arteries and intracranial vessels should be carefully imaged in order to determine the degree of VAOS, VA dominance and flow dynamics that may play a role in the development of symptoms. Moreover, catheter angiography may provide information about the plaque characteristics, such as ulceration, extent and degree of stenosis. Ultimately, an aortic arch angiogram may provide further information about the presence of intra and extracranial collaterals, as well reverse flow, as in the case of subclavian steal.
Duplex ultrasound has a high specificity (93–98%) but a relatively low sensitivity (70%) for the diagnosis of extracranial VA stenosis [19]. Developments in the protocols of imaging modalities, such as CTA and MR angiography, have improved the visualization and diagnosis of VAOS [4]. However, routine clinical time of flight magnetic resonance angiography (MRA) significantly overestimates VAOS, and CTA tends to underestimate the degree and prevalence of VAOS [8]. Contrasted MRA may be more accurate in determining the degree of VAOS when compared with time of flight MRA.
Treatment modalities
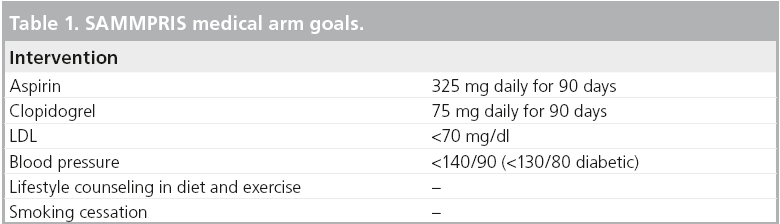
There is no consensus on the optimal therapeutic approach for symptomatic VAOS. Medical management should maximize secondary stroke prevention. The Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) study is a good example of the effectiveness of medical management in the treatment of progressive atherosclerotic lesions [20]. SAMMPRIS achieved a 30-day incidence of stroke and death of 5.8% in the medical arm, which is superior to a previous intracranial atherosclerosis trial that reported a 10.7% incidence of stroke and death [21]. The medical treatment in the SAMMPRIS study had been set rigorous goals (Table 1) in the treatment of atherosclerosis and achieved superior results compared with the interventional arm, which involved angioplasty and stenting of intracranial atherosclerotic stenoses. Although SAMMPRIS is an intracranial atherosclerosis trial, the effectiveness of the medical arm protocol may be extrapolated to the treatment of extracranial atherosclerosis as well. SAMMPRIS also exemplifies the power of medical management in a disease with high morbility and mortality.
Surgical approaches for the treatment of VAOS include endarterectomy, vertebral anastomosis to the subclavian or common carotid arteries and bypass. Open surgical reconstruction of the vertebral artery in the treatment of VAOS carries high mortality (8.4%) and morbidity (13.3%) rates and is generally not recommended [22]. Moreover, surgical repair of the VAOS has a high incidence of cranial neuropathies and non-neurological complications [23]. These surgical procedures are often laborious and technically complex. Nowadays, with the advent of endovascular techniques, an open surgical approach is rarely used.
Percutaneous transluminal angioplasty (PTA) was the first endovascular alternative to open surgery. Common complications with angioplasty are dissection, occlusion, vasospasm and thromboembolism. The first report of a VAOS angioplasty was described by Motarjeme et al. in 1981 [24]. Later Higashida et al. published work on a series of 41 patients treated with angioplasty (34 with V1-segment stenosis) [25]. Three permanent complications (7.1%) were reported in this series, including two strokes and one vessel rupture. Overall, angioplasty is associated with an approximately 9% risk of transient neurological deficits and an up to 15% restenosis rate [15,25–27]. The ostial segment of the vertebral arteries, much like coronary and renal ostia, is characterized by a large amount of elastin and smooth muscle, both of which can produce high recoil forces after angioplasty and stenting [28,29]. PTA of VAOS is associated with approximately 9% of TIAs and has a restenosis rate of up to 15% [25,27].
