Clinical Trial Teport - Interventional Cardiology (2011) Volume 3, Issue 4
The BASKET study program: continued evaluation of the efficacy and safety of drug-eluting stents
- Corresponding Author:
- Christoph Kaiser
Department of Cardiology
University Hospital Basel
Petersgraben 4, 4031 Basel, Switzerland
Tel: +41 61 265 52 25
Fax: +41 61 265 45 98
E-mail: ckaiser@uhbs.ch
Abstract
Keywords
bare-metal stents, coronary artery disease, drug-eluting stents, interventional cardiology
The introduction of drug-eluting stents (DES) in the year 2000 has revolutionized the treatment of coronary artery disease. The first-generation DES significantly reduced the rate of restenosis and thereby the rates of target vessel revascularization (TVR) [1,2]. From an economic standpoint, the use of the far more expensive DES in most patients instead of bare-metal stents (BMS) would pose an enormous strain on hospital budgets. In addition, the pivotal trials showing a benefit of DES have been conducted in highly selected patient cohorts (e.g., in stable low-risk patients, simple lesions and native vessels only) and therefore did not reflect the ‘real world’. Therefore, the question arose whether DES are cost effective in an unselected patient cohort. The Basel Stent Kosten-Effektivitäts Trial (BASKET) was initiated in 2003 to address the question of whether unlimited use of DES is cost effective in preventing major adverse cardiac events (MACE).
BASKET was a single center, prospective, randomized controlled study that included 826 out of 988 (84%) consecutive patients, irrespective of the indication for percutaneous coronary intervention (PCI), from May 2003 until May 2004 [3]. Patients were randomized to receive either a third-generation BMS (cobalt–chromium- based Vision® stent, Abbott Vascular) or one of the two first-generation DES available: the sirolimus-coated Cypher® stent (Cordis) or the paclitaxel-coated Taxus® stent (Boston Scientific). The DES were compared with each other and with the BMS. Exclusion criteria were a target vessel diameter above 4 mm (n = 23), a restenotic lesion (n = 49) or missing informed consent (n = 90). The primary end point was cost–effectiveness after 6 months with effectiveness defined as reduction of MACE including cardiac death, nonfatal myocardial infarction (MI) and TVR. Costs were ascertained on the basis of procedures, stents used and days spent in the hospital at baseline and during followup. Statistical analysis was performed according to the intention-to-treat principle. BASKET confirmed that the use of DES reduces the rate of MACE by 44%, mostly due to a lower rate of TVR. However, the higher initial stent costs were not compensated for by reductions in eventrelated follow-up costs. Nevertheless, subgroup analyses suggested that DES might be more cost effective in certain high-risk patients such as those with three-vessel disease, age above 65 years, more than one segment treated, small stent size or stent length greater than 20 mm [3]. The 18‑month analysis of cost–effectiveness confirmed the findings of the 6-month analysis, showing that an approximation to cost–effectiveness for DES is only given in a high-risk subset of patients, especially those with PCI of small vessels and bypass grafts [4,5].
The initial enthusiasm about DES was further dampened by reports of increased rates of latestent thrombosis [2]. To investigate these phenomenon, patients from the BASKET trial were further followed for another 12 months after discontinuation of clopidogrel in the BASKET Late Thrombotic Events (BASKET-LATE) study – a prospective randomized observational study [6]. The goal was to define the incidence of late (>6 months) clinical events related to stent thrombosis in patients treated with DES compared with BMS-treated patients after discontinuation of clopidogrel. Between 6 and 18 months there was a significantly higher rate of the combination of nonfatal MI and cardiac death in patients after DES compared with BMS implantation. In contrast to the first 6 months, however, the rate of restenosis-related TVR and MACE was not significantly different between DES- and BMS-treated patients. During the entire 18-month follow-up, there was a maintained benefit regarding the reduction of TVR of DES compared with BMS, whereas owing to the increased rates of late events, the rate of MACE was not different between DES and BMS use. The BASKET-LATE data suggested that late clinical events associated with stent thrombosis limit the early clinical benefit of DES [6].
