Short Communication - International Journal of Clinical Rheumatology (2017) Volume 12, Issue 4
The correlation between blood lead levels and knee osteoarthritis: a preliminary Egyptian study
- *Corresponding Author:
- Rania Hamed Abdel-Rahman
Mansoura University, Egypt
E-mail: raniahamed73@gmail.com
Abstract
Background: Lead is a common ubiquitous environmental pollutant. The increasing prevalence of knee Osteoarthritis (OA) among young patients is suggestive of a possible contribution of environmental factors such as lead. This work aimed to assess the correlation between blood lead and hyaluronic acid levels and degree of knee osteoarthritis among a group of young adult Egyptian patients. Methods and findings: This study was conducted on 90 patients aged 20-50 years old, presented with knee OA. Clinical and radiological assessments of OA severity were performed. Blood samples were collected for estimation of lead and hyaluronic acid (HA) levels. The mean age of the study participants was 36.88±8.96 years. The median blood lead level was 6.35 μg/dl. There was a significant correlation between blood lead level and the severity of knee osteoarthritis either clinically or radiologically. The median serum HA level was 46.95 ng/ml (min-max:10.4-184 ng/ml). A significant positive correlation was found between blood lead and HA levels. Conclusions: it could be concluded that lead represents a potential contributing environmental risk factor for osteoarthritis. Further investigation is warranted to clarify the exact mechanisms involved in the pathogenesis of this disease in relation to various environmental pollutants including lead.
Keywords
lead, knee osteoarthritis, hyaluronic acid
Introduction
Lead is a common ubiquitous environmental pollutant distributed all over the world [1]. Cartilage, chondrocytes and osteoblasts seem to be the target cells for toxic effects of lead being accumulated in bones overtime leading to many skeletal adverse effects e.g. osteoporosis, impaired fracture healing, decreased walking speed, etc. [2,3].
Knee Osteoarthritis (OA) is one of the most frequent chronic debilitating skeletal diseases. [4] It is characterized by cartilage degradation and bony overgrowth. Hyaluronic Acid (HA) is one of the useful inflammatory biomarkers signaling the presence and severity of osteoarthritis [5].
It was claimed that there is an increased prevalence of knee OA in the next 10 years in young American adults aged 35-54 years [6] in addition to its earlier appearance among Arab patients in the late 30s and early 40s with a different clinical pattern and a greater severity in comparison to Western world patients [7].
Geographical variation in knee OA prevalence with the increasing number of younger patients [8] is suggestive of a possible contribution of environmental factors. Although some risk factors for knee OA are known, such as advancing age and obesity, the disease process remains poorly understood. Accordingly, the possibility of involvement of metal exposure as an environmental risk factor for OA has been considered [9]. There is insufficient data regarding the relationship between levels of blood lead and knee osteoarthritis in young age.
Hence, the aim of this work is to assess the correlation between the degree of knee osteoarthritis and levels of blood lead and serum hyaluronic acid among a group of young adult Egyptian patients who are environmentally exposed to this toxic metal.
Study design and patients
The present cross-sectional study was approved by the Institutional Review Board of Faculty of Medicine, Mansoura University. An informed written consent was obtained from all patients before enrollment in the research.
Inclusion criteria
Patients aged 20-50 years old, presented with symptomatic or radiographic knee OA at the Rheumatology Clinic, Mansoura University Hospital, in the period between June 2014 to February 2015. Study participants were divided into three groups according to their age (20-30, 30-40 and 40-50 years).
Exclusion criteria
Risk factors for OA or those conditions that may alter the levels of hyalouronic acid are excluded such as obese patients (BMI>25) or those suffering from diabetes, liver, kidney or autoimmune disease, thyroid and parathyroid dysfunction and those passing through bone turnover periods such as postmenopausal and pregnant women or receiving medications that interfere with calcium absorption (e.g. iron, tetracycline, etc.) and lead-exposed workers.
Methods
• Sociodemographic data collection, lifestyle habits such as smoking and alcohol consumption, medical history and past history of trauma.
• Clinical assessment: A set of standardized questionnaire (Western Ontario and McMaster Universities Arthritis Index: WOMAC) covering items of pain, stiffness and physical functioning limitation was applied [10].
