Research Article - Neuropsychiatry (2019) Volume 9, Issue 5
The Complexity of the Factors Increasing the Depressive Symptoms among Patients in the Acute Phase after Stroke
- Corresponding Author:
- Liberacka Donata
Faculty of Medicine
Jagiellonian University Medical College, Kraków, Poland
email: donataliberacka@gmail.com
Abstract
ABSTRACT
Background
Depression is the most prevalent neuropsychiatric disorder among post-stroke patients.Depressive symptoms can be observed already in the first few days following the stroke onset.The aim of this research was to identify specific factors or groups of factors that contribute to
the occurrence of depressive symptoms among patients in the acute phase after ischemic stroke.
Methods and findings
The experimental group consisted of 95 patients after ischemic stroke. The inclusion criteria were strictly specified diagnostic criteria encompassing neuroimaging procedures CT and MRI, physical and neurological examination.The experimental procedure involved neuropsychological cognitive assessment (MMSE, The Clock Drawing Test, selected experimental methods from the Ã…ÂÂÂÂÂÂÂÂÂucki Book), observations, and questionnaires for mood disorders (BDI, HDRS). Depressive symptoms were observed among 57%-69% of the patients in the acute phase after ischemic stroke (dependent on the measure applied). Generalized Regression Model (GRM) was applied to explore relationship between measured variables and the severity of depressive symptoms. A correlation was observed between depressive symptoms measured with HDRS and general cognitive functioning measured with MMSE. Additionally, two other factor groups: 1) gender, consciousness level at the onset, hemisphere damage side, and 2) gender, marital status, hemisphere damage side, were found to be significantly correlated with the severity of depression in the acute phase after
stroke. The specific correlations between these variables are described in this article. However, the analysis did not confirm for instance a simple correlation between depression and the hemispheric localization of stroke.
Conclusion
General, organic and functional risk factors for post-stroke depression do not have an isolated influence on the emotional state of the patients. These factors, from the beginning, are influencing each other. It is therefore necessary to consider the possibility of their complex interactions in the occurrence of post-stroke depressive symptoms from the early stages of diagnosis and rehabilitation
Keywords
Stroke, Post-stroke depression, Risk factors, Acute stroke
Introduction
Depression is one of the most prevalent mental disorders among stroke patients that according to the research, is one of the strongest predictors for decrease in the quality of life [1]. Moreover, post-stroke depression (PSD) is associated with greater disability [2], lower rehabilitation efficacy [3], and higher mortality rates [4,5]. According to the existing research, depressive symptoms can be already observed during the hospitalization period, and the development of those symptoms significantly affects physical recovery [6]. Therefore, it is of clinical importance to investigate the process of depression development in the acute phase after stroke and the factors influencing it.
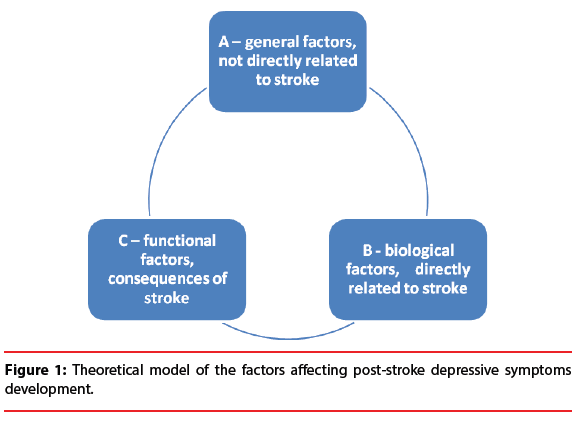
The research regarding risk factors for developing PSD distinguishes three groups of (probable) predictors.
The first, general factors group (A) consists of factors which are directly connected to the stroke. It encompasses demographic factors, for instance gender, age, or education and psychosocial factors such as personality traits, social network, recent adverse life events, or lack of social support [7]. It also includes factors such as personal or family history of mood disorders and comorbidity of other somatic diseases. The second risk factor group (B) involves biological factors that are directly connected to the specificity and consequences of the stroke. The most prevalent in this group is stroke location [8], and the severity of stroke [9], i.e. lesion extent, loss of consciousness.