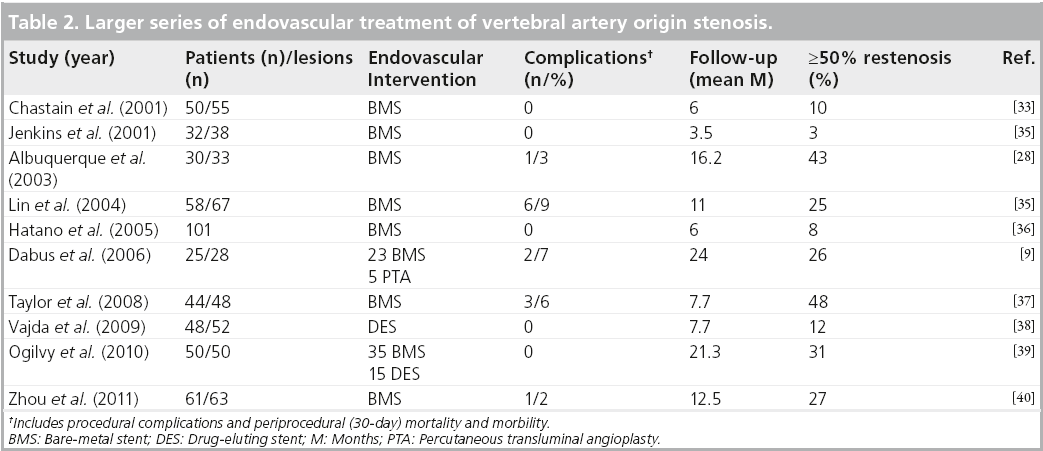
Endovascular treatment of the VAOS gained increasing popularity in the 1990s with the availability of stents. Storey et al. reported the first vertebral artery stenting in 1996 [30]; since then, multiple series have followed (Table 2). Stent placement is believed to decrease the incidence of elastic recoil and restenosis when compared with PTA [12,15]. In addition, it may protect against distal embolism by smoothing luminal irregularities, reconstructing the vessel anatomy and decreasing flow turbulence [15,26]. A large systematic review of the literature regarding the endovascular treatment of VAOS, including 1099 patients, determined that the percutaneous reconstruction of the proximal vertebral artery with stents is a safe and effective procedure, with low perioperative stroke and death rates. In this review, six (0.5%) patients sustained a periprocedural posterior circulation-related stroke, and another six died within 30 days of intervention [31]. Another review, which included 993 patients, found that 11 patients (1.1%) experienced a stroke and eight (0.8%) experienced a TIA within 30 days of the procedure [32]. These retrospective reviews suggest that the overall rate of periprocedural stroke and death after the endovascular treatment of VAOS is low.
In summary, there is no strong evidence to favor medical treatment, surgical reconstruction or endovascular treatment in the treatment of symptomatic VAOS. A randomized controlled trial evaluating PTA and stenting for symptomatic vertebral artery stenosis compared with medical therapy showed no benefit of endovascular treatment: no patient in either arm experienced a recurrent vertebrobasilar territory stroke up to 8 years after randomization [10]. However, these results are limited given the small sample size (eight patients in each arm). A larger trial assessing the best medical treatment with or without stenting for symptomatic vertebral artery stenosis is underway [41].
Treatment considerations
In our opinion, asymptomatic VAOS patients should be treated medically. However, if the patient is experiencing posterior circulation events despite maximized medical therapy, and no other sources of embolism have been identified, endovascular PTA with or without stenting should be considered. Symptomatic patients with bilateral VA disease who fail maximized medical therapy should be treated endovascularly, despite the limited evidence. The low complication rate of an endovascular procedure may justify an intervention when compared with the natural history of the disease.
In our practice, we treat symptomatic patients at the time of their presentation. In The New England Medical Center Posterior Circulation Stroke Registry, 24–59% of patients presented with TIAs before developing a stroke [1,42]. Posterior circulation strokes may also have an insidious course characterized by multiple TIAs that finally culminate in a stroke. The fact that at least a forth of posterior circulation strokes are preceded by TIAs underscores the need for urgent treatment in a disease that can have a recalcitrant course. As has been demonstrated in symptomatic carotid stenosis disease [43], most patients with VAOS may benefit from treatment within 2 weeks of symptom onset.
Restenosis
One caveat of PTA and stenting is the risk of restenosis, which can range from 0 to 48% [4,9,28,37]. The degree of restenosis was quantified in all studies by follow-up catheter angiograms, obtained between 7 and 24 months. Mechanisms involved in the development of restenosis include injury of the arterial wall during angioplasty and stent deployment and artificial straightening of the vessel [28,44]. Mechanical strain in an anatomic location with strong pulsatile movement and unfavorable angulation of the subclavian and VA may play a role in the development of restenosis [38]. Moreover, a tortuous origin of the VA, which arises in a sharp angle from the subclavian artery, may lead to straightening at the time of endovascular stenting and, later, restenosis [45]. Cigarette smoking, vessel tortuosity and small diameter have also been associated with a higher risk of restenosis [37,40].