• Radiological assessment: Anteroposterior knee X-ray was done. Then, grading of OA through Kellgren-Lawrence system which includes five severity grades [11] was performed by a blinded investigator.
• Lead level determination: One ml blood was drawn from each participant into a lead free heparinized polypropylene tube. All samples were stored at -20°C until analysis. Digestion of blood samples was done by acid wet digestion according to the method of Yahaya et al. [12] Samples were analyzed at the central laboratory in Tanta University using Optima™ 7000 DV ICP-OES (Integrated Coupled Plasma-Optical Emission Spectroscopy) apparatus–Perkin Elmer Company, Germany. Wavelength: 220-353 nm and detection limit: 0.14 μg/dl.
• Hyaluronic Acid (HA) level estimation: Three ml blood was drawn, left for serum-coagulation and centrifuged at 3500 rpm. Estimation of HA level was done using Human HA ELISA Kit (SRB/ shanghai-Sunred Biotechnology Comp., Ltd., Catalogue No. 201-12-1375) according to the manufacturer manual. At Biochemistry Department, Faculty of Medicine using ELISA-Reader Sun Rise TECAN 10018 apparatus at wave-length 450 nm. Assay range: 4-600 ng/ml.
Statistical analysis
Data was analyzed using the computer program SPSS (Statistical Package for Social Sciences) version release 16.0 to obtain descriptive statistics including frequency analysis. One way ANOVA, Chi-square, Kruskal-Wallis or Mann-Whitney test was performed for statistical comparison. Correlation was evaluated using Spearman correlation test, algorithmic transformation and linear regression analysis. P value is considered significant at ≤0.05.
Results
The current study included 90 patients (20- 50 years) who have symptomatic or radiographic knee OA. Sociodemographic data and results of clinical and radiological assessment of knee OA are shown in Table 1. All patients were non-smokers and non-alcoholic. All other data were irrelevant.
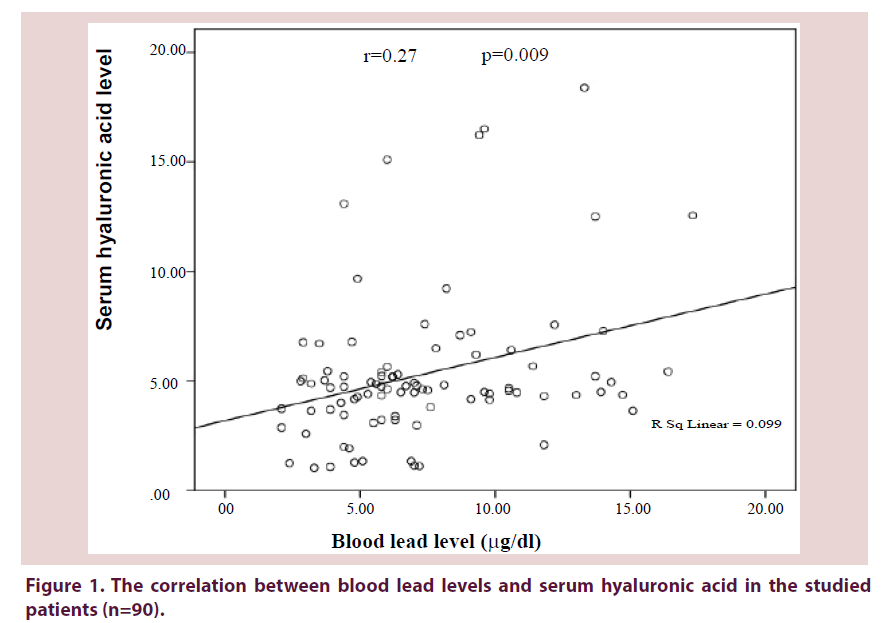
The blood lead and serum hyaluronic acid levels in relation to age and gender are illustrated in Table 2. The median blood lead level was 6.35 μg/dl (min-max: 2.1-17.3 μg/dl). Figure 1 shows that there was a significant weak direct correlation between the levels of blood lead and serum hyaluronic acid (r=0.27, P=0.009).