The third risk factor group (C) for developing depressive symptoms are functional consequences of stroke, such us the independence level in the activities of daily living [10], cognitive impairment or physical disability [11].
The lack of simple and clear relationships between those factors and PSD indicates the complexity of the issue. The analyses need to consider relationships between diverse factors. Despite PSD being the most frequently researched neuropsychiatric consequence of neurological disorders [12], it remains the subject of numerous clinical investigations regarding its mechanisms, clinical image, and risk factors [13,14]. Particularly the development of depressive symptoms in the acute phase after stroke requires more investigation.
Materials and Methods
The research aim
This research aims to distinguish the specific factors increasing the depressive symptoms among patients in the acute phase after stroke.
Participants
The participants were ischaemic stroke patients hospitalized at the Department of Neurology and Stroke Unit of the Ludwik Rydygier Memorial Specialized Hospital in Krakow, Poland. The study was approved by the Bioethical Committe (number 12/KBL/2010, date 26 January 2011). The participants were all in the acute phase after stroke, i.e. within the first 7 days from the onset. The inclusion criteria were: diagnosed ischaemic stroke meeting clinical criteria and confirmed by neuroimagining procedure (CT and/or MRI), and written consent to participate in the neuropsychological assessment.
The exclusion criteria were: consciousness disorders, severe cognitive or speech deficits, prestroke diagnosis and treatment of depression or other psychiatric disorders.
The initial neuropsychological assessment was conducted among 200 participants. The results of 95 participants were included in the final analysis.
Psychological anamnesis
A structured interview was conducted in the form of yes/no questions to enable further statisical analysis.
The interview was designed to inquire about somatic health (permanent diseases, medication, previous strokes or depressive episodes), lifestyle (diet, drugs, and physical activity), psychosocial status (marital status, perceived social support, recent stressful events). Moreover, the information about the psychophysical well-being was collected. The collected data was verified with the medical record.
Mood disorder assessment
The questionnaires used to assess severity of mood disorder were: Hamilton Depression Rating Scale (HDRS), Beck Depression Inventory (BDI), and The Depression Intensity Scale Circles (DISCs). The inclusive approach was implemented in the assessment of depressive symptoms. Therefore, all present symptoms were summed without the analysis of whether they stem from a somatic disease or are the evidence for depressive episode [15].
The Hamilton Depression Rating Scale (HDRS) is a 17-item observational scale for the severity of depressive symptoms. The questionnaire is designed to screen for depression accounting for both mood and self-esteem related symptoms as well as vegetative symptoms. The correlational studies indicate its highly significant correlation with Montgomery-Asberg Depression Rating Scale (MADRS) in the German version of the test (reliability 0.72-0.92; validity 0.81-0.92). The score lower than 10 points was indicative of the lack of depressive symptoms (consistent with the current tendencies to increase the cut-off point for depression diagnosis after brain stroke due to the numerous stroke-related somatic symptoms) [15]. The score below 12 points was considered mild depression, the scores between 13-17 were considered moderate depression, the scores of 18-29 were considered severe depression, and the score above 30 was indicative of very severe depression.
Beck Depression Inventory (BDI) is a 22- item self-report questionnaire. It measures depressive symptoms related to both general psychological condition and self-esteem, as well as vegetative symptoms of depression. The reliability of this questionnaire in Polish research is satisfactory (α=0.87). The score on the BDI scale that was below 12 was not indicative of depressive symptoms. According to the author’s guidelines, scores between 12-26 points were considered mild depression. The scores of 27-49 were indicative of moderately severe depressive symptoms and the outcome between 50-63 was considered very severe depression.
