Research Article - Neuropsychiatry (2018) Volume 8, Issue 4
The Antidepressant-Like Effect of Vanillin Aroma Involves Serum Magnesium and Brain BDNF
- Corresponding Author:
- Guangwu Li
Department of Neurobiology and Anatomy, Anhui Medical University, Hefei, People’s Republic of China
Tel: + 86 551 65161272
Fax: + 86 551 65161272
Abstract
Depression is a major global public health concern. The variable effectiveness and undesirable side effects of antidepressant medication have prompted the search for alternative treatments. Olfaction plays an important role in emotion regulation in our daily life. Pleasant odorants would evoke positive emotions, inducing relaxation and calmness. The beneficial effects of vanillin aromatherapy on depression model rats were investigated in our previous research. Here, biochemical molecules including serum magnesium and brain derived nerve growth factor (BDNF) were further detected to investigate the possible underlying mechanisms. Results showed that the administration of vanillin aromatherapy restored the serum magnesium and brain BDNF which might mediate remission of depression. This suggests that vanillin aromatherapy could be considered as a potential alternative therapy for alleviating psychological responses among people suffering from major depressive disorder.
Keywords
Vanillin, Aromatherapy, Magnesium, Brain-derived neurotrophic factor
Introduction
Major depressive disorder (MDD) is a common psychiatric disorder affecting more than 120 million people worldwide [1]. The unsatisfactory clinical efficacy of antidepressant drugs, further compounded by a variety of adverse effects associated with current antidepressant drugs has made major depression disorder a central focus of medical research and prompted the search for novel alternative treatments.
Aromatherapy has a long history in the treatment of depression [2]. The pilot study indicates positive findings with minimal risk for the use of aromatherapy as a complementary therapy in both anxiety and depression scales with the postpartum woman [3]. Evidences also suggest that aromatherapy is effective in controlling perceived stress and depression [4,5]. Pleasant odorants can be experienced as extremely relaxing and calming, such as rose, lavender, citrus and lemon fragrance [6].
Basing on the antidepressant experience of aromatherapy, we select an odorant named vanillin to investigate its antidepressant effect on depressive model rats. In our previous research, vanillin was demonstrated to ameliorate the depression-like behaviors in depression model rats and elevated dopamine and serotonin in the brain [7]. The aim of the present study is to investigate the other possible underlying mechanisms and provide stronger evidence for alternative antidepressant effect of vanillin aroma.
Methods
▪ Animals
The present study was performed in compliance with the Animal Scientific Procedures Act of 1986 and received local ethics committee approval (license number: LLSC2013006).
The chronic depressive disorder rat model (Male Sprague-Dawley rats, 200-250 g) was induced by chronic unpredictable mild stress (CUMS), which was elaborated well in our previous research [7].
Depression model rats were divided into 3 groups with 12-14 rats per group: the fluoxetine group; the vanillin group and the untreated group. Another normal 8 rats without stress stimulation were used as the control group.
Fluoxetine (10 mg/kg/d, diluted in distilled water; Eli Lilly &Co., Souzhou, China) were chronically administered to the fluoxetine group once daily for 4weeks between 8:00 and 10:00 a.m. by oral. Vanillin (Sangon Biotech, Shanghai co., Ltd, china) was administrated to the vanillin group according to the reference [7]. Rats in the untreated group and the control group received similar treatment to the vanillin group, but without any odorants administrated.
▪ Serum magnesium determination
After treatment for 4 w, 1-2 ml blood was drawn from the tail vein of anesthetized rats. After being centrifuged at 3000 rpm for 10 min at 4°C (Eppendorf, 5810R), the clear serum was obtained and sent to the laboratory department of the First Affiliated Hospital of Anhui Medical University where serum magnesium ([Mg2+]) was detected.
▪ Biological determinations
At the end of the experiment, the rats were deeply anesthetized with 10% chloral hydrate (330 μl/100 g body weight, i.p.). After transcardially perfused with physiological saline, the rats were decapitated and brains were promptly collected. The brains were cut into two equal halves from the longitudinal fissure.