| Parameter | Men (45) | Women (45) | Total (n=90) | Significance |
|---|---|---|---|---|
| Age (years) Mean ± SD | ||||
| 20-29.9 | 26.66 ± 2.68 | F=369.8 P ≤ 0.001* | ||
| 30-39.9 | 36.7 ± 3.1 | 36.88 ± 8.96 | ||
| 40-50 | 47.3 ± 3 | |||
| Residence | N | n | n (%) | |
| Urban | 23 | 33 | 56 (62.3%) | χ2=2.93 P=0.23 |
| Rural | 22 | 12 | 34 (37.7%) | |
| Occupation | ||||
| Professionals and semiprofessionals | 16 | 18 | 34 (38%) | χ2=10.364 P=0.24 |
| Manual workers | 4 | 1 | 5 (24.4%) | |
| Salesmen and traders | 8 | 2 | 10 (21.1%) | |
| Farmers | 17 | 2 | 19 (11%) | |
| Housewives | - | 22 | 22 (5.5%) | |
| WOMAC Osteoarthritis severity index | n | % | χ2=10.27 P=0.114 | |
| Mild | 37 | 41.1 | ||
| Moderate | 39 | 43.3 | ||
| Severe | 13 | 14.5 | ||
| Extreme | 1 | 1.1 | ||
| Kellgren-Lawrence Grading system | ||||
| Grade 0 | 45 | 50% | P=0.08 χ2=11.3 | |
| Grade 1 | 30 | 33.50% | ||
| Grade 2 | 12 | 13.50% | ||
| Grade 3 | 3 | 3% | ||
| n=number, F for one way ANOVA, χ2 for chi-square test. *Significant at P ≤ 0.05 | ||||
Table 1. Statistical results of sociodemographic data, clinical and radiological assessment of the studied knee osteoarthritis patients (n=90).
| Blood Lead level (µg/dl) | Serum hyaluronic acid level (ng/ml) | |||
|---|---|---|---|---|
| Age in years | Median | Min-max | Median | Min-max |
| Overall (20-50) | 6.35 | 2.1-17.3 | 46.95 | 10.4-184 |
| 20-29.9 | 5.9 | 2.1-14.7 | 44.6 | 10.4-76.1 |
| 30-39.9 | 8.5 | 2.8-15.1 | 49.6 | 13.4-162.3 |
| 40-50 | 5.9 | 2.9-17.3 | 47.5 | 10.8-184 |
| Statistical significance | Kruskal-Wallis test | P=0.03* | P=0.18 | |
| Gender | ||||
| Women | 5.8 | 2.1-17.3 | 48.8 | 10.4-184 |
| Men | 7 | 2.1-15.1 | 44.9 | 10.8-162.3 |
| Statistical significance | Kruskal-Wallis test | P=0.37 | Mann Whitney test | P=0.21 |
| *P value significant at <0.05 | ||||
Table 2. Blood lead and serum hyaluronic acid levels in the studied knee osteoarthritic patients according to the age group and gender (n=90).