The severity of depressive symptoms was additionally assessed by The Depression Intensity Scale Circles (DISCs). It consists of six circles that are shaded to various degrees. The participants are asked to select the circle that best depicts their mood. This measure is characterized by high, satisfactory diagnostic validity. Its correlation with BDI is 0.66, and correlation with diagnostic criteria of DSMIV is 0.59. This scale is also highly reliable. It is recommended for the assessment of patients with communication problems, for instance can be used in the diagnosis of patients with aphasia or alexia. According to the literature guidelines which were applied in this study, already the figures (Figures 1 and 2) with shaded area are considered as indicative of depressive symptoms [16].
Figure 1:Theoretical model of the factors affecting post-stroke depressive symptoms development.
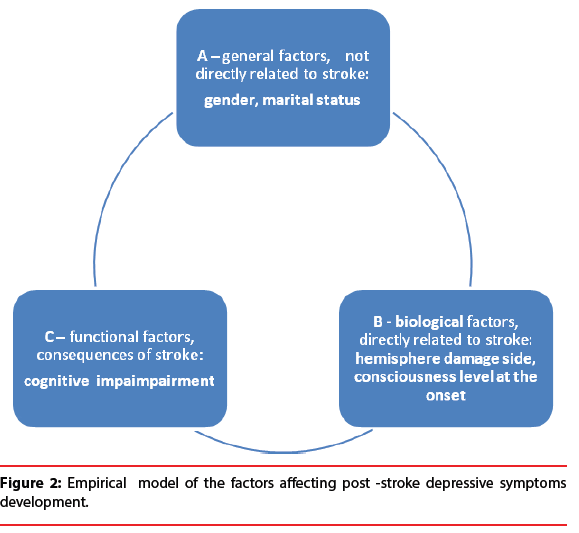
Figure 2:Empirical model of the factors affecting post -stroke depressive symptoms development.
Cognitive function assessment
The cognitive assessment was conducted with Mini-Mental State Examination scale (MMSE) developed by Marshal M. Folstein. It is the most common screening measure for the assessment of cognitive functioning. The maximum score is 30 points and the test assesses 19 different aspects of cognitive performance. The score below 26 points is indicative of cognitive disorder, according to the diagnostic criteria of ICD-10 and DSM-IV. The scale is characterized by high validity and reliability measures and is considered to be very accurate in detecting cognitive impairment. Its test-retest reliability in the period of a few weeks is 0.98. The validity of MMSE as compared with CT outcomes and Wechsler’s test gave over 90% sensitivity and 80% specificity of this method among patients with dementia over the age of 40.
Furthermore, the participants were assessed with the Clock Drawing Test and chosen experimental methods from the Łucki Book for investigating the cognitive functioning of patients with organic brain damage. (Assessing working memory capacity, gnosia, praxia, and executive functions).
Statistical analysis
Following data transformations, statistical analysis was conducted using Statistica 12. The significance level was set at α=0.05. In total, data from 95 observations was analysed.
Generalized Regression Model (GRM) was applied to explore relationship between measured variables and the severity of depressive symptoms. Generalized linear model was applied to Analysis of Covariance (ANCOVA), which involved both qualitative predictors and continuous data. The sigma-restricted parametrization was applied.
Results
The data from 95 participants was analysed. Their average age was 66 years (SD=10.67). There were 37 women and 58 men among the participants. 22 people had primary education, 37 vocational educations, 30 secondary educations, and 6 higher educations. The most relevant demographic data is depicted in Table 1.
| N | % | |
|---|---|---|
| Age at stroke (MD=65.98) 37-50 years 51-70 years 71-87 years |
95 8 50 37 |
100% 8% 53% 39% |
| Gender: Male Female |
58 37 |
61% 39% |
| Education level: Primary education Vocational education Secondary education Higher education |
22 37 30 6 |
23% 39% 32% 6% |
| Marital status: Married Single (bachelor, widowed, divorced) |
69 26 |
73% 27% |
Table 1: Demographic data of stroke population (N=95).
