Review Article - Interventional Cardiology (2023) Volume 15, Issue 1
The development of vaccines against PCSK9: A promising approach to the treatment of atherosclerosis
- Corresponding Author:
- Alexander V. Blagov Laboratory of Angiopathology, Institute of General Pathology and Pathophysiology, 8 Baltiiskaya Street, Moscow 125315, Russia, E-mail: al.blagov2014@gmail.com;
- Alexander N. Orekhov Laboratory of Angiopathology, Institute of General Pathology and Pathophysiology, 8 Baltiiskaya Street, Moscow 125315, Russia, E-mail: a.h.opexob@gmail.com
Received date: 13-Jan-2023, Manuscript No. FMIC-22-87135; Editor assigned: 16-Jan-2023, PreQC No. FMIC-22-87135 (PQ); Reviewed date: 30-Jan-2023, QC No. FMIC-22-87135; Revised date: 06-Feb-2023, Manuscript No. FMIC-22-87135 (R); Published date: 16-Feb-2023, DOI: 10.37532/1755- 5310.2023.15(1).615
Abstract
Despite the success of statins, which have proven to be the main line of therapy for atherosclerosis, there is still a need to develop new types of drugs, because it is associated with the risk of severe side effects from taking statins in some groups of patients, as well as the requirement for even greater reduction in total cholesterol levels. One of the promising therapeutic targets is the molecule of the enzyme Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9), which plays an important role in lipid metabolism. One of the most tested groups of drugs aimed at PCSK9 is monoclonal antibodies, which nevertheless have a few limitations in their use. This review will look at a newer group of therapeutics, the PCSK9 vaccines. In addition to assessing the available studies on the efficacy and safety of PCSK9 vaccines, the prospects for the development of this therapeutic area will be analyzed, as well as an assessment of the multiple roles of PCSK9 in the development of atherosclerosis and the action mechanism of these vaccines.
Keywords
Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) • Atherosclerosis • Low-Density Lipoprotein (LDL)
Introduction
Atherosclerosis is a chronic inflammatory disease in which plaque builds up inside the arteries. Many processes are involved in atherogenesis. One of the most significant developmental factors is the concentration of cholesterol in plasma. There have been several studies demonstrating a direct correlation between plasma concentrations of cholesterol and low-density lipoprotein and the prevalence of serious cardiovascular events, as well as morbidity and mortality associated with cardiovascular diseases [1].
Atherosclerosis is the main cause of about 50% of all deaths in a Europeanized society. Atherosclerotic cardiovascular diseases mainly affect the heart and brain: Coronary Heart Disease (CHD) and ischemic stroke. Coronary artery disease and stroke are respectively the first and fifth causes of death in the world. The most common risk factors include hypercholesterolemia, hypertension, diabetes mellitus, cigarette smoking, age (men over 45 and women over 55), male gender, and strong family history. In addition, a sedentary lifestyle, obesity, diets high in saturated and Tran’s fatty acids and some genetic mutations contribute to the risk [2].
Therapeutic strategies to lower LDL cholesterol levels are the gold standard in the treatment of patients with cardiovascular disease [3]. According to the World Health Organization, up to 80% of CHD cases can be prevented, and progression and possible complications can be reduced [4]. Several studies show that lipid-lowering statins (HMG-CoA reductase inhibitors) are the most effective treatment. In the case of indicated drugs that lower LDL cholesterol, and with proper adherence to the therapy regimen, the risk can be reduced by up to 40%. However, statin therapy may be associated with negative pleiotropic effects such as myopathy or even rhabdomyolysis, and this may contribute to the fact that at least 20% of high-risk patients will not achieve target LDL cholesterol levels with statin monotherapy [5].
Persistent cardiovascular risk, as well as the need to achieve even lower LDL-C targets and reduce cardiovascular risk, further motivate the development and research of new potential therapeutic drugs.
Literature Review
The role of PCSK9 in the development of dyslipidemia
Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) plays an important role in the regulation of lipid exchange between Low-Density Lipoprotein (LDL) particles and the LDL receptor. Within the hepatocyte, the presence of PCSK9 prevents the dissociation of the LDL particle from the receptor, leading to lysosomal degradation. This process prevents the continuous transport of free LDL receptors back to the surface of hepatocytes, where it continues to remove LDL particles and the cholesterol, they contain from circulation [6].