Pre- and post-intervention antiplatelet management is crucial in preventing restenosis. Most series have described dual antiplatelet therapy for at least 3 months following the intervention [31]. Albuquerque et al. reported one of the highest restenosis (43%) rates of all the published series, probably because the investigators used dual antiplatelets for only 3 weeks [28].
The interventional cardiology experience in the use of sirolimus or paclitaxel-eluting stents (drug-eluting stents [DES]) has shown a significant reduction in the rate of restenosis by inhibiting neointimal hyperplasia [46]. The largest series of DES in the treatment of vertebral artery ostial stenosis reported a restenosis rate of 12% at a mean follow-up of 7 months [38]. This is one of the lowest rates of restenosis reported in the literature (Table 2). Moreover, a systematic review of the literature found that DES are associated with lower restenosis rates (11%) compared with bare-metal stents (30%) at a mean of 24 months follow-up [32].
Technical considerations of VAOS stenting
Patients are preloaded with dual antiplatelet therapy at least 24 h before the intervention: 300–600 mg of clopidogrel and 325 mg of aspirin. Most procedures are performed under conscious sedation. The procedure can be performed through different approaches: transfemoral, transbrachial and transradial access [47]. Anticoagulation is administered with intravenous heparin to achieve an activated clotting time that is two–three times the baseline value. We usually use balloon-expandable coronary stents because of their radial force, low crossing profile and limited foreshortening. DES may be used in diabetic patients and may decrease the rate of restenosis [4,38].
In our practice, we oversize the length of the stent by at least 3 mm at both ends of the stenotic arterial segment. The proximal end of the stent is usually deployed within the subclavian artery (1 mm below the origin of the VA) in order to cover the whole atherosclerotic plaque that usually originates at this level. Leaving the proximal end of the stent ‘hanging’ in the subclavian (by more than 2 mm) is not advised by other authors owing to the theoretical shearing force at the ostium of the VA, originated by pulsation [38].
Distal protection devices to prevent cerebrovascular events during wire/catheter manipulation and stent placement are rarely used. In one study, 12 consecutive patients undergoing PTA for subclavian and VAOS of atherosclerotic origin were studied using transcranial Doppler sonography monitoring before, during and after angioplasty for 30 min at a time [48]. No embolic signals were detected in any patient before angioplasty. During angioplasty, one embolic signal was detected immediately after balloon deflation in one of 12 patients. Several embolic signals were detected immediately after the procedure in six of 12 patients. These signals were detected despite the routine administration of antiplatelets and heparin. Qureshi et al. used distal protection devices in 12 patients and visualization of embolic debris occurred in eight devices [49]. In view of the low procedural risks of PTA and stenting demonstrated in the vast majority of case series published, we do not advocate the use of protection devices in the treatment of VAOS at this time.