The overview of the observed depressive symptoms levels after the stroke is presented in Table 2. In the outcomes of HDRS scale, the presence of depressive symptoms was observed among 66 participants (69% of the patients) of which 23 were mildly depressed (24%), 23 moderately depressed (24%) and 20 severely depressed (21%). In the BDI scale 52 patients had a score indicative of depression (57%), of which 39 patients showed mild depressive symptoms (43%), 11 moderately severe depressive symptoms (12%), and 2 of the patients were considered severely depressed (2%). Four of the patients were unable to fill out the questionnaire due to alexia. In DISCs scale 72 patients (76%) indicated circles that are interpreted as characteristic for depressive symptoms.
| HDRS | BDI | DISCs | ||||||
|---|---|---|---|---|---|---|---|---|
| No depressive symptoms | Score <10 |
N 29 |
% 31% |
Score <12 |
N 39 |
% 43% |
N 23 |
% 24% |
| Depressive symptoms Mild Moderate Severe |
10-12 13-17 18-29 |
66 23 23 20 |
69% 24% 24% 21% |
12-26 27-49 50-63 |
52 39 11 2 |
57% 43% 12% 2% |
72 | 76% |
Table 2: The severity of depressive symptoms among patients in the acute phase after ischaemic stroke.
In this research the three factors that might influence the severity of depression among patients in the acute phase after stroke were distinguished:
A) General factors
B) Biological factors – directly related to the stroke
C) Functional consequences of stroke
In order to observe the relationship between analysed factors and the severity of depression among patients in acute stroke phase a Generalized Regression Model (GRM) was applied with sigma-restricted parametrization. This method was chosen to account for the specificity of both qualitative and continuous predictors. BDI and DISCs scales will not be further described due to the lack of significant correlations. Table 3 depicts the outcomes of the final model of depression severity measured with HDRS scale. Statistically significant measures were observed in:
| Hamilton - Param. | Hamilton - SD | Hamilton - t | Hamilton – p | Hamilton - Beta (ß) | Hamilton - SD.ß | |
|---|---|---|---|---|---|---|
| Intercept | 19.50888 | 2.664993 | 7.32042 | 0.000000 | ||
| MMSE - general | -0.27999 | 0.107094 | -2.61444 | 0.010532* | -0.254092 | 0.097188 |
| Gender martial status hemisphere damage side | -1.68205 | 0.660412 | -2.54696 | 0.012625* | -0.273748 | 0.107480 |
| Gender no consciousness loss hemisphere damage side | -2.36865 | 0.661081 | -3.58299 | 0.000559* | -0.385303 | 0.107537 |
| The SS test for the full model (the analysis of 95 patients) | ||||||
| Multiple- R | Multiple- R2 | Corrected - R2 | F | p | ||
| Hamilton | 0.428030 | 0.183210 | 0.155045 | 6.504842 | 0.000506* | |
Table 3: Generalized Regression Model (GRM).
I. Cognitive functioning level measured with MMSE
II. Extracted clusters of variables:
A. Gender, no conciousness loss at the stroke onset, hemisphere damage side
B. Gender, martial status, hemisphere damage Sid
The obtained model allows to explain around 18% of the variability of the dependent variable, that is severity of depression symptoms among patients in the acute phase after ischaemic stroke. The interpretation of the estimated value of the respective parameters allows to conclude that increasing the score of MMSE by 1 results in a decrease in Hamilton scale by 0.28% (all other variables remained constant). A mild, however, significant relationship between general cognitive performance and emotional functioning was observed in correlation matrix (r=-0.227542). The greater severity of depression symptoms observed in HDRS scale is correlated with lower cognitive performance confirmed with MMSE test among patients in the acute phase after stroke (Table 4).
| Mean | SD | MMSE - general | Hamilton | |
|---|---|---|---|---|
| MMSE - general | 24.28191 | 5.545297 | 1.000000 | -0.227542* |
| Hamilton | 12.50000 | 6.176177 | -0.227542* | 1.000000 |
Table 4: The correlation between the severity of depression symptoms among patients in the acute phase after ischemic stroke (HDRS) and a general cognitive performance (MMSE).
