Mini Review - Interventional Cardiology (2022) Volume 14, Issue 4
The Albumin-Bilirubin (ALBI) score as a new model to assess liver function in acute heart failure
- Corresponding Author:
- Takayuki Kawata
Department of Cardiovascular Medicine,
Sassa General Hospital, 4-24-15, Tanashicho,
Nishi Tokyo-City,
Tokyo 188-0011,
Japan,
E-mail: dqh07724@nifty.ne.jp
Received date: 01-Jun-2022, Manuscript No. FMIC-22-65548; Editor assigned: 03-Jun-2022, PreQC No. FMIC-22-65548 (PQ); Reviewed date: 20-Jun-2022, QC No. FMIC-22-65548; Revised date: 27-Jun-2022, Manuscript No. FMIC-22-65548 (R); Published date: 04-Jul-2022, DOI: 10.37532/1755- 5310.2022.14(4).526
Abstract
Liver dysfunction is one of the most recognized complications in patients with acute Heart Failure (HF) and therefore, a reliable liver function score may be useful to help risk stratify these patients. A number of measures of liver function have been proposed as predictors of operative risk in patients with liver disease, such as the Child-Pugh grade and the Model of End-Stage Liver Disease (MELD). However, there are several limitations to both and they may not be as clinically useful in fully assessing patients with HF.
More recently, the Albumin-Bilirubin (ALBI) score was developed as a new model to assess liver function in liver disease. The ALBI score incorporates two variables, serum albumin and total bilirubin. Although there have been scattered reports recently examining the association between ALBI scores and HF, the relationship between ALBI scores and HF and cardiovascular disease has not yet been elucidated. This article reviews and discusses the findings on ALBI and HF reported to date and future perspectives.
Keywords
Acute heart failure •Liver function marker • Albumin-bilirubin
Introduction
Heart Failure (HF) is a major cause of death among elderly individuals in many countries. Since Japan and many Western countries have entered an era of an ultraaging society, HF has become a significant public health problem. It has been reported that each episode of acute HF may lead to dysfunction or injury of end-organs other than the heart, such as the liver, kidneys, brain, or lungs. All of these organs interact with each other, and because of this, many subsequent consequences may result [1- 3]. Therefore, acute HF is not simply a failure of a single organ. In particular, liver dysfunction is a well-known major complication in patients with acute HF, and interactions between the heart and the liver are becoming increasingly recognized.
Literature Review
Hepatic dysfunction score and HF
Various scoring models have been used to assess risk in patients with HF. For example, the Heart Failure Survival Score, the Seattle Heart Failure Model and the Get With The Guidelines Heart Failure (GWTG-HF) risk score have all been shown to effectively identify patients at high risk for clinical events and death in cohorts of HF patients [4- 6]. While these models incorporate a multitude of variables, they fail to fully address the impact of liver dysfunction. The cardio-hepatic syndrome, a condition characterized by the development of congestive hepatopathy and subsequent cirrhosis in patients with advanced HF, has long been recognized in clinical settings [7]. Moreover, abnormal liver function test results in patients with HF have been linked to poor outcomes and a higher risk of death vs. those without abnormal liver function tests [8-10].
There are several reports on the usefulness of composite scoring systems of liver dysfunction in patients with HF. One scoring system is the established Model for End-Stage Liver Disease (MELD), which was developed for patients with hepatic cirrhosis awaiting liver transplantation. The MELD may provide information on HF patients by measuring the progression of liver dysfunction based on a patient’s creatinine, total bilirubin, and International Normalized Ratio (INR) [11]. These 3 laboratory parameters are non-cardiac biomarkers that represent hepatic and renal dysfunction and their impact on coagulation [12]. This makes the MELD score suitable for the prognosis of advanced HF, a state of multiorgan dysfunction secondary to impaired cardiac function, with known hepatic and renal dysfunction in advanced stages of the disease. Indeed, MELD scores reflect dynamic changes in liver function that may respond to HF therapies and hemodynamic stabilization [13,14]. In addition to its established role in determining the urgency for liver transplantation, the MELD scoring system has also been shown to be a versatile tool for predicting outcomes in patients with cirrhosis who are undergoing cardiac surgery [15,16], patients with advanced HF undergoing left ventricular assist device implantation [17], and patients undergoing heart transplantation [18]. Furthermore, alternative MELD scoring systems may offer improved prognostic efficacy [19-21]. This is particularly true for the MELD excluding INR (MELD XI) score, which excludes INR as a variable and is therefore, a more reliable marker of risk in patients with elevated INR secondary to anticoagulation medications [22].