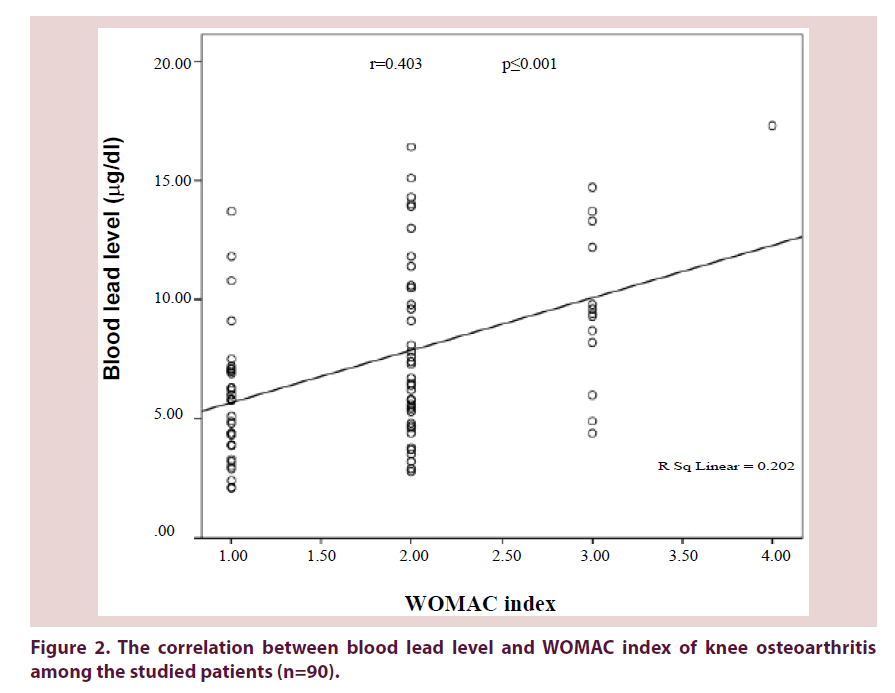
Blood lead levels were significantly correlated with both the degree of symptomatic knee osteoarthritis as seen in Figure 2 (r=0.403, P ≤ 0.001) and radiological grading of OA severity as seen in Table 3 (r=0.643, P ≤ 0.001).
Figure 2. The correlation between blood lead level and WOMAC index of knee osteoarthritis among the studied patients (n=90).
| Items | #Blood lead levels (µg/dl) according to age groups | |||
|---|---|---|---|---|
| 20-29.9 years | 30-39.9 years | 40-50 years | ||
| Kellgren Lawrence grading system Kruskal-Wallis test | 0 | 4.9 (2.1-7.5)a | - | - |
| 1 | 8.6 (3.2-14.7)a | 11.4 (5.8-15.1)c | 5.8 (3.5-7.3)e | |
| 2 | 9.3 (9.3-9.3)a | 9.5 (8.2-13.9)c | 9.8 (6-16.4)de | |
| 3 | - | - | 13.3 (4.4-17.3)de | |
| P=0.01* | P=0.001* | P=0.02* | ||