The relationship between the severity of depression and the clusters of variables that are present in the model is complex and therefore depicted in the form of Figures presented below. The visual representation below demonstrates that the relationship between the severity of depression symptoms and the side of hemisphere damage during the stroke is ambiguous and depends on coexistence of diverse variables.
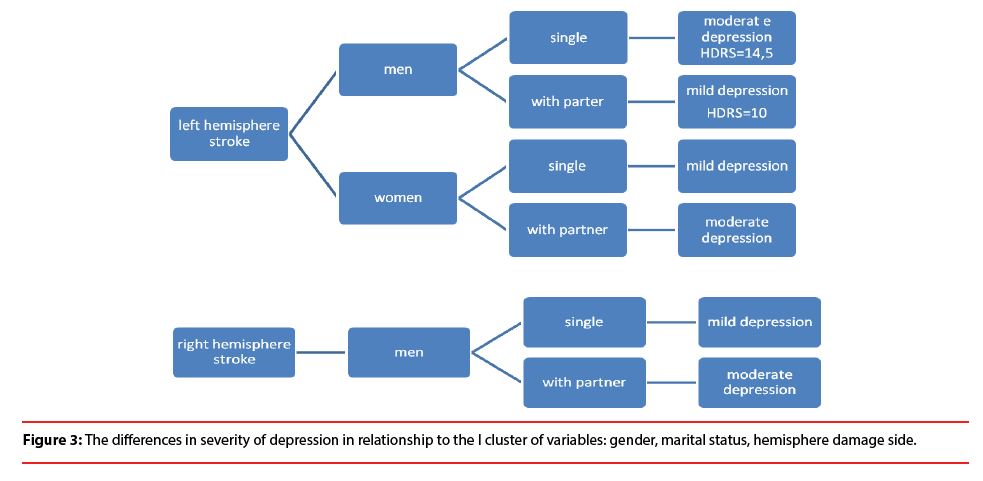
The analysis showed the difference in severity of depression among patients in the acute phase after stroke depending on the I cluster of variables, that is: gender, marital status, hemisphere damage side. The results demonstrate that single men with stroke localized in left hemisphere are more depressed (HDRS=14.5) than married men (HDRS=10). However, married women with left-hemisphere lesion show more severe depression than divorced, single, or widowed women. Similar relationship is observed among men with stroke localized in right hemisphere. In case of right hemisphere stroke no significant differences were observed among women (Figure 3).
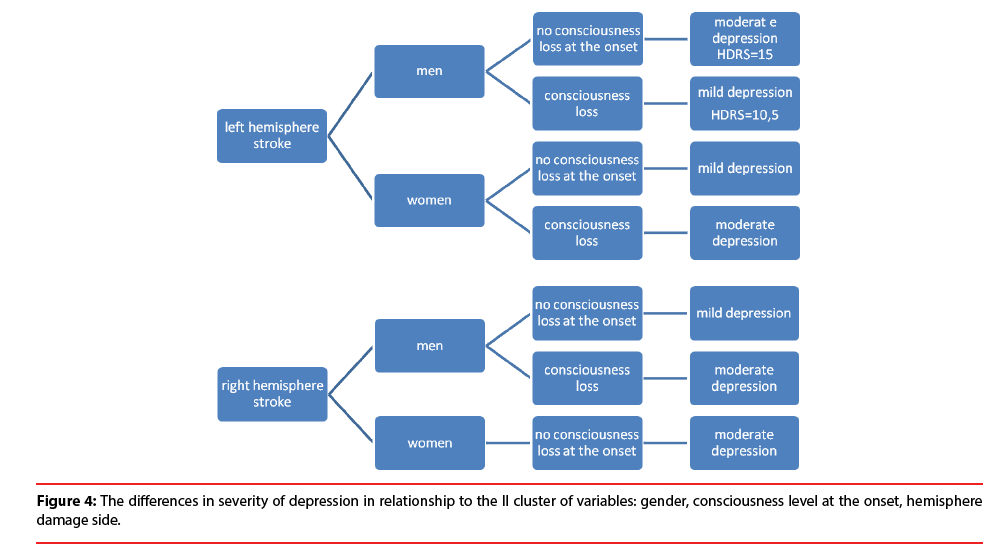
Further results show the difference (Figure 4) between the severity of depressive symptoms among patients in the acute phase after stroke and the II cluster of variables: gender, consciousness level at the onset, hemisphere damage side. Men with the left hemisphere stroke are characterized with more severe depression (HDRS=15), when they remained conscious at the onset of stroke. On the other hand, no consciousness loss at the onset is related to the milder depression (HDRS=10,5) among men with stroke lesion located in the right hemisphere. Moreover, women with stroke lesion located in their left hemisphere are more depressive when they lost their consciousness at the onset. On the other hand, remaining conscious at the onset is related to higher depression level among women with stroke lesion located in right hemisphere. The described relationships are depicted in Figure 4.