However, some reports have also found this alternative MELD score to be useful for patients with HF due to its incorporation of renal function indicators, a reasonable HF prognostic marker [23]. Moreover, there may be limitations to alternative MELD, as [24] many studies do not adjust the prognostic value of MELD XI scores with other potential confounders. More specifically, for example, there are no studies examining the prognostic ability of the MELD XI score independent of blood urea nitrogen. This is important because blood urea nitrogen has been strongly confirmed as a prognostic factor in patients with acute and chronic HF. The prognostic ability of the MELD XI score independent of blood urea nitrogen should be more thoroughly examined, because blood urea nitrogen is an easily available biomarker in clinical practice [24]. Furthermore, the usefulness of MELD Na, another modification of MELD, may also be attributed to its incorporation of sodium, which is closely related to the prognosis of HF [23].
ALBI literature review
The Albumin-Bilirubin (ALBI) score was developed in 2015 as a newer parameter to assess liver function in patients with liver disease. The ALBI score incorporates serum albumin and total bilirubin, and it was developed in patients with Hepatocellular Carcinoma (HCC) as a response to problems encountered with the Child-Pugh (C-P) grade [25]. Those problems were that some factors were subjective (i.e., ascites and encephalopathy), while other factors such as serum albumin and ascites were interrelated. Based on 1,313 patients with HCC of all stages from Japan, proponents of the ALBI [25] developed a simple model to assess liver function that involved only serum and albumin levels. The model was evaluated using similar cohorts from other geographical regions (n=5,097) and other clinical situations (patients undergoing resection (n=525) or sorafenib treatment for advanced HCC (n=1,132)). The specificity of the model for liver dysfunction was also assessed in patients with chronic liver disease but without HCC (n=501). The model, the ALBI grade, performed at least as well as the C-P grade in all geographic regions. The majority of patients with HCC had C-P grade A disease at presentation. Yet, within this C-P grade, ALBI revealed 2 classes with clearly different prognoses. Therefore, the utility of the ALBI in patients with chronic liver disease alone supports the contention that the ALBI grade is an index of liver dysfunction. Indeed, the ALBI grade offers a simple, evidence-based, objective, and discriminatory method of assessing liver function in HCC that has been extensively tested in an international setting. This new model also eliminates the need for subjective variables such as ascites and encephalopathy, which are requirements in the conventional C-P grading system.
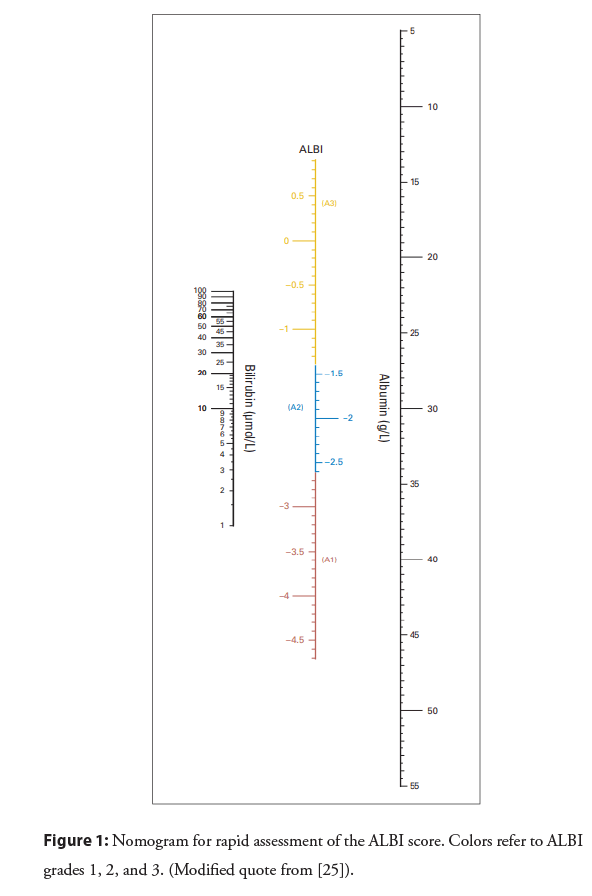
The ALBI score is calculated using the following formula: (log 10 total bilirubin (mmol/L) × 0.66)+(albumin (g/L) × -0.085). ALBI grade categories are: ≤ -2.60=grade 1, >-2.60 to ≤ -1.39=grade 2, >-1.39=grade 3. Some countries and regions may have units that differ from those used in daily clinical practice and therefore, the ALBI may require close attention to units and detailed calculations. Indeed, the ALBI scores are not easy to calculate, so therefore, a published nomogram for the ALBI score may aid clinicians in the use of this score in clinical settings [25] (Figure 1).