One half brain was weighed and homogenized with an Ultrasonic Turax T25 (Bio-block Scientific, France) in 1 × PBS (10 ml PBS/g brain tissue). After centrifuged at 10000 rmp for 10 min at 4°C, the supernatants were collected and were stored at -80°C. The total soluble protein in supernatants was used as the internal reference standard for the quantification of BDNF. They were determined by improved concentration BCA protein assay kits (Sangon Co., Ltd, Shanghai, China) and the BDNF enzyme-linked immunosorbent assay (ELISA) kits (Cusabio Co., Ltd, Wuhan, China) respectively.
The other half brain was used for immunohistochemical examination of brain BDNF in CA3 area of the hippocampus (see supplementary information). The other half brain was fixed in 10% neutral formalin and desiccated and embedded in paraffin. Subsequently, the tissues were cut into 5 μm thick serial sections. The sections including hippocampus area were selected for immunohistochemical examination for BDNF. After dewaxed by dimethylbenzene and hydrated by gradient alcohol, the sections washed by PBS for 3 times. Then the sections were treated with 3% hydrogen peroxide to block endogenous peroxidases for 20 min. After antigen repaired by microwave in 0.01 m citrate buffer solution, the sections were blocked with goat serum for 1 h at room temperature, then incubated at 4°C overnight with rabbit antibody against human BDNF (1: 200 dilution, Bioss Co., Ltd, Beijing, China). Afterwards, the sections were washed with PBS and subjected to immunohistochemical staining using a SP96000 kit (ZSGB Co., Ltd, Beijing, China). Next, the sections were washed with PBS and developed with 3,3’-diaminobenzidine (DAB) for 5 min. Finally the sections imaged under light microscopy.
▪ Data analysis
Data were analyzed by Spss11.5 software using one-way ANOVA followed by least significant difference test for the parameters among the different groups (two-tailed). Data were expressed as the mean ± S.E.M (standard error of the mean). The statistical significance was set at P<0.05.
Results
▪ Vanillin increased serum magnesium
Serum magnesium in the vanillin group was significantly higher than that of the untreated group and the fluoxetine group [F (3,43)=4.868, P=0.036; F (3,43)=4.868, P=0.016, respectively]. But, no obvious difference was found between the untreated group and the fluoxetine group. Serum magnesium in the fluoxetine group and the untreated group was still lower than the control group [F (3,43)=4.868, P<0.01; F (3,43)=4.868, P<0.01, respectively]. No obvious difference was found between the vanillin group and the control group.
▪ Brain BDNF was normalized by vanillin aroma
BDNF levels in both the vanillin and fluoxetine groups were significantly higher than that of the untreated group [F (3,43)=5.702, P=0.012; F (3,43)=4.703, P=0.014, respectively]. No obvious difference was found between the vanillin group and the fluoxetine group. When compared with the control group, BDNF in the untreated group was significantly lower [F (3,43)=5.702, P<0.01]. No similar result was found in the vanillin group and the fluoxetine group (Table 1).
| Biochemical molecules | Vanillin (n=13) |
Fluoxetine (n=13) |
Untreated (n=12) |
Control (n=8) |
|---|---|---|---|---|
| Serume [Mg2+] | 0.983 ± 0.025* | 0.871 ± 0.032## | 0.885 ± 0.024## | 1.035 ± 0.055 |
| BDNF/Pr | 0.524 ± 0.041* | 0.520 ± 0.038* | 0.371 ± 0.039## | 0.644 ± 0.062 |
Table 1: Serum magnesium and brain BDNF among groups after treatment.