Discussion
The results obtained in this study are in line with the existing reports on early occurrence of depressive symptoms among patients after the brain stroke [6]. the analyses indicate that the symptoms related to the development of emotional disorders are present among 57- 59% patients within 2-7 days after the stroke onset. The differences in scores between applied scales result from the difficulties in unequivocal assessment of depressive symptoms in organic diseases. Therefore, the diagnostic process requires considering several crucial factors such as the assessment of somatic depressive symptoms [10], disorders of consciousness and anosognosia [17], In the current study, the limited criticism of the patients towards their own mood disturbances and the difficulties in verbalizing emotional states were observed to affect the depression assessment. The increase in severity of symptoms indicating depression was present among over half of the participants, in both self-report and observational scales. These observations are in line with some of the existing research [18], which indicates that in some of the patients those symptoms will progress into major depression episode that will likely interrupt the treatment and rehabilitation process. The results of this research demonstrate that the assessment of depressive symptoms in the acute phase after stroke is possible, similarly to the conclusion of Karamchandani and the colleagues. Thus, it is implicated that early diagnostic and therapeutic procedures are justified and necessary among the brain stroke patients.
The relationship between cognitive impairment and severity of depression in the acute phase after stroke was demonstrated in the analyses, which is consistent with some of the existing research [19]. However, the observed relationship was very mild and therefore it is difficult to assess its direction. In the literature, cognitive impairment is considered as both the risk factor for the afterstroke depression [9] as well as the derivative and crucial clinical factor of the depression itself. Nonetheless, the presence of that relationship yields several clinical conclusions. It emphasizes the importance of extending the diagnostic process whenever either of the related variables is observed, which in turn increases the effectiveness of diagnosis and then further improves the treatment and rehabilitation of patients in the acute phase after stroke.
The results did not demonstrate simple relationships or single predictors for depressive symptoms in the acute phase after brain stroke. However, the analyses led to distinguishing clusters that encompass several variables. The differences in severity of depression were observed in relationship to the variables: gender, martial status, hemisphere damage side (cluster 1). The differences were also observed in the relationship between severity of depression and the variables: gender, no conciousness loss at the stroke onset, hemisphere damage side (cluster 2). The obtained results are difficult to compare with existing research as they indicate a considerable complexity of the relationship between the observed variables. Thus, they require the assumption that the risk factors do not act in isolated manner but rather influence each other. Therefore, careful consideration of the correlations between general factors, that are indirectly related to the stroke, as well as organic and functional factors, directly dependent on the stroke is required to draw any conclusions.
The hypothesis of the relationship between hemisphere damage side and the after-stroke depression is frequently considered in the literature. However, majority of the current research does not demonstrate that relationship [10]. Nevertheless, some of the research indicates higher severity of depression in patients with left hemisphere damage in the acute stroke phase [20]. Other research, on the other hand, demonstrates that in the later stage –several months following the onset-the symptoms of depression increase in severity in patients with right hemisphere stroke [21]. these relationships were considered in the literature relatively early and consequently supported in research on male patients after brain stroke. This study further indicates that the differences between left and right hemisphere damage are indeed present, however they should be considered in a broader view accounting for other variables, such as patients’ gender.