The ALBI score is calculated using the following formula: (log 10 total bilirubin (mmol/L) × 0.66)+(albumin (g/L) × -0.085). ALBI grade categories are: ≤ -2.60=grade 1, >-2.60 to ≤ -1.39=grade 2, >-1.39=grade 3. Some countries and regions may have units that differ from those used in daily clinical practice and therefore, the ALBI may require close attention to units and detailed calculations. Indeed, the ALBI scores are not easy to calculate, so therefore, a published nomogram for the ALBI score may aid clinicians in the use of this score in clinical settings [25] (Figure 1).
To date, 5 papers regarding the ALBI score and HF have been published from Asia. In late 2019, Matsue and colleagues published a study on 1,190 cases of acute HF [24]. The authors evaluated ALBI and MELD XI as indicators of liver function and their associations with clinical profiles and one-year prognoses. They hypothesized that liver dysfunction in acute HF is more strongly associated with fluid overload than with fluid redistribution. Therefore, the authors examined the association between a “Simple Fluid Overload” (SFO) score, the ALBI and the MELD XI. The SFO was calculated by counting the number of the following signs that represent fluid overload: Peripheral edema, jugular venous distention, pulmonary rales, and pleural effusions on chest x-ray. Overall, the authors found that the ALBI score was associated with the SFO score and with one-year prognosis, but the MELD XI was not. They concluded that even though the pathophysiological background should be evaluated in future studies, the ALBI score may be a promising liver dysfunction score that incorporates information on fluid overload and prognosis in acute HF.
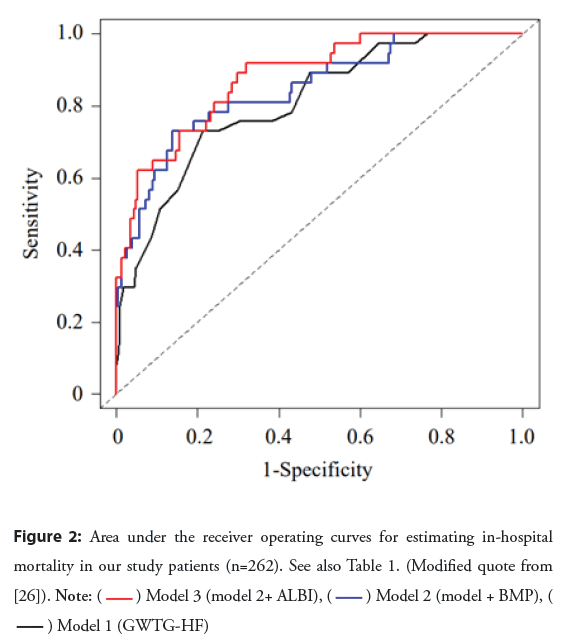
Two other papers have reported on the association between ALBI score and short-term prognosis of acute HF, i.e., in-hospital mortality. One of these papers, published from our institution (Sassa General Hospital, Tokyo, Japan), was a study of 262 Japanese elderly patients (median age, 86 years) who were admitted to our hospital for treatment of acute HF [26]. A GWTG-HF risk score was calculated as an established risk model for each patient. During hospitalization, 37 patients (14.1%) died. The in-hospital mortality rate was significantly higher in patients with ALBI scores >-2.25 compared with patients with ALBI scores ≤ -2.25 (21.1% vs. 4.5%, respectively; P=0.0001). Multivariate analysis revealed that the GWTG-HF scores, the B-type Natriuretic Acid (BNP) levels and ALBI scores were independently associated with inhospital mortality. Table 1 and Figure 2 show the receiver operating curve analysis of each model that estimated in-hospital mortality based on the results of the multivariate analysis. Compared with the GWTG-HF model (Model 1), a significant net reclassification improvement occurred with the addition of the BNP level (Model 2). Compared with Model 2, there was a further significant increase in net reclassification improvement with the addition of the ALBI score (Model 3). The results of our study indicated a high ALBI score to be independently associated with in-hospital mortality in patients hospitalized for acute HF.