PCSK9 targets the degradation of Low-Density Lipoprotein (LDL-R) receptors, which is an important target for lowering Low-Density Lipoprotein (LDL-C) cholesterol in circulation [7]. R-LDL is critical for the metabolism of LDL particles. LDL-C binds to LDL-R at the surface of the liver cell membrane and enters human cells via endocytosis. A decrease in intracellular pH separates LDL-C from LDL-R [8]. After separation, LDL-C is cleaved in lysosomes, and R-LDL returns to the surface of the liver membrane to continue binding the remaining LDL-C. After PCSK9 binds to R-LDL, the PCSK9-LDL-R-LDL complex is formed, which causes PCSK9, LDL-C, and R-LDL to enter the lysosome together for degradation, R-LDL on the cell surface is reduced, and X degradation-LDL decreases accordingly [9]. Based on this process, studies have shown that a PCSK9-mediated increase in circulating LDL is strongly associated with the progression of cardiovascular diseases such as coronary artery disease [10]. A meta-analysis evaluating a large sample of patients (n=28319) found a significant positive association between circulating PCSK9 concentration and the risk of serious adverse cardiovascular events, suggesting that inhibition of PCSK9 expression reduces serum LDL levels and the risk of cardiovascular disease [11]. In addition, the level of circulating PCSK9 protein is independent of known risk factors, including LDL, and can be used to predict future cardiovascular events [12]. Intravascular ultrasound virtual histological imaging has shown a linear correlation between serum levels of PCSK9 and the amount of necrotic central tissue in coronary atherosclerosis [13]. Similarly, in ApoE -/- mice, PCSK9 overexpression increased plaque size in the aortic sinus and aortic root without altering plasma cholesterol [14]. This suggests that PCSK9 may also influence vascular biology and the progression of cardiovascular disease through other mechanisms.
PCSK9 influences plasma lipid and lipoprotein levels not only by reducing hepatic lipoprotein clearance, but also by stimulating hepatic lipogenesis, a phenomenon mediated by both LDL-β and ApoE, and influenced by transcriptional and post-transcriptional events in hepatic lipogenesis [14].
Plasma LDL cholesterol concentration is a balance between hepatic secretion of triglyceride-rich Very Low-Density Lipoproteins (VLDL), peripheral conversion of VLDL to LDL, and LDL clearance [15]. ApoB-100 is the main protein component of LDL and is required for the binding of LDL particles to R-LDL. Some evidence has shown that PCSK9 increases ApoB-100 secretion in hepatocytes and intestinal cells and reduces VLDL levels in adipocytes, providing a mechanistic basis for the LDL-independent effect of PCSK9 on cholesterol levels [16].
Studies of the pleiotropic effects of PCSK9 evaluated in P-LDL-/- mice have shown that human PCSK9 overexpression does not affect the expression of genes and proteins involved in hepatic lipogenesis, but rather increases the intestinal source of plasma cholesterol and triglycerides. These data suggested that LDL-β-independent effects of PCSK9 on the secretion of triglyceride-rich lipoproteins and specific for enterocytes. Thus, PCSK9 increases the production of triglyceride-rich lipoproteins in the gut through both β-LDL dependent and independent mechanisms [17].
The role of PCSK9 in the development of inflammation in atherosclerosis
PCSK9 is mainly produced in the liver, kidneys, and small intestine [6]. However, it is also expressed in vascular cells, including Endothelial Cells (ECs) and vascular Smooth Muscle Cells (SMCs) [18]. Vascular cells are affected by such hemodynamic factors as blood flow, which, by causing wall shear stress, plays a crucial role in the development and progression of atherosclerosis [19]. Human ECs and SMCs under low blood flow have a higher PCSK9 protein expression than cells under high blood flow, and this effect persists even after stimulation with Lipopolysaccharides (LPS). Indeed, mouse aortas showed significantly higher expression of PCSK9 in areas of high shear stress, an effect further enhanced by LPS administration [19]. Furthermore, in rabbits fed a high-fat diet, low-flow aortic regions had higher PCSK9 expression, while high-flux regions, such as the aortic arch, showed lower PCSK9 expression in vessels [20]. Therefore, there is a negative correlation between PCSK9 expression levels in vessels and blood flow.