In a further analysis of the BASKET 18‑month data, which sought to delineate predictors of outcome and their association with the type of stent used, large vessel diameter (≥3 mm) was identified as the single independent predictor of late nonfatal MI and cardiac death after DES implantation [5]. These results further suggested that in large vessels, the late clinical benefit of DES is possibly diminished by stent thrombosis whereas the initial angiographic success was not an independent predictor. Clearly, the analysis was not designed to provide angiographic proof of stent thrombosis. However, various studies showed that late cardiac death and nonfatal MI may be attributed to stent thrombosis [7–9]. The clinical 3‑year follow-up investigation of BASKET took the 18‑month data into consideration by adding (among others) a subgroup comparison of the ‘small-stent’ (<3.0 mm) and ‘large-stent’ (≥3 mm) population [10]. In patients after stenting of large native vessels, the cardiac death/MI rate was significantly higher beyond 6 months in patients with DES compared with those with BMS, with no significant difference in the rate of restenosis-related TVR. In contrast, there was a significantly reduced rate of restenosis, nonfatal MI and cardiac death in patients with small native vessel stenting or stenting of saphenous vein grafts with a DES when compared with BMS. In the overall population, owing to the opposing results in the two subgroups, the rate of MACE (cardiac death, nonfatal MI and non-MI-related TVR) was not significantly different. However, the reduced need for revascularization due to restenotic events was maintained long-term for DES in the overall population.
However, these were the results of a retrospective analysis of an underpowered monocenter trial. In addition, there have only been a few other retrospective subgroup analyses or registries that have compared BMS and DES in large vessels [7,11,12]. To further examine this hypothesis in an adequately powered, prospective, international multicenter trial, the BASKET-Prospective Validation Examination (BASKET-PROVE) trial was initiated in 2007. The aim of this study was to test the hypothesis that in large native vessel stenting, first-generation DES provide only a small reduction in TVR and may increase late cardiac death/MI rate when compared with BMS. Furthermore, the goal was to evaluate the benefit–risk ratio for second-generation DES [13].
BASKET-PROVE
▪ Design
Between March 2007 and May 2008, patients were screened for enrollment in 11 centers in four different European countries. Patients were eligible for the BASKET-PROVE study if they required a stent larger than 3 mm in diameter, irrespective of the indication for PCI. Patients were randomly assigned in a 1:1:1 fashion to receive a first-generation sirolimus-eluting stent (SES; Cypher Select), a BMS (Vision) or a secondgeneration everolimus-eluting stent (EES; Xience V®, Abbott Vascular). Exclusion criteria were cardiogenic shock, in-stent restenosis, stent thrombosis, unprotected left main coronary artery or a bypass graft to be stented, planned surgery within the next 12 months, increased risk of bleeding or oral anticoagulation, vessel diameter above 4 mm or suspected noncompliance with long-term dual antiplatelet therapy. Clinical follow-up was performed after 12 and 24 months with specifically designed questionnaires. Repeat angiography was only performed if clinically indicated. All patients received a dual antiplatelet therapy with aspirin and clopidogrel for 12 months.
The primary end point was cardiac death or nonfatal MI at 24 months. Comparisons were performed between the BMS group and the two DES groups, as well as between the two DES groups. The main secondary end points were non-MI-related TVR as an efficacy measure, and late cardiac death and nonfatal MI as a safety end point. Late events were defined as those occurring between 7–24 months. Further secondary end points were death from any cause, cardiac death, nonfatal MI, stent thrombosis and the composite end point of nonfatal MI, cardiac death and TVR. Statistical analysis was performed according to the intention-to-treat principle.
▪ Results
A total of 2314 patient were randomly assigned to receive a SES (n = 775), BMS (n = 765) or an EES (n = 774). The 2-year follow-up was completed for 97% of patients. There were no significant differences between the three stent groups with regard to clinical, target vessel and procedural baseline characteristics. Two-thirds of all patients had an acute coronary syndrome and one-half had an ST-elevation infarction. The majority of patients (76%) were treated for off-label indications regarding DES use. The average total stent length per patient was approximately 30 mm.