Figure 2: Area under the receiver operating curves for estimating in-hospital mortality in our study patients (n=262). See also Table 1. (Modified quote from [26]). 
| Variables | AUC (95% CI) | NRI (95% CI) | p |
|---|---|---|---|
| Model 1 (GWTG-HF risk score) | 0.81 (0.73-0.88) | ||
| Model 2 (Model 1+BNP level) | 0.85 (0.77-0.92) | 0.55 (0.21-0.89) * | 0.0017 |
| Model 3 (Model 2+ALBI score) | 0.88 (0.82-0.94) | 0.46 (0.12-0.80) ** | 0.0085 |
Note: AUC: Area Under the Curve; NRI: Net Reclassification Improvement; GWTG-HF: Get With The Guidelines Heart Failure; BNP: B-type Natriuretic Peptide; ALBI: Albumin-Bilirubin
*vs. Model 1.
**vs. Model 2.
Table 1: Area under the curve analysis of each model and associated net reclassification improvement (Modified quote from [26]).
Similarly, Han, et al. [27] reported the relationship between inhospital mortality and ALBI in acute HF in a large number of patients (n=9749) in China. The authors reported that in-hospital mortality was higher in those patients with higher ALBI scores. The ALBI score was not only independently associated with outcome, but there was also a stronger association between inhospital mortality and a combination of BNP and ALBI vs. BNP alone. Unfortunately, the GWTG-HF score is lacking as a wellestablished predictive model, but this is a valuable study of a large number of cases of acute HF.
Two other studies on the ALBI examined the relationship between ALBI, Cardiac Resynchronization Therapy (CRT) response, and prognosis in HF reduced Ejection Fraction (HFrEF) patients undergoing CRT [28,29]. Saito, et al. [28] showed that in 274 HFrEF patients, a high ALBI score before CRT implantation was associated with HF severity. The definition of HF severity in this study was based on New York Heart Association functional class, BNP level, tricuspid regurgitation severity, pulmonary hypertension, and inferior vena cava diameter. A high ALBI score before CRT implantation was also associated with a worse prognosis after implantation. Finally, the authors found an improved ALBI in CRT responders evaluated after 6 months, and adding an ALBI score to the existing VALID-CRT score strengthened the association with one-year prognosis. Similarly, Yamada, et al. [29] compared ALBI scores and left ventricular ejection fraction before and six months after CRT implantation in 180 HFrEF patients. They also evaluated subsequent prognosis. The subjects were divided into 4 groups according to ALBI score (high/high, low/high, high/low, low/low). At a median follow-up of 50 months, the high/high and low/high groups had a poorer prognosis. These results showed that a lower ALBI score after, but not before, implantation is associated with a better prognosis. Since there were no significant differences in improvement of left ventricular ejection fraction after CRT implantation in the 4 groups, the authors concluded that left ventricular contractility was not associated with ALBI score.
Discussion
In the discussion of these studies, it is considered that a decrease in serum albumin, an increase in serum bilirubin, and the subsequent increase in ALBI score are brought about mainly by hepatic congestion due to right HF, but also partly by hepatic hypoperfusion. Earlier reports have shown that both albumin [30,31] and bilirubin [32,33] alone are prognostic factors in patients with HF. Therefore, the ALBI score, as a combined measurement of albumin and total bilirubin, may become a more attractive prognostic index in patients with HF, and not just for patients with HCC.
In the field of liver diseases, liver function tests and ALBI assume roles as indicators of liver function by themselves. On the other hand, in the field of circulation, these tests can be regarded as indices that reflect “how the liver feels” as a result of low cardiac output and congestion, i.e., forward and backward failure of the failing heart. Comprehensive HF prognostic models such as the GWTG-HF risk score or the Seattle Heart Failure Model mainly include blood pressure, heart rate, and left ventricular ejection fraction, which are essentially “sender” indicators. The same is true for echocardiography, cardiac magnetic resonance imaging, and other cardiac function indices. On the other hand, ALBI, which is evaluated as a receiver index of circulation, may reflect hemodynamics that cannot be fully evaluated by the so-called “sender indices” alone. In fact, in the final paper [29] introduced in this review, it was reported that the prognosis of HF patients was better in cases with improved ALBI, even though the change in left ventricular ejection fraction after 6 months with CRT was the same. This is interesting because it suggests that improvement in the receiving index, not the sending index, is better associated with prognosis.