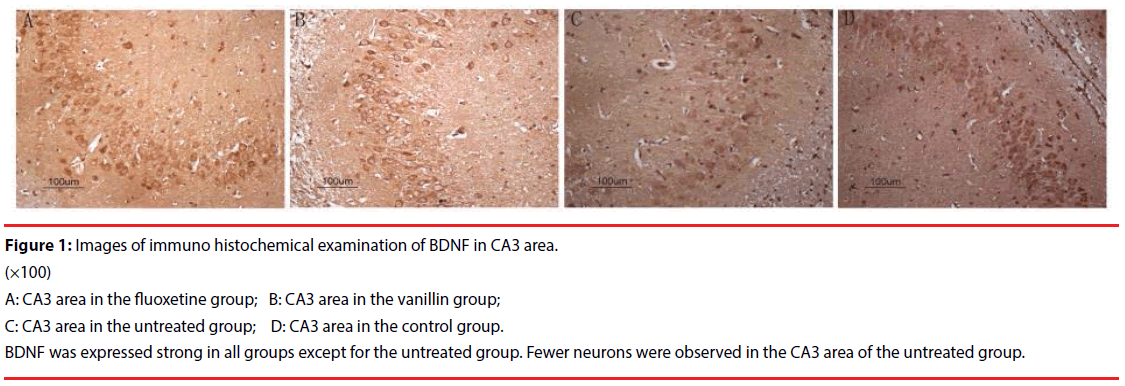
By immunohistochemical staining, the expression of BDNF in the fluoxetine group, the vanillin group and the control group was strong. Relatively speaking, it was weak in the untreated group. At the same time, fewer neurons were observed in CA3 area in the untreated group. No similar result was found in the vanillin group (Figure 1).
Figure 1: Images of immuno histochemical examination of BDNF in CA3 area.
(×100)
A: CA3 area in the fluoxetine group; B: CA3 area in the vanillin group;
C: CA3 area in the untreated group; D: CA3 area in the control group.
BDNF was expressed strong in all groups except for the untreated group. Fewer neurons were observed in the CA3 area of the untreated group.
Discussion
Complementary therapies for the treatment of depression has revealed that odorant aroma can relieve depression and anxiety, restoring both physical and emotional well-being [8,9]. As an odorant, vanillin is generally rated as pleasant and correspondingly evokes positive moods [10]. It has even been used to combat depression by oral [11,12]. In our previous studies, we had demonstrated that vanillin could alleviate depressive symptoms in the depression model rats by elevating both serotonin and dopamine levels in brain tissue [7]. In present study, we would further explore other underlying mechanisms.
NMDA receptor antagonists and brain BDNF appear to be involved in the mechanism of depression [13]. NMDA receptor is a type of ionotropic glutamate receptors playing a unique role in synaptic functions. Persistent activation of NMDA receptor would lead to a large amount of calcium ions inflow, causing cytotoxicity and leading to depression. So, NMDA receptor antagonists, such as magnesium, may have antidepressant efficacy [14,15].
Magnesium is one of the most important elements and is involved in a number of biochemical processes crucial for the proper functioning of living organisms [16]. It also plays a vital modulatory role in brain biochemistry, influencing several neurotransmission pathways associated with the development of depression. Magnesium ions are inhibitors of NMDA receptor by competing with calcium ions and blocking the glycine site on the NMDA receptor [17]. A decline of magnesium is believed associated with ensuing neuronal cell death and subsequent functional impairment [18]. Magnesium deficiency increases the susceptibility of animals to stress and leads to many personality changes including depression [19,20]. On the contrary, supplementary of magnesium is demonstrated antidepressant-like effect [21,22]. Screening for compounds to increase neuronal magnesium concentration could be a promising instrument to identify new classes of antidepressants [13]. The antidepressant-like activity of magnesium involves the AMPA/BDNF pathway [23]. BDNF is linked to the pathophysiology of depression [24,25]. There is accumulating evidence for the role of BDNF in the pathophysiology of depression [26]. CUMS procedure induces cognitive impairments including depression as well as the reduction of BDNF level [27,28]. On the contrary, an intensified release of BDNF may mediate antidepressant effect via BDNF-TrKB signaling pathway [29,30]. In present study, after treatment for 4 w, vanillin aroma increased serum magnesium significantly compared with that of the untreated group. Increase of magnesium predicted the antidepressant effect of vanillin. Correspondingly, BDNF levels of the vanillin group were also increased remarkably which would mediate the antidepressant effect of magnesium by AMPABDNF pathway [23,31]. In the fluoxetine group, magnesium was not found increased significantly. However, fluoxetine itself also has an inhibitory effect on Ca2+-permeable AMPA receptors [32]. As a result, BDNF was also remarkably increased in the fluoxetine group, which was one of the mechanisms fluoxetine exerting the antidepressant effect [30]. We also noticed that the number of neurons in the CA3 were highly consistent with the level of brain BDNF (Figure 1). Fewer neurons were found in the untreated group since its BDNF level was lower. BDNF deficiency combined with a decrease in the number of neurons may be one of the reasons why depression cannot be recovered [33].