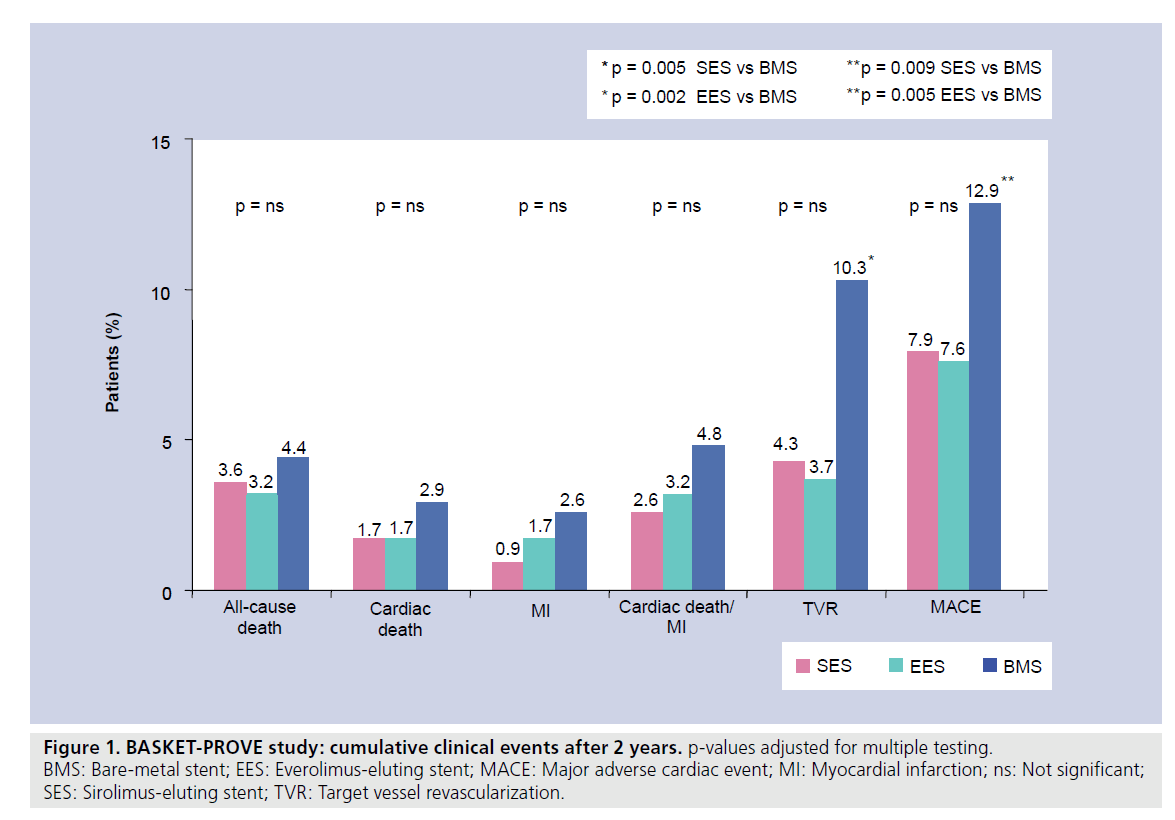
Regarding the primary composite end point of cardiac death or nonfatal MI, there were no significant differences between either of the DES groups and the BMS group or between the two DES groups. There were also no significant differences when cardiac death and nonfatal MI were analyzed separately. Strikingly, there were no significant differences between the groups in either early or late rates of stent thrombosis. However, there was a significant reduction of TVR in the DES groups when compared with the BMS group (SES 4.3% vs EES 3.7% vs BMS 10.3%; Figure 1). Consecutively, the composite end point of MACE was equally reduced in the DES groups as compared with BMS (Figure 1) [13].
Figure 1. BASKET-PROVE study: cumulative clinical events after 2 years. p-values adjusted for multiple testing.
BMS: Bare-metal stent; EES: Everolimus-eluting stent; MACE: Major adverse cardiac event; MI: Myocardial infarction; ns: Not significant;
SES: Sirolimus-eluting stent; TVR: Target vessel revascularization.
Conclusion
In the BASKET-PROVE trial, concerns about late safety problems with DES in large coronary arteries could not be confirmed. On the contrary, there was a trend towards a reduction of late cardiac death or nonfatal MI in DES-treated patients. Both DES showed superior efficacy in large vessels when compared with the BMS since TVR was reduced by over 50%. There was no significant difference in either safety or efficacy between the second-generation EES and the first generation SES at 2 years of follow-up [13].