To date, only retrospective studies have been reported. There are still many unknowns, such as the meaning of short-term and longterm changes during the course of the disease, the significance of the value of ALBI in acute exacerbations of chronic HF, and the usefulness of ALBI in patients with coexisting liver disease. For example, the association between nonalcoholic fatty liver disease, the most common liver disease in Western countries, and coronary artery disease has been widely reported [34], however, the association between nonalcoholic fatty liver disease and HF is not fully understood, and the usefulness of the ALBI score is unknown. Vascular endothelial function is known to be associated with prognosis of HF and exercise tolerance, but patients with hepatitis C virus-related chronic hepatitis have impaired vascular endothelial function [35]. There is also report [36] of an association between serum total bilirubin level and coronary endothelial function in obese patients. Therefore, whether ALBI score as well as vascular endothelial function is a useful prognostic factor in HF patients with hepatitis C virus-related chronic hepatitis awaits further investigation.
Conclusion
Based on previous reports, we have outlined the relationship between ALBI and HF. There have been no reports of prospective studies examining the relationship between ALBI score and HF. Therefore, future prospective studies are needed to determine whether the new liver function index, ALBI, can be useful in the management or risk stratification of HF patients with various comorbidities and conditions such as nonalcoholic fatty liver disease and hepatitis C virus-related chronic hepatitis.
Conflicts of Interest
The authors have no conflicts of interest directly relevant to the content of this article.
References
- Harjola VP, Mullens W, Banaszewski M, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 19(7): 821-36 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: Systematic review and meta-analysis. J Am Coll Cardiol. 47(10): 1987-96 (2006).
[CrossRef] [Google Scholar] [PubMed]
- Biegus J, Hillege HL, Postmus D, et al. Abnormal liver function tests in acute heart failure: Relationship with clinical characteristics and outcome in the PROTECT study. Eur J Heart Fail. 18(7): 830-9 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Aaronson KD, Schwartz JS, Chen TM, et al. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 95(12): 2660-7 (1997).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Levy WC, Mozaffarian D, Linker DT, et al. The Seattle heart failure model: Prediction of survival in heart failure. Circulation. 113(11): 1424-33 (2006).
[CrossRef] [Google Scholar] [PubMed]
- Peterson PN, Rumsfeld JS, Liang L, et al. American Heart Association Get With the Guidelines-Heart Failure Program. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 3(1): 25-32 (2010).
[CrossRef] [Google Scholar] [PubMed]
- van Deursen VM, Damman K, Hillege HL, et al. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. 16(1): 84-90 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Poelzl G, Ess M, Mussner-Seeber C, et al. Liver dysfunction in chronic heart failure: Prevalence, characteristic and prognostic significance. Eur J Clin Invest. 42(2): 153-63 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Shinagawa H, Inomata T, Koitabashi T, et al. Prognostic significance of increased serum bilirubin levels coincident with cardiac decompensation in chronic heart failure. Circ J. 72(3): 364-9 (2008).
[CrossRef] [Google Scholar] [PubMed]
- Allen LA, Felker GM, Pocock S, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. 11(2): 170-7 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 31(4): 864-71 (2000).
[CrossRef] [Google Scholar] [PubMed]
- Nagarajan V, Tang WH. Biomarkers in advanced heart failure: Diagnostic and therapeutic insights. Congest Heart Fail. 17(4): 169-74 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Choksi A, Cheema F, Shahzad K, et al. Hepatic dysfunction and survival after heart transplantation: Application of the MELD scoring system for outcome prediction. J Heart Lung Transplant. 31(6): 591-600 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Yang JA, Kato TS, Shulman BP, et al. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: Use of the Model of End-stage Liver Disease (MELD) and MELD excluding INR (MELD-XI) scoring system. J Heart Lung Transplant. 31(6): 601-10 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Suman A, Barnes DS, Zein NN, et al. Predicting outcome after cardiac surgery in patients with cirrhosis: A comparison of Child-Pugh and MELD scores. Clin Gastroenterol Hepatol. 2(8): 719-23 (2004).