| N.B. #Blood lead levels are expressed as median (min-max) *Significant at P ≤ 0.05 Similar letters denote significant difference in between groups |
||||
Table 3. Association between the levels of blood lead and Kellgren-Lawrence grading system of knee osteoarthritis in the studied patients (n=90).
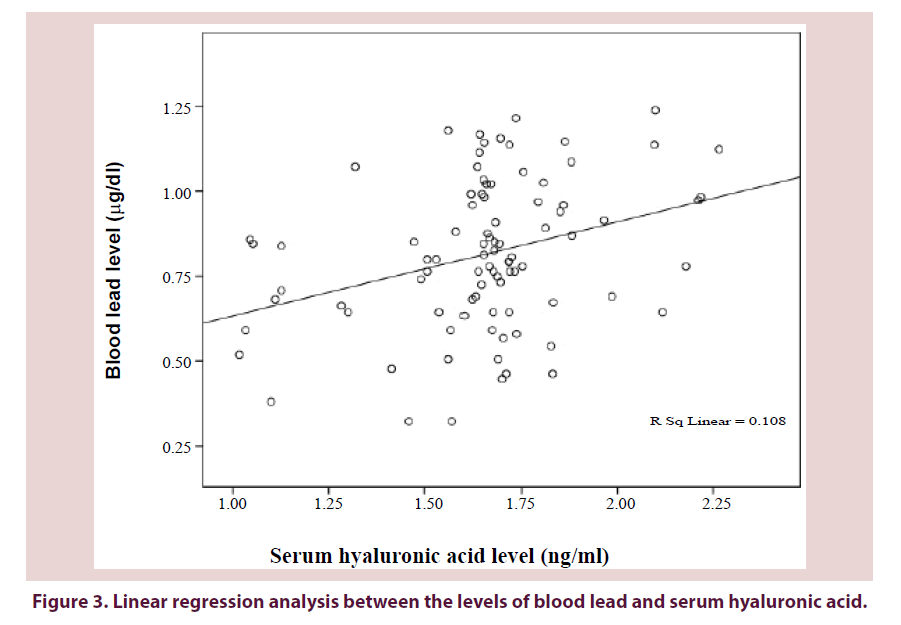
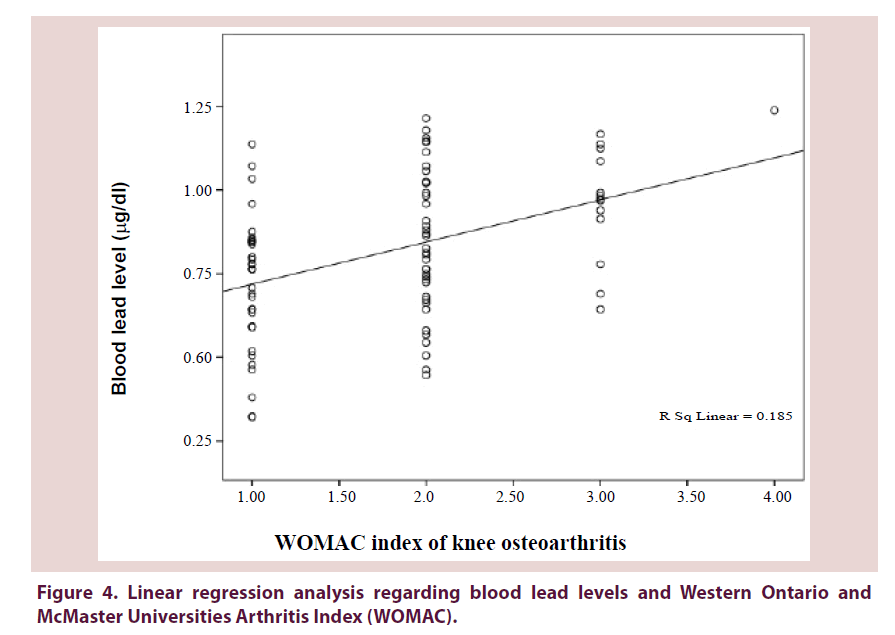
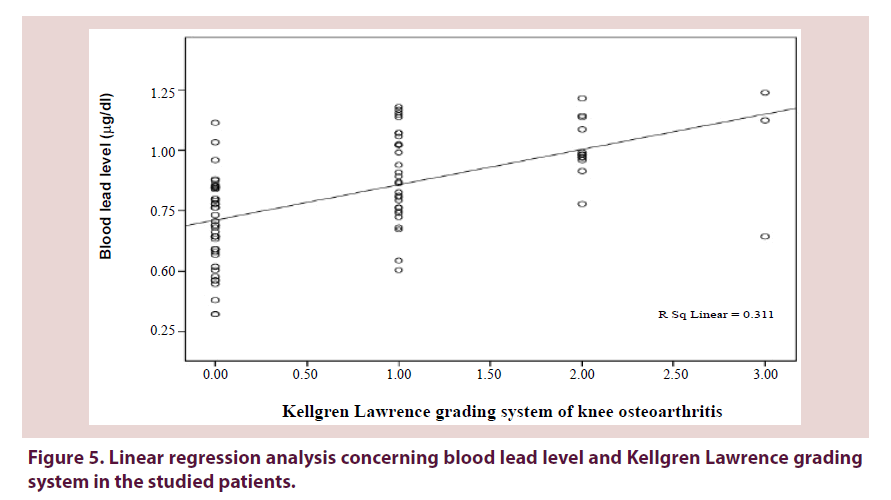
Figures 3-5 show a significant linear regression between lead and hyaluronic acid levels (P ≤ 0.003), WOMAC index (P ≤ 0.001) as well as Kellgren-Lawrence grading system (P ≤ 0.001) where blood lead levels or degree of OA could be predicted and calculated from one of the following equations (Lead level=HA level × 0.034+5.52 or WOMAC index × 0.126+0.593 or K-L grade of KOA × 0.311+0.712).
Figure 4. Linear regression analysis regarding blood lead levels and Western Ontario and McMaster Universities Arthritis Index (WOMAC).