PCSK9 has been shown to promote vascular inflammation. The binding of PCSK9 to the inflammatory receptor TLR4 was first hypothesized based on structural homology between the C-terminal domain of PCSK9 and the TLR4 resisting ligand in a model in silico [21]. TLRs are transmembrane proteins defined by cytoplasmic domains to recognize ligands of extracellular domains and interact with TLR signaling proteins. TLRs recognize pathogens and regulate the expression of pro-inflammatory cytokines as well as the early immune response to infection. Among TLRs, TLR4 acts as a receptor for LPS and activates NF-κB, promoting an inflammatory response [22]. PCSK9 expression in ECs and SMCs is dependent on the TLR4/NFκB signaling pathway, as inhibition of various components of the activation cascade indicates that PCSK9 expression is dependent on the TLR4-MyD88-NFkB axis and is independent of TLR4/TRIF signaling, making the MyD88 pathway a possible target for future research. Methods of treatment and prevention of excessive production of PCSK9 in the vascular medicine was described by Liu, et al., [20]. Therefore, PCSK9 synthesis is regulated by the TLR4 receptor signaling pathway through MyD88 and NFκB activation, and soluble PCSK9 can act as an inflammatory mediator by TLR4 binding and recognition, as shown in ApoE knockout mice [23].
Vascular stability depends on cellular apoptosis. PCSK9 modulates the expression of the apoptosis inducer Bax and the apoptosis inhibitor Bcl-2. The balance between these two proteins is key to preventing or triggering apoptosis [24]. Lipid-loaded endothelial cells exhibit increased levels of the Bax protein and reduced levels of Bcl-2, which leads to the activation of caspase 3 and caspase 9, which induces cell apoptosis [25]. Silencing PCSK9 with siRNA inhibits apoptosis because silencing PCSK9 cannot phosphorylate p38 and JNK (both members of the MAPK signaling pathway), which allows activation of the apoptosis inhibitor Bcl-2 [26]. Interestingly, p38 and JNK are also responsible for Bax and Bad phosphorylation, which activates programmed cell death. Therefore, PCSK9 may promote activation of the MAPK signaling cascade and endothelial cell apoptosis, a mechanism that has already been described in cancer cells [27].
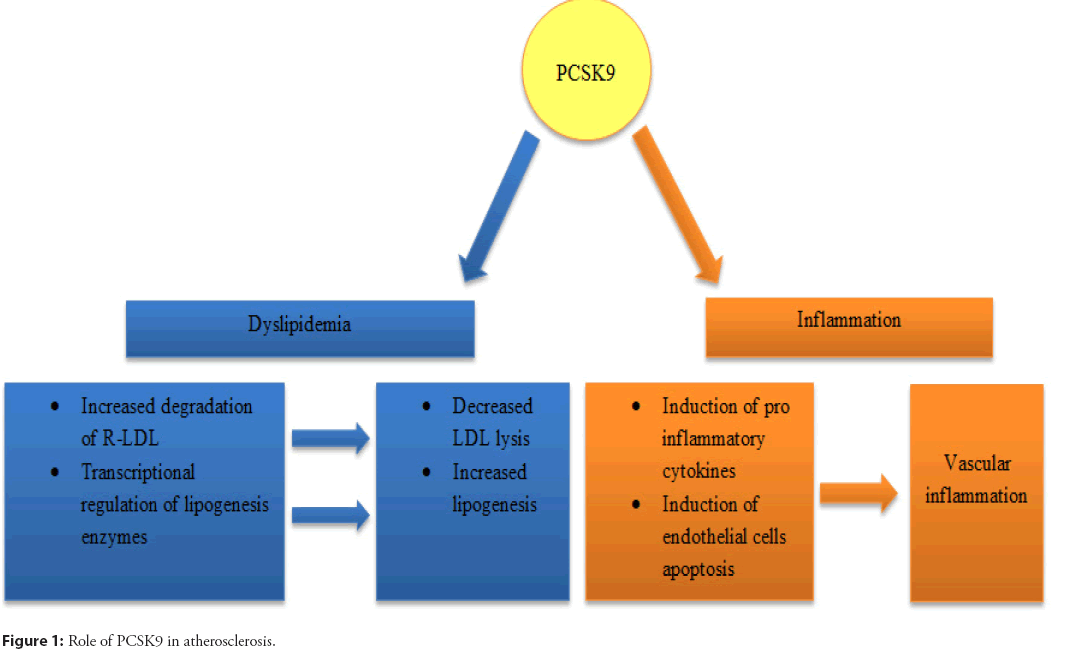
In conclusion, PCSK9 can combine with TLR4 to regulate the expression of inflammatory factors and influence the inflammatory disease process. The role of PCSK9 in atherosclerosis is shown schematically in Figure 1.