The relationship between after-stroke depression and gender appears to be equally complex. The majority of research considers the severity of depression in relation to female gender [10]. Among males the depressive reaction tends to occur later, even 18 months after the stroke onset [22]. The results of this study indicate the differences in depression severity dependent on gender, lesion location and one other factor. However, among male patients the differences are present regardless of lesion location and in females the differences were observed only in case of the left hemisphere lesions. The relationship between left hemisphere stroke and depression in women was previously discussed [23]. It was also indicated that depression is more frequent among men at the age between 51-60 as compared to women of the same age group. It was argued that the after-stroke depression among men is often related to the strong feeling of guilt that is associated with the perceived responsibility for socioeconomical situation and the social and financial status of the family related to male earnings.
The results of this study indicate two possibilities for how being married is related to the severity of depression in the acute stroke phase. The fact of having a partner may either increase or decrease the depressive symptoms in the patients, which is perhaps related to the perceived involvement and role in the relationship. Marriage can either act as a buffer for potential mood disturbances or create an environment for grieving lost health demonstrated as depressive symptoms. The fact of having a partner can also increase the severity of depression due to the feelings of responsibility for another person or for the socioeconomical situation of the family which is consistent with the findings of Angelori and Chakraborty Men and women may benefit marriage differently depending on the coping style. The research supports a general mechanism which indicates that women use strategies directed at emotions and social support, while men focus on goal-oriented strategies [24,25]. In the future research with stroke patients, it would be worth consideration to investigate the relationship between coping strategies at use and the availability of the source of support such as having a partner or another close member of the family.
The results of this study also indicate a relationship between the state of consciousness at the onset of stroke and the depression severity in the acute phase of stroke. The state of consciousness at the onset is one of the variables indicating severity of stroke The recent research also underlines the relationship between the severity of depressive symptoms early after the onset of brain stroke and the severity of stroke itself and of the disabilities that follow [26]. In this research the discussed relationship is not that strong which might be due to the mediating influence of the consequences of the disease itself such as increase of dysfunction and disability. These factors should be considered in the future research.
Conclusion
The results of this study contribute to the understanding of depression at the early stage following the brain stroke. They indicate the possibility of examining depressive symptoms within the first 2-7 days of hospitalization. The analysis suggests the need to rethink the understanding of the factors co-occurring with increased depressiveness in the acute phase after brain stroke. The risk factors discussed in the literature appear to influence each other rather than act in an isolated manner. The results of this study demonstrate that the symptoms of depression among patients after brain stroke are influenced by:
– General factors, not directly related to the stroke i.e. gender, marital status,
– Organic factors, directly related to the stroke i.e. lesion location, no loss of consciousness at the onset of stroke,
– Functional factors, i.e. cognitive functioning following the stroke.
The results obtained in this study are hoped to inspire further research that will take a more comprehensive approach toward the examination of patients and their biological and psychosocial functioning. The analysis of these results, however challenging, allows a new perspective at this undoubtedly complex relationship. Distinguishing between complex risk factors allows to improve the understanding of the underlying mechanisms behind the development of depressive symptoms in the acute phase after brain stroke. Thus, it contributes to the improvements in prevention as well as early diagnosis and treatment.
References
- Žikić TR, Divjak I, Jovićević M, et al. The effect of post stroke depression on functional outcome and quality of life. Acta. Clinica. Croatica 53(3), 294-301 (2014).
- Willey JZ, Disla N, Moon YP, et al. Early depressed mood after stroke predicts long-term disability: The Northern Manhattan Stroke Study (NOMASS). Stroke. J. Cereb. Circul 41(9), 1896-1900 (2010).
- Farner L, Wagle J, Engedal K, et al. Depressive symptoms in stroke patients: A 13month follow-up study of patients referred to a rehabilitation unit. J. Aff. Disord 127(1-3), 211-218 (2010).