Discussion and conclusion
To the best of our knowledge, there are very few studies investigating the correlation between the levels of blood lead and serum hyaluronic acid in young patients with osteoarthritis. In the present study, the mean age of the patients was 36.88±8.96 years. Nearly similar, MacDonald et al. [13] reported that 37% of Canadians aged 20- 50 years old were diagnosed as osteoarthritic and 29% of them presented with knee osteoarthritis. In addition, Ackerman et al. [14] stated that 30% of persons less than 45 years old are affected with knee OA.
The blood lead levels detected in the studied patients are considered toxic as reported by CDC [15] which mentioned that health problems were observed at substantially low blood lead concentrations (2-10 μg/dl). Studies evaluating lead levels in osteoarthritis are very scarce. For instance, Nelson et al. [16] reported lower median blood lead levels in women and men (1.9 and 2.2 μg/dL respectively). The higher lead concentrations could be attributed to more environmental pollution in Egypt with more exposure to lead than other populations [17].
In the current research, there is a significant difference regarding the age of the studied OA cases (P ≤ 0.001) as well as lead levels which also increased significantly with age (P=0.03). These findings could be explained by the aging process rendering the joint more susceptible to oxidative and catabolic damage and less responsive to anabolic factors [18] in addition to the cumulative effects of environmental exposures including lead.
Men in the present study had higher median blood Pb levels than women (7 and 5.8 μg/dl respectively). It is known that men had higher hematocrit values (42-54%) as testosterone stimulates erythropoietin hormone secretion from the kidney resulting in more synthesis of RBCs [19] and since Pb binds to erythrocytes, this would explain the higher blood lead levels. It could be also due to gender-related difference in lead toxicokinetics as in premenopausal women, the release of lead from bone into blood is slower than men [20].
In line with our results, Nelson et al. [21] stated that there was an association between lead level and severity of symptomatic and radiographic knee OA. It was assumed that low blood lead levels may cause a local toxic effect to bones and joints [22,23]. Moreover, it was claimed that Pb exposure may increase the susceptibility of osteoblasts to environmental toxins and once the blood Pb levels are increased; a cycle of increased tendency to toxic damage may begin regardless of causality [24].
The positive correlation between blood Pb level and pain in symptomatic knee OA observed in our work could be related to Pb neurotoxic effects leading to modulation of pain perception [21]. In other words, lead has the ability to replace Ca2+ and Mg2+, which are involved in cellular signaling, ionic transportation and the release of neurotransmitters with affection of Ca2+-dependent protein kinase C, which regulates neural excitation [25,26] which could increase the severity of pain as evidenced in the current analysis.
Additionally, the positive correlation between lead levels and radiographic OA reported in the current study could be explained by the detrimental effect of Pb on joints leading to articular degeneration. It was found that the likelihood of a person having an x-ray showing knee OA was 20% higher for each one-unit increase of blood lead level [21]. This may be attributed to lead-induced decrease of type II-collagen which is an essential constituent of articular cartilage [22] or increased type X-collagen which facilitates endochondral ossification and hypertrophy of chondrocytes [27]. These changes are closely parallel to those occur in osteoarthritis suggesting a potential role of lead in this debilitating disease.
In the present work, the median hyaluronic acid level was 46.95 ng/ml (min- max: 10.4-184 ng/ml). Serum HA level is known to be lower in healthy human adults (mostly less than 40 ng/ ml). It is elevated in OA as well as major diseases such as liver dysfunction, kidney diseases, rheumatoid arthritis and malignant tumors [28]. Those conditions were excluded in the present study to accurately investigate the relationship between knee OA and HA.
This finding is nearly comparable to the reports of Singh et al. [29] who found that the mean HA level was 41.94±68.79 ng/dl in knee OA and Golightly et al. [30] who stated that higher serum HA levels predict the presence of early stage radiographic knee OA as the degenerated cartilage produce more HA locally which will be forced into blood by the elevated intra-articular pressure.
Contradictory to the present observation, Nelson et al. [21] found no association between Pb and serum HA. However, patients with higher Pb levels were more likely to have more severe knee OA. This discrepancy may be due to different population sample, study design, environmental influence, genetic and ethnic factors.
The positive correlation between lead and HA levels may highlight an indirect relationship between lead and OA. It is worthy-mentioning that inflammation and oxidative stress are the most common mechanisms proposed for the pathogenesis of OA [31]. Inflammation has been associated with accumulation of HA which is a marker of localized joint inflammation and early Knee OA [32].