In present study, we investigated the underlying mechanisms of antidepressant effect of vanillin aromatherapy. Our results indicated that vanillin aromatherapy could increase serum magnesium, which elevated brain BDNF and mediated the antidepressant effect as NMDA receptor antagonists. Further investigations would be carried out around the related signaling pathway and receptors. The present evidences further support vanillin as a potential antidepressant by olfactory pathway.
Acknowledgments
This research was supported by National Natural Science Foundation of China (81000589).
References
- Freudenberg F, Celikel T, Reif A. The role of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in depression: central mediators of pathophysiology and antidepressant activity? Neurosci. Biobehav. Rev 52(1), 193-206 (2015).
- Okamoto A, Kuriyama H, Watanabe S, et al. The effect of aromatherapy massage on mild depression: A pilot study. Psychiat. Clin. Neurosci 59(3), 363 (2005).
- Conrad P, Adams C. The effects of clinical aromatherapy for anxiety and depression in the high risk postpartum woman - a pilot study. Complement. Therap. Clin. Pract. 18(3), 164-168 (2012).
- Kim S, Song JA, Kim ME, et al. Effects of Aromatherapy on Menopausal Symptoms, Perceived Stress and Depression in Middle-aged Women: A Systematic Review. J. Korean. Acad. Nurs. 46(5), 619-629 (2016).
- Tang SK, Tse MY. Aromatherapy: Does it help to relieve pain, depression, anxiety, and stress in community-dwelling older persons? Biomed. Res. Int. 14(1), 430195 (2014).
- Bahrami T, Rejeh N, Heravi-Karimooi M, et al. Effect of aromatherapy massage on anxiety, depression, and physiologic parameters in older patients with the acute coronary syndrome: A randomized clinical trial. Int. J. Nurs. Pract (2017).
- Xu J, Xu H, Liu Y, et al. Vanillin-induced amelioration of depression-like behaviors in rats by modulating monoamine neurotransmitters in the brain. Psychiat. Res. 225(3), 509-514 (2015).
- Motomura N, Sakurai A, Yotsuya Y. Reduction of mental stress with lavender odorant. Perceptual. Motor skill. 93(3), 713-718 (2001).
- Edge J. A pilot study addressing the effect of aromatherapy massage on mood, anxiety and relaxation in adult mental health. Complement. Ther. Nurs. Midwifer. 9(2), 90-97 (2003).
- Seubert J, Rea AF, Loughead J, et al. Mood induction with olfactory stimuli reveals differential affective responses in males and females. Chem. Senses. 34(1), 77-84 (2009).
- Shoeb A, Chowta M, Pallempati G, et al. Evaluation of antidepressant activity of vanillin in mice. Indian. J. Pharmacol. 45(2), 141-144 (2013).
- Saad HB, Kharrat N, Driss D, et al. Effects of vanillin on potassium bromate-induced neurotoxicity in adult mice: impact on behavior, oxidative stress, genes expression, inflammation and fatty acid composition. Arch. Physiol. Biochem. 123(3), 165-174 (2017).
- Murck H. Ketamine, magnesium and major depression--from pharmacology to pathophysiology and back. J. Psychiat. Res. 47(7), 955-965 (2013).
- Allen AP, Naughton M, Dowling J, et al. Serum BDNF as a peripheral biomarker of treatment-resistant depression and the rapid antidepressant response: A comparison of ketamine and ECT. J. Affect. Disord. 186, 306-311 (2015).