- Williams LS, Sushmita SG, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am. J. Psychiatr 161(6), 1090-1095 (2004).
- Bartoli F, Di Brita C, Crocamo C, et al. Early post-stroke depression and mortality: Meta-analysis and meta-regression. Front. Psychiatry 9(1), 1-7 (2018).
- Matsuzaki S, Hashimoto M, Yuki S, et al. The relationship between post-stroke depression and physical recovery. J. Affec. Disord176(1), 56-60 (2015).
- Lewin A, Jöbges M, Werheid K. The influence of self-efficacy, pre-stroke depression and perceived social support on self-reported depressive symptoms during stroke rehabilitation. Neuropsychol. Rehabil 23(4), 546-562 (2013).
- Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. J. Neuropsychiatr. Clin. Neurosci 15(4), 422-430 (2003).
- Hackett ML, Anderson CS. Predictors of depression after stroke: A systematic review of observational studies. Stroke 36(10), 2296-2301 (2005).
- Hadidi N, Treat-Jacobson DJ, Lindquist R. Poststroke depression and functional outcome: a critical review of literature. Heart. Lung 38(2), 151-162 (2009).
- Kutlubaev MA, Hackett ML. Part II: predictors of depression after stroke and impact of depression on stroke outcome: An updated systematic review of observational studies. Int. J. Stroke 9(8), 1026-1036 (2014).
- Byars JA, Jorge RE. Neuropsychiatric sequelae of stroke: Issues and implications for clinicians. Psychiatric. Times 32(3), 1-5 (2015).
- Hackett ML, Köhler S, O'Brien JT, et al. Neuropsychiatric outcomes of stroke. Lan. Neurol 13(5), 525-534 (2014).
- Towfighi A, Ovbiagele B, El Husseini N, et al. Postroste depression: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 48(1), e30-e43 (2016).
- Wichowicz H, Kozera G, Gąsecki D, et al. Diagnostic difficulties associated with post-stroke depression: A case report. Psychogeriatria. Polska 3(4), 211-220 (2006).
- Turner-Stokes L, Kalmus M, Hirani D, et al. The Depression Intensity Scale Circles (DISCs): A first evaluation of a simple assessment tool for depression in the context of brain injury. J. Neurol. Neurosur. Psychiatry 76(9), 1273-1278 (2005).
- Biran I, Chatterjee A. Depression with anosognosia following a left subcortical stroke. Clin. Neurol. Neurosur 105(2), 99-101 (2003).
- Johnson JL, Minarik PA, Nyström KV, et al. Poststroke depression incidence and risk factors: An integrative literature review. J. Neurosci. Nurs 38(4), 316-327 (2006).
- Terroni L, Sobreiro MF, Conforto AB, et al. Association among depression, cognitive impairment and executive dysfunction after stroke. Dement. Neuropsycho 6(3), 152-157 (2012).
- Jiang XG, Lin Y, Li YS. Correlative study on risk factors of depression among acute stroke patients. Eur. Rev. Med. Pharmacol. Sci 18(9), 1315-1323 (2014).
- Wei N, Yong W, Li X, et al. Post-stroke depression and lesion location: A systematic review. J. Neurol 262(1), 81-90 (2015).
- Berg A, Palomäki H, Lehtihalmes M, et al. Post stroke depression: An 18-month follow-up. Stroke 34(1), 138-143 (2003).
- Paradiso S, Robinson RG. Gender differences in post stroke depression. J. Neuropsychiat. Clini. Neurosci. 10(1), 41-47 (1998).
- Melendez JC, Mayordomo T, Sancho P, et al. Coping strategies: Gender differences and development throughout life span (English). Span. J. Psychol 15(3), 1089-1098 (2012).
- Lee HS, Mason D. Cultural and gender differences in coping strategies between Caucasian American and Korean American older people. J. Cross. Cult. Gerontol 29(4), 429-446 (2014).
- Karakus K, Kunt R, Memis CO, et al. The factors related to early-onset depression after first stroke. Psychogeriatrics 17(1), 414-422 (2017).