Various studies support the current finding as they stated that lead could increase many inflammatory mediators [33,34] including highsensitivity C-reactive protein which is found to be elevated four times higher than normal in lead-exposed subjects [35] and positively correlated with synovitis [36].
The current results could be explained by lead-induced production of reactive oxygen species such as Nitric Oxide (NO) with depletion of antioxidant defense systems [1,26]. Increased production of NO has been noted in OA joints resulting in differential effects on pain [37]. A reciprocal relationship between inflammation and oxidative stress has been identified where free radicals can lead to cell membrane damage which in turn triggers the release of many inflammatory mediators [38]. Thus, Pb may contribute to more severe symptomatic and radiographic OA, as seen in the current analysis.
As far as we know, there are no previously reported studies assessing the value of blood lead level in prediction of knee osteoarthritis. The increased levels of blood lead in the present research in conjunction with high serum hyaluronic acid may predict not only the degree of knee pain but also the severity of radiographic knee osteoarthritis.
The present work is one of the very few studies that investigates the correlation between lead and knee osteoarthritis in a group of young adult patients. Limitations of the current work include its small sample size and cross-sectional design which does not contain healthy controls as it is unethical to do radiography to diagnose early stage osteoarthritis in healthy individuals.
In conclusion, the present findings are not simple coincidence and lead seems a possible predictive environmental risk factor for osteoarthritis in young age. Further cohort and experimental studies are recommended to clarify the exact mechanisms involved in the pathogenesis of OA in relation to various environmental pollutants including lead.
Conflict of interest
The authors report no declarations for conflict of interest.
Funding
This work is a part of MSc thesis which is supported by chemicals and equipment from Faculty of Medicine, Mansoura University, Egypt.
References
- Jan AT, Azam M, Siddiqui K et al. Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 16(12), 29592–29630 (2015).
- Ji JS, Elbaz A, Weisskopf MG. Association between blood lead and walking speed in the National Health and Nutrition Examination Survey (NHANES 1999-2002). Environ. Health. Perspect. 121(6), 711–716 (2013).
- Khalil N, Faulkner KA Greenspan SL et al. Associations between bone mineral density, grip strength, and lead body burden in older men. J. Am. Geriatr. Soc. 62, 141–146 (2014).
- Pereira D, Ramos E, Branco J. Osteoarthritis. Acta. Med. Port. 28(1), 99–106 (2015).
- Mobasheri A. Osteoarthritis year 2012 in review: biomarkers. Osteoarthr. Cartil. 20(12), 1451–1464 (2012).
- Sutton PM, Holloway ES. The young osteoarthritic knee: dilemmas in management. BMC Med. (2010).
- Hawamdeh ZM, Al-Ajlouni JM. The clinical pattern of knee osteoarthritis in Jordan: a hospital based study. Int. J. Med. Sci. 10(6), 790–795 (2013).
- Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum. Dis. Clin. North. Am. 39(1), 1–19 (2013).
- Zhang Y, Jordan JM (2008) Epidemiology of osteoarthritis. Rheum. Dis. Clin. North. Am. 34(3), 515–529 (2008).
- Gandek B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: a systematic review. Arthritis. Care. Res. 67(2), 216–229 (2015).
- Litwic A, Edwards MH, Dennison EM et al. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 105, 185–199 (2013).
- Yahaya MI, Shehu A, Dabai FG. Efficiency of extraction of trace metals from blood samples using wet digestion and microwave digestion techniques. J. Appl. Sci. Environ. Manag. 17(3), 365–369 (2013).
- MacDonald KV, Sanmartin C, Langlois K et al. Symptom onset, diagnosis and management of osteoarthritis. Health. Rep. 25(9), 10–17 (2014).
- Ackerman IN, Page RS, Schoch P. Investigating well-being, work limitations and preferences for self-management education and peer support among younger people with hip and knee osteoarthritis: Protocol for a cross sectional study. BMJ. 3(8), 1–8 (2013).