Limitations of currently used PCSK9 inhibitors
Many approaches to pharmacological inhibition of PCKS9 are currently under investigation. Clearly, the most advanced of these is the use of antibodies. Initially, three antibodies were tested in clinical programs. While evolocumab and alirocumab are fully human antibodies, bococizumab is humanized, but not fully human. In the later stages of development, neutralizing antibodies against bococizumab were found, which disrupted the long-term effects of the drug on LDL-C and led to the termination of its further development [28].
PCSK9 is a secreted protein involved in the regulation of the liver β-LDL life cycle. By inhibiting circulating PCSK9, monoclonal antibodies reduce plasma LDL-C, which has benefited many patients, and their effectiveness in lowering LDL-C has also been proven in clinical trials in high-risk CVD patients [29].
Currently, monoclonal antibodies to PCSK9 include alirocumab and evolocumab, which are approved by the US Food and Drug Administration for the treatment of hypercholesterolemia, including primary hypercholesterolemia and familial hypercholesterolemia. In a clinical study in patients with heterozygous familial Hypercholesterolemia (HSH), evolocumab (140 mg/2 weeks or 420 mg/month) reduced LDL-C levels by 60%-65%, and more than 60% of patients were able to achieve their target LDL-cholesterol level in 70 mg/dL [30]. In another clinical study, 57% of patients with refractory severe familial hypercholesterolemia achieved an LDL cholesterol level of 100 mg/ dl after treatment with alirocumab (75/150 mg every 2 weeks) [31].
In addition, alirocumab in combination with statins demonstrated a significant reduction in LDL cholesterol compared with patients receiving a double dose of statins alone or receiving ezetimibe [32]. Alirocumab and evolocumab have been approved for the treatment and prevention of cardiovascular events. However, there are limited data on anti-PCSK9 monoclonal antibodies in Homozygous Familial Hypercholesterolemia (HoFH), so only evolocumab has been labeled for this indication [8].
Excessive muscle symptoms or elevated liver enzymes are unfavorable symptoms of statin therapy, but so far. no similar side effects have been found in PCSK9 monoclonal antibody studies. Nasopharyngitis and mild self-limiting reactions at the injection site (eg, itching, redness, and swelling) are considered the most common adverse reactions with PCSK9 monoclonal antibodies [33].
Despite the great progress in the treatment of dyslipidemia with PCSK9 inhibitors, and the low rates that have been achieved with the combination of statins and antibodies, some disadvantages of this treatment remain. A good example is the short half-life of antibodies in vivo, which can reduce long-term adherence to treatment (however, higher than in the case of statins): According to studies, the number of patients who discontinued or violated the anti-PCSK9 therapeutic regimen can be up to 8.5%. However, this number may be higher as most of the studies were conducted during clinical trials, which may mean that adherence may be lower in real data and long-term follow-ups. The high cost of treatment may particularly limit the full use of the clinical benefits of these drugs. The typical dosing regimen for PCSK9 antibodies is twice a month, which, combined with high monthly and annual costs, limits the widespread use of this category of drugs, despite all recommendations [34].
Immunological features for PCSK9 vaccination
Classically, vaccines have been used to prevent and treat infectious diseases caused primarily by pathogenic viruses, but also by bacteria. Non-infectious disease vaccines target mutant or overexpressed proteins whose activity directly contributes to disease progression, in this case, the PCSK9 enzyme.
The main difficulty in developing such vaccines is that their targets are endogenous proteins, that is, proteins of one’s own body. On the one hand, the development of an immune response is hindered by the tolerance of the body’s immune system to its own proteins, which can be expressed in low production of antibodies, which, in turn, may be insufficient to neutralize the interaction between PCSK9 and LDL-R. On the other hand, the reaction of the body to the vaccine may be autoimmune in nature, so it is extremely important that the vaccine is not able to induce a specific T-cell response and cause cross-reactivity of antibodies with other endogenous proteins and antigens [34]. The danger associated with the induction of a T-cell immune response, which is an important component of the antiviral response of the immune system, is explained by the fact that PCSK9 is secreted by healthy cells of the body, mainly hepatocytes, and a T-cell response against PCSK9 will be accompanied by infiltration of T-lymphocytes into the liver and destruction hepatocytes [35,36]. Therefore, in the process of creating a vaccine, it is important to identify endogenous peptide antigens contained in the PCSK9 protein, which are capable of inducing a T-cell immune response. However, it should be noted that the maturation, proliferation, and differentiation of B cells into antibody-producing plasma cells require the participation of follicular T-helper lymphocytes [37].