- Stewart CA, Reid IC. Antidepressant mechanisms: functional and molecular correlates of excitatory amino acid neurotransmission. Mol. Psychiat. 7(1), S15-22 (2002).
- Serefko A, Szopa A, Poleszak E. Magnesium and depression. Magnesium. Res. 29(3), 112-119 (2016).
- Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Med. Hypotheses. 67(2), 362-370 (2006).
- Fromm L, Heath DL, Vink R, et al. Magnesium attenuates post-traumatic depression/anxiety following diffuse traumatic brain injury in rats. J. Am. Coll. Nutr. 23(5), 529S-533S (2004).
- Li B, Lv J, Wang W, et al. Dietary magnesium and calcium intake and risk of depression in the general population: A meta-analysis. Aus. New. Zealand. J. Psychiat. 51(3), 219-229 (2017).
- Rajizadeh A, Mozaffari-Khosravi H, Yassini-Ardakani M, et al. Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: A randomized, double-blind, placebo-controlled trial. Nutr. 35(1), 56-60 (2017).
- Bambling M, Edwards SC, Hall S, et al. A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: an intestinal anti-inflammatory response is suggested. Inflammopharmacology. 25(2), 271-274 (2017).
- Mehdi SM, Atlas SE, Qadir S, et al. Double-blind, randomized crossover study of intravenous infusion of magnesium sulfate versus 5% dextrose on depressive symptoms in adults with treatment-resistant depression. Psychiat. Clin. Neurosci. 71(3), 204-211 (2017).
- Pochwat B, Sowa-Kucma M, Kotarska K, et al. Antidepressant-like activity of magnesium in the olfactory bulbectomy model is associated with the AMPA/BDNF pathway. Psychopharmacol. 232(2), 355-367 (2015).
- De Sousa CN, Meneses LN, Vasconcelos GS, et al. Reversal of corticosterone-induced BDNF alterations by the natural antioxidant alpha-lipoic acid alone and combined with desvenlafaxine: Emphasis on the neurotrophic hypothesis of depression. Psychiat. Res. 230(2), 211-219 (2015).
- Kreinin A, Lisson S, Nesher E, et al. Blood BDNF level is gender specific in severe depression. PloS one. 10(5), e0127643 (2015).
- Hui LY, Wang YW, Zhou FL, et al. Association Between MKP-1, BDNF, and Gonadal Hormones with Depression on Perimenopausal Women. J. Women's. Health. 25(1):71-77 (2016).
- Kielstein H, Suntharalingam M, Perthel R, et al. Role of the endogenous nitric oxide inhibitor asymmetric dimethylarginine (ADMA) and brain-derived neurotrophic factor (BDNF) in depression and behavioural changes: clinical and preclinical data in chronic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association. Eur. Renal. Assoc. 30(10), 1699-1705 (2015).
- Sahin TD, Karson A, Balci F, et al. TNF-alpha inhibition prevents cognitive decline and maintains hippocampal BDNF levels in the unpredictable chronic mild stress rat model of depression. Behav. Brain. Res. 292(1), 233-240 (2015).
- Liu WX, Wang J, Xie ZM, et al. Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacol. 233(3), 405-415 (2016).
- Mendez-David I, Tritschler L, Ali ZE, et al. Nrf2-signaling and BDNF: A new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci. lett. 597, 121-126 (2015).
- Moshe H, Gal R, Barnea-Ygael N, et al. Prelimbic Stimulation Ameliorates Depressive-Like Behaviors and Increases Regional BDNF Expression in a Novel Drug-Resistant Animal Model of Depression. Brain. Stimulation. 9(2), 243-250 (2016).
- Barygin OI, Komarova MS, Tikhonova TB, et al. Non-classical mechanism of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor channel block by fluoxetine. Eur. J. Neurosci 41(7), 869-877 (2015).
- Caviedes A, Lafourcade C, Soto C, et al. BDNF/NF-kappaB Signaling in the neurobiology of depression. Curr. Pharm. Des. 23(21), 3154-3163 (2017).