- Nelson AE, Renner JB, Schwartz TA et al. Differences in multi-joint radiographic osteoarthritis phenotypes among African Americans and Caucasians: the Johnston County Osteoarthritis project. Arthritis. Rheum. 63(12), 3843–3852 (2011).
- Osman AG, Kloas W. Water quality and heavy metal monitoring in water, sediments, and tissues of the African catfish Clarias gariepinus (Burchell, 1822) from the river Nile, Egypt. J. Environ. Prot. 1(4), 389–400 (2010).
- Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 23(11), 1966–1971 (2015).
- Murphy WG. The sex difference in haemoglobin levels in adults- mechanisms, causes and consequences. Blood. Rev. 28(2), 41–47 (2014).
- Hwang HS, Lee SB, Jee D. Association between blood lead levels and age-related macular degeneration. PLoS ONE. 10(8), 1–15 (2015).
- Nelson AE, Shi XA, Schwartz TA et al. Whole blood lead levels are associated with radiographic and symptomatic knee osteoarthritis: a cross-sectional analysis in the Johnston County Osteoarthritis Project. Arthritis. Res. Ther. 13(2), 37–46 (2011).
- Holz JD, Beier E, Sheu TJ et al. Lead induces an osteoarthritis-like phenotype in particular chondrocytes through disruption of TGF-beta signaling. J. Orthop. Res. 30, 1760–1766 (2012).
- Beier EE, Inzana JA, Sheu TJ et al. Effects of combined exposure to lead and high-fat diet on bone quality in juvenile male mice. Environ. Health. Persp. 123(10), 935–943 (2015).
- Ryan EP, Holz JD, Mulcahey M et al. Environmental toxicants may modulate osteoblast differentiation by a mechanism involving the aryl hydrocarbon receptor. J. Bone. Miner. Res. 22(10), 1571–1580 (2007).
- Flora G, Gupta D, Tiwari A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 5(2), 47–58 (2012).
- Neal AP, Stansfield KH, Guilarte TR. Enhanced Nitric Oxide production during lead (Pb2+) exposure Recovers Protein Expression but not Presynaptic localization of synaptic proteins in developing hippocampal. Brain. Res. 1439, 88–95 (2012).
- He Y, Siebuhr AS, Brandt-Hansen NU et al. Type X collagen levels are elevated in serum from human osteoarthritis patients and associated with biomarkers of cartilage degradation and inflammation. BMC Musculoskelet. Disord. 15, 309–319 (2014).
- Cowman MK, Schmidt TA, Raghavan P, Stecco A. Viscoelastic properties of hyaluronan in physiological conditions. F1000Res. 4, 622–635 (2015).
- Singh S, Kumar D, Sharma NR. Role of hyaluronic acid in early diagnosis of knee osteoarthritis. J. Clin. Diagn. Res. 8(12), LC04–LC07 (2014).
- Golightly YM, Marshall SW, Kraus VB et al. Biomarkers of incident radiographic knee osteoarthritis: do they vary by chronic knee symptoms? Arthritis. Rheum. 63(8), 2276–2283 (2011).
- Casey G. Arthritis: joints inflamed. Nurs. NZ. 21(5), 20–24 (2015).
- Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 11(5), 101–114 (2014).
- Farkhondeh T, Boskabady MH, Koohi MK et al. The effect of lead exposure on selected blood inflammatory biomarkers in guinea pigs. Cardiovasc. Hematol. Disord. Drug. Targets. 13(1), 45–49 (2013).
- Sirivarasai J, Wananukul W, Kaojarern S et al. Association between inflammatory marker, environmental lead exposure and glutathione-s-transferase gene. BioMed. Res. Int. (2013).
- Khan DA, Qayyum S, Saleem S et al. Lead-induced oxidative stress adversely affects health of the occupational workers. Toxicol. Ind. Health. 24(9), 611–618 (2008).
- Pearle AD, Scanzello CR, George S et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthr. Cartil. 15, 516–523 (2007).
- Hancock CM, Riegger-Krugh C. Modulation of pain in osteoarthritis: the role of nitric oxide. Clin. J. Pain. 24(4), 353–365 (2008).
- Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 11(1), 35–44 (2015).