Case examples
▪ Case 1
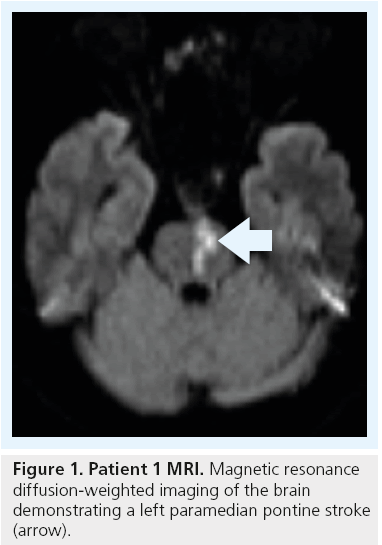
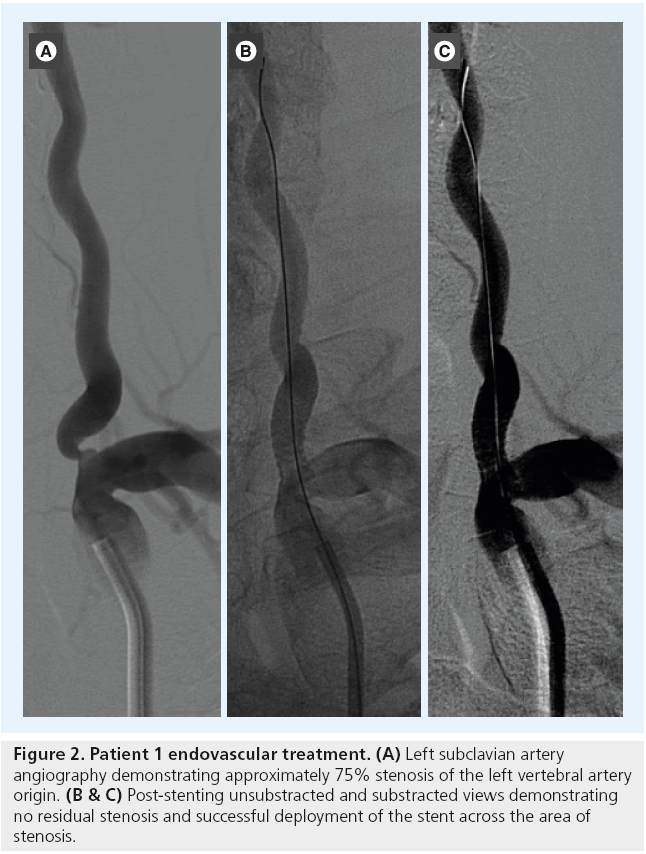
A 57-year-old man with hypertension, diabetes and hyperlipidemia presented with an acute onset of right hemiparesis. A brain MRI demonstrated a left paramedian pontine infarction (Figure 1). A diagnostic cerebral angiogram demonstrated the absence or occlusion of the right vertebral artery and an approximately 75% left VAOS (Figure 2A).
Figure 2: Patient 1 endovascular treatment. (A) Left subclavian artery angiography demonstrating approximately 75% stenosis of the left vertebral artery origin. (B & C) Post-stenting unsubstracted and substracted views demonstrating no residual stenosis and successful deployment of the stent across the area of stenosis.
This type of stroke is generally associated with occlusion of one of the BA perforators due to intrinsic small-vessel ischemic disease in the perforator or the presence of an atherosclerotic plaque in the BA. However, a thromboembolic event from the left VAOS could not be excluded. Since the left VA was dominant and enlarged, and the contralateral VA was not visualized, it was decided to proceed with endovascular PTA and stenting of the left VAOS in order to prevent further strokes. The patient was loaded with 600 mg of clopidogrel and 325 mg of aspirin and underwent endovascular stenting (Figure 2B and C) 7 days after presentation. After successful stenting of the left VAOS, he was discharged on clopidogrel for 3 months and aspirin indefinitely.
▪ Case 2
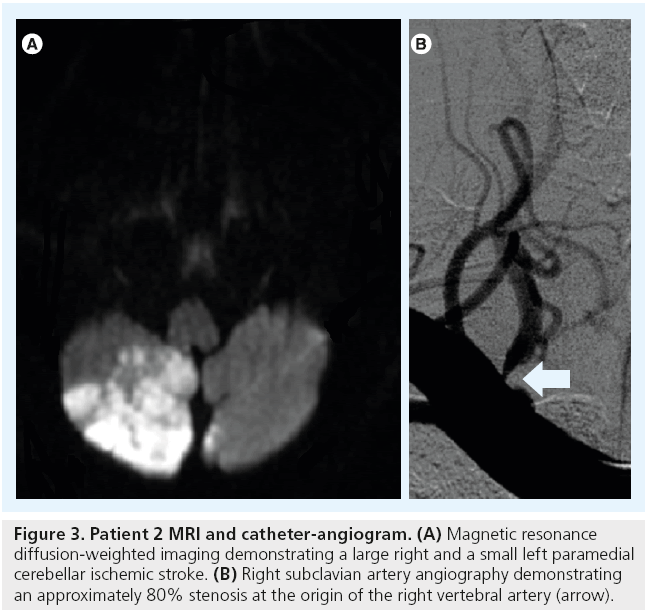
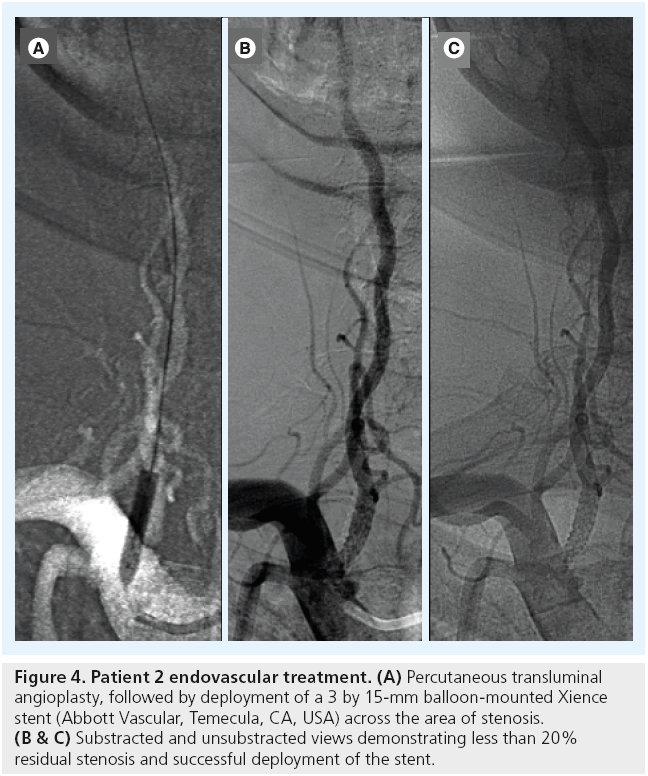