Thus, the dominant branch of the post-vaccination immune response upon immunization with the PCSK9 vaccine should be humoral immunity. Based on this, when developing, it is worth choosing the type of potential vaccine correctly. Live and attenuated vaccines are undestroyed viral or bacterial particles and, due to their origin, can only be used in the prevention of infectious diseases, since they contain exclusively pathogenic proteins. Care should be taken when using vector vaccines, which are viral vectors lacking some of the genes encoding pathogenic proteins, and at the same time having an insertion of the target gene, in this case, the gene encoding PCSK9, since the mechanism of action of these vaccines is based on the predominant activation of the cytotoxic T-cell immune response. This occurs due to the delivery of the target gene into the cell, its subsequent expression, processing, and exposure of peptide antigens formed after degradation of the target protein, and their interaction with MHCI receptors located on the surface of antigen-presenting cells, which triggers the cellular branch of the immune response. For the same reason, DNA and RNA vaccines are not suitable vaccine candidates for PCSK9. In our opinion, subunit protein vaccines and peptide vaccines can be the most effective and safe types of PCSK9 vaccines. Moreover, protein vaccines are able to bind to B cells (playing the role of antigen-presenting cells in this case) in the native state, without degradation into peptide epitopes. At the same time, an important advantage of epitope vaccines is the most narrowly targeted post-vaccination immune response, which minimizes the risks of developing cross-reactivity of produced antibodies. A difficulty in developing such vaccines is the need for precise knowledge of the sequence and three-dimensional structure of PCSK9 epitopes.
Another problem, in addition to the risk of developing an autoimmune reaction, as already noted, is the tolerance of the immune system that is, the very protection that protects the body from the attack of lymphocytes and immune complexes on its own tissues. First of all, such tolerance limits the effect of vaccination on the part of B-cell activation, but it can be overcome by T-helpers, which are direct inducers of the humoral adaptive immune response (Th2 population). In the next section, experimental examples will show that PCSK9 vaccines are able to induce antibody production. To increase the immunogenicity of vaccines against PCSK9, various adjuvants that have proven their effectiveness when used as part of other vaccines can be additionally included in the composition of the vaccine. For example, a study [38], showed the feasibility of an adjuvant supplement in the form of an epitope of tetanus toxin, which was chemically linked to a fragment of the PCSK9 protein. The tetanus toxin epitope, not being a human protein, triggered the immune system to react as a foreign antigen to the entire vaccine, thus circumventing the issue of immune tolerance. In addition, it predominantly stimulated the activation of T-helpers, rather than T-killers, which reduced the risk of developing a cytotoxic autoimmune reaction on liver cells and other cells producing PCSK9. Other compounds, both organic and inorganic, can be used as such adjuvants, for example incomplete Freund’s adjuvant and aluminum alum [39].
Vaccine development targeting PCSK9
This Vaccination strategy may be a better alternative to monoclonal antibodies because their approach is less expensive and does not require frequent injection intervals [40]. The first approach to developing a PCSK9 vaccine was described by Fattori et al. [41]. This study focused on two methods for immunizing mice: DNA-based and protein-based vaccinations. In the first series of experiments in this study, a DNA plasmid expressing murine PCSK9 under the human CMV promoter was introduced and used in conjugation with an oligonucleotide cpg. However, no antibody response was observed in the first set. In the second stage, a xenogenic approach was planned with the expression of the human PCSK9 plasmid and the native protein. This approach proved successful and resulted in high titers of antibodies against hPCSK9. In the first step, protein immunization was tested and compared with the previous DNA immunization protocol. Highly purified human PCSK9 was used along with the oligonucleotide CpG, which resulted in a strong production of antibodies against hPCSK9. In both cases, high titers of antibodies against hPCSK9 led to a significant decrease in LDL levels by up to 40% after 2 weeks. After 42 days, cholesterol reduction was maintained by 28% [41].
According to animal studies, the peptide-based PCSK9 vaccine induces the production of antibodies that improve the lipid profile for a period of 24 to 40 weeks [42]. According to a study, the PCSK9 vaccine reduced plasma lipid levels and systemic and vascular inflammation by reducing plasma inflammatory markers and vascular endothelial growth factor, which led to the attenuation of atherosclerotic lesions in the aorta [43]. Similarly, in mice vaccinated with the peptides, an effective immune response was associated with a significantly improved lipid profile. Vaccinated mice with hypercholesterolemia have been shown to reduce total cholesterol and LDL-C. Recently, a phase I clinical trial was conducted to evaluate the safety and tolerability of the PCSK9 vaccine in healthy volunteers. The results have not yet been published [43].