These findings of the BASKET-PROVE study implicate that in patients in need of large native coronary artery stenting, DES may be used without evidence of increased late cardiac events or late-stent thrombosis. BMS may still be used since they showed similar rates of cardiac death and nonfatal MI as DES. However, one has to take into consideration a higher rate of TVR for BMS. The performance of both DES (first and second generation) appears to be similar.
In concordance, other studies have also demonstrated a superior performance of DES with respect to BMS, even in high-risk populations such as diabetic patients. Several randomized trials comparing SES to BMS in patients with diabetes have all shown significant reductions in in-segment late loss and target lesion revascularization [14–17]. Furthermore, a collaborative network analysis that included 3850 patients demonstrated no significant difference in mortality between SES, BMS and paclitaxel-eluting stents in the treatment of diabetic patients who received dual antiplatelet therapy for over 6 months [18]. Likewise, in patients with chronic renal failure, another population with increased risk for restenosis, it could be shown that the effectiveness of SES in decreasing restenosis compared with BMS was preserved and rates of death and MI were not adversely affected [19]. Similarly, in patients with two- or threevessel disease, the Arterial Revascularization Therapies Study II demonstrated a significant reduction in repeat revascularizations [20]. However, randomized data are scarce in this population and data on the use of DES in patients with multivessel disease are mainly derived from subgroup analyses, registries and nondedicated trials.
Another concern regarding DES is that although most studies point to a favorable result regarding TVR, there have been some reports that describe a delayed intimal hyperplasia (referred to as late catch-up phenomenon) beyond 6 months for DES [21–25]. This phenomenon could diminish the early benefit and needs further investigation.
Future perspective
The field of DES technology is constantly evolving. New stent designs have been developed with different metal alloys replacing stainless steel, thinner stent struts, more flexible designs and biocompatible coatings for BMS, newer and biodegradable polymers, newer drugs with lower drug doses or drug kinetics for DES and, most recently, totally absorbable stents and antibodycoated stents [26,27]. Although some of the newest generation stents have received market approval, data on the efficacy and safety of these nextgeneration stents with the potential advantage of lower ischemic event rates are still scarce.
The BASKET-PROVE platform provides an excellent opportunity to test and compare such new stents in unselected patients in large long-term clinical outcome settings. The recently started BASKET-PROVE II trial will compare a new biolimus-eluting DES with a bioabsorbable polyactic acid coating (Nobori®) with the currently most widely used everolimus- eluting DES (Xience V®) and the newest generation BMS with thin cobalt–chromium struts and a biocompatible silicone carbide layer (ProKinetic®). Inclusion and exclusion criteria will be the same as in the BASKET-PROVE trial. However, owing to the dual antiplatelet regime with aspirin and prasugrel in all patients, those with a history of stroke must be excluded as well.
This study will test the superiority of DES over a newest-generation BMS with biocompatible coating and the noninferiority of a DES with a biodegradable polymer to a DES with a durable polymer. In addition, the results will allow a comparison of a dual antiplatelet therapy with aspirin and prasugrel against aspirin and clopidogrel as assessed by a historical comparator cohort in an all-comer population. In conclusion, BASKET-PROVE II will test the newestgeneration stents on the market in daily practice and will therefore provide insights into the efficacy and safety of current stent designs in an all-comer population.
With further improvements expected in implantation techniques, stent designs, drugcoatings and platelet-inhibition regimes, coronary artery stenting will become increasingly safe and effective during the coming years. The final goal of an individual targeted use of specific devices for each patient becomes more and more realistic.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
BASKET (2005)
▪ Drug-eluting stents (DES) are effective in reducing major adverse cardiac events by 44% when compared with bare-metal stents (BMS).
▪ Higher initial stent costs for DES are not compensated for by reduction in event-related follow-up costs.
▪ In subgroups of high-risk elderly patients with three-vessel disease, treatment of multiple segments, long-treated segments or small vessels, the use of DES was more cost effective.