A 60-year-old man with a history of hypertension presented with a 3-day headache and gait instability. Brain MR diffusion-weighted imaging demonstrated the presence of a right cerebellar stroke and a small left paramedian cerebellar stroke (Figure 3A). A diagnostic cerebral angiogram demonstrated approximately an 80% right VAOS (Figure 3B) and occlusion of the distal branches of the right and left posterior inferior cerebellar arteries. The patient underwent PTA followed by stenting of the right VAOS (Figure 4).
Figure 3: Patient 2 MRI and catheter-angiogram. (A) Magnetic resonance diffusion-weighted imaging demonstrating a large right and a small left paramedial cerebellar ischemic stroke. (B) Right subclavian artery angiography demonstrating an approximately 80% stenosis at the origin of the right vertebral artery (arrow).
Figure 4: Patient 2 endovascular treatment. (A) Percutaneous transluminal angioplasty, followed by deployment of a 3 by 15-mm balloon-mounted Xience stent (Abbott Vascular, Temecula, CA, USA) across the area of stenosis. (B & C) Substracted and unsubstracted views demonstrating less than 20% residual stenosis and successful deployment of the stent.
▪ Case 3
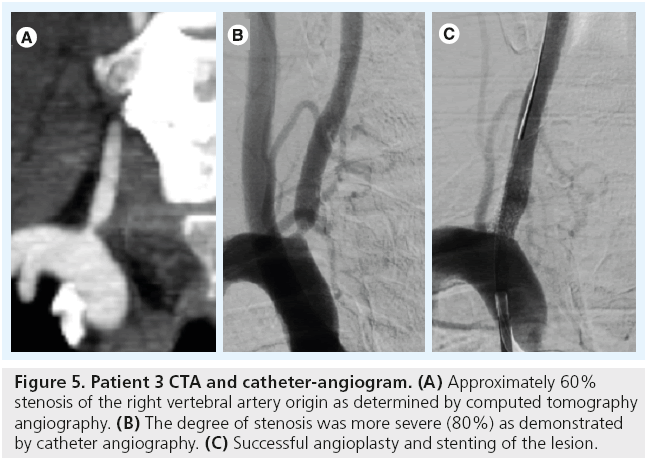
A 48-year-old with hypertension and diabetes presented with left hemiparesis. Brain MRI demonstrated a right pontine stroke. A CTA suggested the presence of a 60% right VAOS and occlusion of the left VA (Figure 5A). A diagnostic cerebral angiogram demonstrated that the degree of VAOS was underestimated by CTA. The VA had approximately 80% stenosis at the origin. The patient underwent successful angioplasty and stenting of this symptomatic lesion (Figure 5B and C).
Figure 5: Patient 3 CTA and catheter-angiogram. (A) Approximately 60% stenosis of the right vertebral artery origin as determined by computed tomography angiography. (B) The degree of stenosis was more severe (80%) as demonstrated by catheter angiography. (C) Successful angioplasty and stenting of the lesion.