[CrossRef] [Google Scholar] [PubMed]
- Teh SH, Nagorney DM, Stevens SR, et al. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 132(4): 1261-9 (2007).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Matthews JC, Pagani FD, Haft JW, et al. Model for end-stage liver disease score predicts left ventricular assist device operative transfusion requirements, morbidity, and mortality. Circulation. 121(2): 214-20 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Chokshi A, Cheema FH, Schaefle KJ, et al. Hepatic dysfunction and survival after orthotopic heart transplantation: Application of the MELD scoring system for outcome prediction. J Heart Lung Transplant. 31(6): 591-600 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Kim MS, Kato TS, Farr M, et al. Hepatic dysfunction in ambulatory patients with heart failure: Application of the MELD scoring system for outcome prediction. J Am Coll Cardiol. 61(22): 2253-61 (2013).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Inohara T, Kohsaka S, Shiraishi Y, et al. Prognostic impact of renal and hepatic dysfunction based on the MELD-XI score in patients with acute heart failure. Int J Cardiol. 176(3): 571-3 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Biegus J, Zymlinski R, Sokolski M, et al. Impaired hepato-renal function defined by the MELD XI score as prognosticator in acute heart failure. Eur J Heart Fail. 18(12): 1518-21 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Heuman DM, Mihas AA, Habib A, et al. MELD-XI: A rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl. 13(1): 30-7 (2006).
[CrossRef] [Google Scholar] [PubMed]
- Grodin JL, Gallup D, Anstrom KJ, et al. Implications of alternative hepatorenal prognostic scoring systems in acute heart failure (from DOSE-AHF and ROSE-AHF). Am J Cardiol. 119(12): 2003-9 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Matsue Y, Kagiyama N, Yamaguchi T, et al. Clinical and prognostic values of ALBI score in patients with acute heart failure. Heart Lung Circ. 29(9): 1328-37 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J Clin Oncol. 33(6): 550-8 (2015).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Kawata T, Ikeda A, Masuda H, et al. Association between albumin-bilirubin score at admission and in-hospital mortality in patients with acute heart failure. Int Heart J. 62(4): 829-836 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Han S, Wang C, Tong F, et al. Prognostic impact of albumin-bilirubin score on the prediction of in-hospital mortality in patients with heart failure: A retrospective cohort study. BMJ Open. 12(1): e049325 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Saito Y, Nakai T, Ikeya Y, et al. Clinical significance of the albumin-bilirubin score in patients with heart failure undergoing cardiac resynchronization therapy. Heart Vessels. 37(7): 1136-1145 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Yamada S, Kaneshiro T, Yoshihisa A, et al. Albumin-Bilirubin score for prediction of outcomes in heart failure patients treated with cardiac resynchronization therapy. J Clin Med. 10(22): 5378 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Ancion A, Allepaerts S, Oury C, et al. Serum albumin level and hospital mortality in acute non-ischemic heart failure. ESC Heart Fail. 4(2): 138-45 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Horwich TB, Kalantar-Zadeh K, MacLellan RW, et al. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. 155(5): 883-9 (2008).
[CrossRef] [Google Scholar] [PubMed]
- Allen LA, Felker GM, Pocock S, et al. Liver function abnormalities and outcome in patients with chronic heart failure: Data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. 11(2): 170-7 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Samsky MD, Dunning A, DeVore AD, et al. Liver function tests in patients with acute heart failure and associated outcomes: Insights from ASCEND-HF. Eur J Heart Fail. 18(4): 424-32 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Ciccone MM, Principi M, Ierardi E, et al. Inflammatory bowel disease, liver diseases and endothelial function: Is there a linkage? J Cardiovasc Med (Hagerstown). 16(1): 11-21 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Barone M, Viggiani MT, Amoruso A, et al. Endothelial dysfunction correlates with liver fibrosis in chronic HCV infection. Gastroenterol Res Pract. 2015: 682174 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Yoshino S, Hamasaki S, Ishida S, et al. Relationship between bilirubin concentration, coronary endothelial function, and inflammatory stress in overweight patients. J Atheroscler Thromb. 18(5): 403-12 (2011).
[CrossRef] [Google Scholar] [PubMed]