BASKET-LATE (2007)
▪ After discontinuation of clopidogrel, the rate of cardiac death/myocardial infarction (MI) was significantly higher in patients treated with a DES when compared with a BMS (4.9 vs 1.3%).
▪ The net clinical benefit of DES after discontinuation of clopidogrel appeared to be reduced by increased rates of cardiac death and nonfatal MI, possibly owing to late stent thrombosis, whereas the positive effect on target vessel revascularization was maintained beyond 6 months.
BASKET 18 months & 36 months data analysis (2007, 2009)
▪ Large vessel diameter (≥3 mm) single most independent predictor of late clinical events, most likely due to late-stent thrombosis.
BASKET-PROVE (2010): use of contemporary techniques & stents for large vessels
▪ Late safety problems of DES could no longer be confirmed.
▪ Both DES showed superior efficacy compared with BMS (TVR reduction >50%).
▪ No difference in either safety or efficacy was found between second-generation EES and first-generation SES.
BASKET-PROVE II (prospectively 2014): aims
▪ To evaluate the superiority of DES over a newest-generation BMS with biocompatible coating.
▪ To test the noninferiority of a DES with a biodegradable polymer to a DES with a durable polymer.
▪ Comparison of prasugrel versus clopidogrel in an all-comer population.
▪ First results will be available by the beginning of 2014.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Morice MC, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346, 1773–1780 (2002).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxeleluting coronary stents. N. Engl. J. Med. 356, 998–1008 (2007).
- Kaiser C, Brunner-La Rocca HP, Buser PT et al. Incremental cost–effectiveness of drug-eluting stents compared with a third-generation bare-metal stent in a real-world setting: randomised Basel Stent Kosten Effektivitats Trial (BASKET). Lancet 366, 921–929 (2005).
- Brunner-La Rocca HP, Kaiser C, Bernheim A et al. Cost–effectiveness of drug-eluting stents in patients at high or low risk of major cardiac events in the Basel Stent KostenEffektivitats Trial (BASKET): an 18-month analysis. Lancet 370, 1552–1559 (2007).
- Brunner-La Rocca HP, Kaiser C, Pfisterer M; BASKET Investigators. Targeted stent use in clinical practice based on evidence from the Basel Stent Cost Effectiveness Trial (BASKET). Eur. Heart J. 28, 719–725 (2007).
- Pfisterer M, Brunner-La Rocca HP, Buser PT et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J. Am. Coll. Cardiol. 48, 2584–2591 (2006).
- Steinberg DH, Mishra S, Javaid A et al. Comparison of effectiveness of bare metal stents versus drug-eluting stents in large (> or =3.5 mm) coronary arteries. Am. J. Cardiol. 99, 599–602 (2007).
- Ellis SG, Colombo A, Grube E et al. Incidence, timing, and correlates of stent thrombosis with the polymeric paclitaxel drug-eluting stent: a TAXUS II, IV, V, and VI meta-analysis of 3,445 patients followed for up to 3 years. J. Am. Coll. Cardiol. 49, 1043–1051 (2007).
- Weisz G, Leon MB, Holmes DR Jr et al. Two-year outcomes after sirolimus-eluting stent implantation: results from the Sirolimus-Eluting Stent in de Novo Native Coronary Lesions (SIRIUS) trial. J. Am. Coll. Cardiol. 47, 1350–5 (2006).
- Pfisterer M, Brunner-La Rocca HP, Rickenbacher P et al. Long-term benefit-risk balance of drug-eluting vs. bare-metal stents in daily practice: does stent diameter matter? Three-year follow-up of BASKET. Eur. Heart J. 30, 16–24 (2009).
- Gordon PC, Applegate RJ, Hermiller JB et al. Clinical and angiographic outcomes with an everolimus-eluting stent in large coronary arteries: the SPIRIT III 4.0 mm registry. Catheter Cardiovasc. Interv. 75, 179–186 (2010).
- Akiyama T, Moussa I, Reimers B et al. Angiographic and clinical outcome following coronary stenting of small vessels: a comparison with coronary stenting of large vessels. J. Am. Coll. Cardiol. 32, 1610–1618 (1998).