Conclusion
VAOS is a treatable cause of posterior circulation strokes. PTA and stenting of the VAOS appear to be safe with a 30-day stroke and death rate of approximately 1% based on recent large systematic reviews of the literature [31,32]. A lower rate of restenosis is observed with the use of DES [31,32,38]. The best treatment for symptomatic VAOS when patients fail maximized medical therapy is yet to be determine. PTA and stenting are endovascular options that should be considered.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Incidence & natural history of vertebral artery origin stenosis
▪ The incidence of vertebral artery origin stenosis (VAOS) is between 5 and 7%.
▪ The New England Posterior Circulation Stroke Registry suggests that 9-–20% of posterior circulation strokes are caused by VAOS.
Pathophysiology of VAOS strokes
▪ Artery–artery thromboembolism and hemodynamic hypoperfusion are the main physiological processes in VAOS strokes.
Treatment modalities of symptomatic VAOS
▪ Optimized medical management is the first treatment option.
▪ Surgical procedures are often laborious and technically complex; nowadays they are rarely used.
▪ Percutaneous transluminal angioplasty is associated with an approximately 9% risk of transient neurological deficits and up to a 15% restenosis rate.
▪ Endovascular stenting of VAOS has a 1% complication risk and appears to have less restenosis than percutaneous transluminal angioplasty.
Restenosis
▪ Mechanisms involved in the development of restenosis include injury of the arterial wall during angioplasty and stent deployment, and artificial straightening of the vessel.
▪ Cigarette smoking, vessel tortuosity and small diameter have been associated with a higher risk of restenosis.
▪ DES have shown a significant reduction in the rate of restenosis by inhibiting neointimal hyperplasia.
Endovascular treatment considerations of symptomatic VAOS
▪ Patients should be preloaded with dual antiplatelets before the procedure and then continue taking double antiplatelet therapy for at least 3 months.
▪ Distal protection devices are not routinely used during the procedure.
▪ The stent is usually oversized by 3 mm at both ends of the stenotic arterial segment.
▪ Balloon-expandable coronary stents are usually used for stenting because of their radial force, low crossing profile and limited foreshortening.
References
Papers of special note have been highlighted as:
▪ of interest
- Caplan LR, Wityk RJ, Glass TA et al. New England medical center posterior circulation registry. Ann. Neurol. 56(3), 389–398 (2004).
- Wityk RJ, Chang HM, Rosengart A et al. Proximal extracranial vertebral artery disease in the New England medical center posterior circulation registry. Arch. Neurol. 55(4), 470–478 (1998).
- Gulli G, Khan S, Markus HS. Vertebrobasilar stenosis predicts high early recurrent stroke risk in posterior circulation stroke and TIA. Stroke 40(8), 2732–2737 (2009).
- Dabus G, Moran CJ, Derdeyn CP, Cross DT 3rd. Endovascular treatment of vertebral artery-origin and innominate/subclavian disease: indications and technique. Neuroimaging Clin. N. Am. 17(3), 381–392, ix (2007).
- Wehman JC, Hanel RA, Guidot CA, Guterman LR, Hopkins LN. Atherosclerotic occlusive extracranial vertebral artery disease: indications for intervention, endovascular techniques, short-term and long-term results. J. Interv. Cardiol. 17(4), 219–232 (2004).
- Compter A, Van Der Worp HB, Algra A, Kappelle LJ. Prevalence and prognosis of asymptomatic vertebral artery origin stenosis in patients with clinically manifest arterial disease. Stroke 42(10), 2795–2800 (2011).
- Hennerici M, Hulsbomer HB, Hefter H, Lammerts D, Rautenberg W. Natural history of asymptomatic extracranial arterial disease. Results of a long-term prospective study. Brain 110(Pt 3), 777–791 (1987).
- Kumar Dundamadappa Md S, Cauley Md Phd K. Vertebral artery ostial stenosis: prevalence by digital subtraction angiography, MR angiography, and CT angiography. J. Neuroimaging doi: 10.1111/j.1552- 6569.2011.00692.x. (2012) (Epub ahead of print).
- Dabus G, Gerstle RJ, Derdeyn CP, Cross DT 3rd, Moran CJ. Endovascular treatment of the vertebral artery origin in patients with symptoms of vertebrobasilar ischemia. Neuroradiology 48(12), 917–923 (2006).
- Coward LJ, Mccabe DJ, Ederle J, Featherstone RL, Clifton A, Brown MM. Long-term outcome after angioplasty and stenting for symptomatic vertebral artery stenosis compared with medical treatment in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Stroke 38(5), 1526–1530 (2007).
- Amin-Hanjani S, Du X, Zhao M, Walsh K, Malisch TW, Charbel FT. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke 36(6), 1140–1145 (2005).
- Malek AM, Higashida RT, Phatouros CC et al. Treatment of posterior circulation ischemia with extracranial percutaneous balloon angioplasty and stent placement. Stroke 30(10), 2073–2085 (1999).
- Caplan LR, Amarenco P, Rosengart A et al. Embolism from vertebral artery origin occlusive disease. Neurology 42(8), 1505–1512 (1992).
- Weber W, Mayer TE, Henkes H et al. Efficacy of stent angioplasty for symptomatic stenoses of the proximal vertebral artery. Eur. J. Radiol. 56(2), 240–247 (2005).
- Hauth EA, Gissler HM, Drescher R, Jansen C, Jaeger HJ, Mathias KD. Angioplasty or stenting of extra- and intracranial vertebral artery stenoses. Cardiovasc. Interv. Radiol. 27(1), 51–57 (2004).
- Fisher CM, Gore L, Okabe N, White PD. Atherosclerosis of the carotid and vertebral arteries-extracranial and intracranial. J. Neuropathol. Exp. Neurol. 24, 455–476 (1965).
- Fisher CM. Occlusion of the vertebral arteries causing transient basilar symptoms. Arch. Neurol. 22, 13–19 (1970).
- Searls DE, Pazdera L, Korbel E, Vysata O, Caplan LR. Symptoms and signs of posterior circulation ischemia in the New England medical center posterior circulation registry. Arch. Neurol. 69(3), 346–351 (2012).
- Khan S, Cloud GC, Kerry S, Markus HS. Imaging of vertebral artery stenosis: a systematic review. J. Neurol. Neurosurg. Psychiatry 78(11), 1218–1225 (2007).
- Chimowitz MI, Lynn MJ, Derdeyn CP et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N. Engl. J. Med. 365(11), 993–1003 (2011).
- Chimowitz MI, Lynn MJ, Howlett-Smith H et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N. Engl. J. Med. 352(13), 1305–1316 (2005).
- Ausman JI, Diaz FG, Vacca DF, Sadasivan B. Superficial temporal and occipital artery bypass pedicles to superior, anterior inferior, and posterior inferior cerebellar arteries for vertebrobasilar insufficiency. J. Neurosurg. 72(4), 554–558 (1990).
- Imparato AM. Vertebral arterial reconstruction: a nineteen-year experience. J. Vasc. Surg. 2(4), 626–634 (1985).
- Motarjeme A, Keifer JW, Zuska AJ. Percutaneous transluminal angioplasty of the vertebral arteries. Radiology 139(3), 715–717 (1981).
- Higashida RT, Tsai FY, Halbach VV et al. Transluminal angioplasty for atherosclerotic disease of the vertebral and basilar arteries. J. Neurosurg. 78(2), 192–198 (1993).
- Piotin M, Spelle L, Martin JB et al. Percutaneous transluminal angioplasty and stenting of the proximal vertebral artery for symptomatic stenosis. AJNR Am. J. Neuroradiol. 21(4), 727–731 (2000).
- Bruckmann H, Ringelstein EB, Buchner H, Zeumer H. Percutaneous transluminal angioplasty of the vertebral artery. A therapeutic alternative to operative reconstruction of proximal vertebral artery stenoses. J. Neurol. 233(6), 336–339 (1986).
- Albuquerque FC, Fiorella D, Han P, Spetzler RF, McDougall CG. A reappraisal of angioplasty and stenting for the treatment of vertebral origin stenosis. Neurosurgery 53(3), 607–614 (2003).
- Moufarrij NA, Little JR, Furlan AJ, Williams G, Marzewski DJ. Vertebral artery stenosis: long-term follow-up. Stroke 15(2), 260–263 (1984).
- Storey GS, Marks MP, Dake M, Norbash AM, Steinberg GK. Vertebral artery stenting following percutaneous transluminal angioplasty. Technical note. J. Neurosurg. 84(5), 883–887 (1996).