- Kaiser C, Galatius S, Erne P et al. Drugeluting versus bare-metal stents in large coronary arteries. N. Engl. J. Med. 363, 2310–2319 (2010).
- Kirtane AJ, Ellis SG, Dawkins KD et al. Paclitaxel-eluting coronary stents in patients with diabetes mellitus: pooled analysis from 5 randomized trials. J. Am. Coll. Cardiol. 51, 708–715 (2008).
- Chan C, Zambahari R, Kaul U et al. A randomized comparison of sirolimuseluting versus bare metal stents in the treatment of diabetic patients with native coronary artery lesions: the DECODE study. Catheter Cardiovasc. Interv. 72, 591–600 (2008).
- Baumgart D, Klauss V, Baer F et al. One-year results of the SCORPIUS study: a German multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. J. Am. Coll. Cardiol. 50, 1627–1634 (2007).
- Maresta A, Varani E, Balducelli M et al. Comparison of effectiveness and safety of sirolimus-eluting stents versus bare-metal stents in patients with diabetes mellitus (from the Italian Multicenter Randomized DESSERT Study). Am. J. Cardiol. 101, 1560–1566 (2008).
- Stettler C, Allemann S, Wandel S et al. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ 337, a1331 (2008).
- Garg P, Charytan DM, Novack L et al. Impact of moderate renal insufficiency on restenosis and adverse clinical events after sirolimus-eluting and bare metal stent implantation (from the SIRIUS trials). Am. J. Cardiol. 106, 1436–1442 (2010).
- Serruys PW, Onuma Y, Garg S et al. 5-year clinical outcomes of the ARTS II (Arterial Revascularization Therapies Study II) of the sirolimus-eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. J. Am. Coll. Cardiol. 55, 1093–1101 (2010).
- Nakagawa Y, Kimura T, Morimoto T et al. Incidence and risk factors of late target lesion revascularization after sirolimus-eluting stent implantation (3-year follow-up of the j-Cypher Registry). Am. J. Cardiol. 106, 329–336 (2010).
- Cosgrave J, Corbett SJ, Melzi G et al. Late restenosis following sirolimus-eluting stent implantation. Am. J. Cardiol. 100, 41–44 (2007).
- Hong MK, Mintz GS, Lee CW et al. Late target lesion revascularization after implantation of sirolimus-eluting stent. Catheter Cardiovasc. Interv. 71, 299–303 (2008).
- Rodriguez AE, Maree AO, Mieres J et al. Late loss of early benefit from drug-eluting stents when compared with bare-metal stents and coronary artery bypass surgery: 3 years follow-up of the ERACI III registry. Eur. Heart J. 28, 2118–2125 (2007).
- Claessen BE, Beijk MA, Legrand V et al. Two-year clinical, angiographic, and intravascular ultrasound follow-up of the XIENCE V everolimus-eluting stent in the treatment of patients with de novo native coronary artery lesions: the SPIRIT II trial. Circ. Cardiovasc. Interv. 2, 339–347 (2009).
- Windecker S, Serruys PW, Wandel S et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet 372, 1163–1173 (2008).
- Nakazawa G, Granada JF, Alviar CL et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc. Interv. 3, 68–75 (2010).
▪▪ This patient-based meta-analysis using the four principal Taxus® randomized trials (3445 patients) with a follow-up duration of over 1 year raised concerns about the safety of drug-eluting stents since there were more stent thromboses in the Taxus group beyond 6 months.
▪ The analysis of pooled patient data from five prospective, double-blind, randomized trials of paclitaxel-eluting stent (PES) versus bare-metal stents (n = 3513) demonstrated that treatment with PES compared with treatment with bare-metal stents was safe and effective, resulting in markedly lower rates of target lesion revascularization at 4 years, with similar rates of death, myocardial infarction and stent thrombosis.
▪ The SPIRIT II trial confirmed the noninferiority of second-generation everolimus-eluting stents in terms of in-stent late loss when compared with the PES up to 2-year follow-up.
▪▪ The LEADERS trial demonstrated for the first time that a stent eluting biolimus from a biodegradable polymer represents a safe and effective alternative to a stent eluting sirolimus from a durable polymer in patients with chronic stable coronary artery disease or acute coronary syndromes.