- Antoniou GA, Murray D, Georgiadis GS et al. Percutaneous transluminal angioplasty and stenting in patients with proximal vertebral artery stenosis. J. Vasc. Surg. (2011).
- Stayman AN, Nogueira RG, Gupta R. A systematic review of stenting and angioplasty of symptomatic extracranial vertebral artery stenosis. Stroke 42(8), 2212–2216 (2011).
- Chastain HD 2nd, Campbell MS, Iyer S et al. Extracranial vertebral artery stent placement: in-hospital and follow-up results. J. Neurosurg. 91(4), 547–552 (1999).
- Jenkins JS, White CJ, Ramee SR et al. Vertebral artery stenting. Catheter Cardiovasc. Interv. 54(1), 1–5 (2001).
- Lin YH, Juang JM, Jeng JS, Yip PK, Kao HL. Symptomatic ostial vertebral artery stenosis treated with tubular coronary stents: clinical results and restenosis analysis. J. Endovasc. Ther. 11(6), 719–726 (2004).
- Hatano T, Tsukahara T, Ogino E, Aoyama T, Nakakuki T, Murakami M. Stenting for vertebrobasilar artery stenosis. Acta Neurochir. (Suppl. 94), 137–141 (2005).
- Taylor RA, Siddiq F, Suri MF, Martin CO, Hayakawa M, Chaloupka JC. Risk factors for in-stent restenosis after vertebral ostium stenting. J. Endovasc. Ther. 15(2), 203–212 (2008).
- Vajda Z, Miloslavski E, Guthe T et al. Treatment of stenoses of vertebral artery origin using short drug-eluting coronary stents: improved follow-up results. AJNR Am. J. Neuroradiol. 30(9), 1653–1656 (2009).
- Ogilvy CS, Yang X, Natarajan SK et al. Restenosis rates following vertebral artery origin stenting: does stent type make a difference? J. Invasive Cardiol. 22(3), 119–124 (2010).
- Zhou Z, Yin Q, Xu G et al. Influence of vessel size and tortuosity on in-stent restenosis after stent implantation in the vertebral artery ostium.Cardiovasc. Intervent. Radiol. 34(3), 481–487 (2011).
- Compter A, Van Der Worp HB, Schonewille WJ et al. VAST: vertebral artery stenting trial. Protocol for a randomised safety and feasibility trial. Trials 9, 65 (2008).
- Caplan L, Chung CS, Wityk R et al. New England Medical Center Posterior Circulation Stroke Registry: I. Methods, data base, distribution of brain lesions, stroke mechanisms, and outcomes. J. Clin. Neurol. 1(1), 14–30 (2005).
- Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 363(9413), 915–924 (2004).
- Hayashi K, Kitagawa N, Morikawa M, Kaminogo M. A case of intimal hyperplasia induced by stenting for vertebral artery origin stenosis: assessed on intravascular ultrasound. Neurol. Res. 25(4), 357–360 (2003).
- Mukherjee D, Roffi M, Kapadia SR et al. Percutaneous intervention for symptomatic vertebral artery stenosis using coronary stents. J. Invasive Cardiol. 13(5), 363–366 (2001).
- Stone GW, Ellis SG, Cox DA et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- Fessler RD, Wakhloo AK, Lanzino G, Guterman LR, Hopkins LN. Transradial approach for vertebral artery stenting: technical case report. Neurosurgery 46(6), 1524–1527 (2000).
- Sawada M, Hashimoto N, Nishi S, Akiyama Y. Detection of embolic signals during and after percutaneous transluminal angioplasty of subclavian and vertebral arteries using transcranial Doppler ultrasonography. Neurosurgery 41(3), 535–540 (1997).
- Qureshi AI, Kirmani JF, Harris-Lane P et al. Vertebral artery origin stent placement with distal protection: technical and clinical results. AJNR Am. J. Neuroradiol. 27(5), 1140–1145 (2006).
▪ Extensive review on the diagnosis and treatment of vertebral artery origin stenosis.
▪ First randomized study comparing medical therapy with endovascular treatment of vertebral artery origin stenosis.
▪ Comprehensive systematic review of most of the studies in vertebral artery origin stenosis.
▪ Systematic review of studies about vertebral artery origin stenosis.
▪ Study using drug-eluting coronary stents in the treatment of vertebral artery origin stenosis.