Another study presented a nanoliposomal vaccine against PCSK9, and in the first approach, it was administered to healthy animals to monitor antibody production [44]. After the detection of antibody production, streptozotocin was intraperitoneally administered to immunized rats to induce diabetes mellitus. It is reported that vaccinated rats showed lower plasma LDL-C levels compared to unvaccinated diabetic rats. Hyperglycemia was suppressed in vaccinated rats [45]. In mice, with hypercholesterolemia the nanoliposomal PCSK9 vaccine stimulated the production of antibodies that inhibited the interaction between PCSK9 and the LDL receptor and also led to a decrease in LDL and triglycerides. In addition, a decrease in inflammatory cells was observed, as well as a decrease in the size of atherosclerotic lesions [46].
Several other groups have also explored the potential of PCSK9 vaccines with other approaches and methods [47]. Vaccines have been developed that use bacteriophage virus-like particles (QBeta- VLP) that expose PCSK9 peptides on the surface along with Freund’s adjuvant and aluminum. The mechanism for displaying a self-antigen on a virus-like particle has been tested in several studies on vaccines for Alzheimer’s disease, hypertension, and cancer. This method has proven to be capable of inducing a strong humoral response against autoantigens. In this study, there was a significant reduction in total cholesterol by up to 55%, as well as other lipid parameters. The results of the study were consistent with Alzheimer’s disease clinical trials with the same QBetal-VLP vaccine, which showed the same antibody response and a weak T-cell response [48].
Currently, active immunization methods directed against PCSK9 represent an interesting alternative to passive immunization by administration of antibodies. Active immunization could have interesting applications due to the supposed better adherence and significantly lower cost of treatment. However, there are currently no significant clinical studies investigating the possibility of active immunization against PCSK9 that could provide us with a clinically relevant treatment in the near future [34]. The above studies are systematized in Table 1.
| Type of vaccine | Model | Immunogenicity | Clinical effect |
|---|---|---|---|
| Combination of protein and DNA vaccines [43] | Mice | Antibody detection | Up to 40% reduction in LDL levels 2 weeks after immunization. |
| Peptide [44,45] | Mice | Antibody detection | Decreased plasma lipid levels (up to 40 weeks), reduced vascular and systemic inflammation. |
| Nanoliposomal vaccine with exposed peptides [46,48] | Mice | Antibody detection | Lowering LDL levels, reducing inflammation and atherosclerotic lesions. |
| Virus-like particles with exposed peptides [50] | Mice | Antibody detection | Reducing total cholesterol levels up to 55%. |
Table 1: PCSK9 vaccine developments.
Discussion
Despite the prospects of the currently available developments aimed at creating vaccines against PCSK9, there are a number of important questions, primarily related to the safety of this type of drug. Since PCSK9 is directly a protein of the human body, there is a certain probability of an autoimmune reaction to its own proteins that have a similar molecular structure to PCSK9, which can lead to the development of persistent disorders in the tolerance of the immune system. This issue should be investigated in experimental conditions in suitable animal models with an assessment of the effect of the drug on certain proteins potentially dangerous for the occurrence of an autoimmune reaction and an assessment of the likelihood of developing such reactions over time. Particularly important for evaluating the safety of PCSK9 vaccines is the need to understand how the components of these vaccines act on multifunctional proteins critical to human health. These proteins, in particular, include the Sirtuin 1 protein, which regulates food intake, glucose and cholesterol metabolism, and is also involved in genomic DNA repair, cell proliferation, and inhibition of apoptosis [49]. Disruption of Sirtuin 1 activity caused by various factors, including xenobiotics, may be a prerequisite for the development of a number of chronic diseases, including obesity, cardiovascular diseases, Parkinson’s disease, etc [50,51]. Sirtuin 1 has also been found to be able to reduce the severity of atherosclerosis by decreasing PCSK9 secretion in hepatocytes [52], as well as modulating its plasma activity [53]. The possibility of Sirtuin 1 binding to PCSK9 at the acetylation sites [53], provides the prerequisites for Sirtuin 1 binding to the exogenous vaccine form of PCSK9, while containing these binding sites. This, in turn, suggests some risk associated with the potential ability of neutralizing antibodies to PCSK9 to interact with binding sites in the Sirtuin 1 molecule. To understand this risk, it is necessary to assess the actual ability of such binding in vitro and in vivo. Additional questions regarding the potential safety of PCSK9 vaccine preparations are the risk of reactogenicity and severe allergic reactions. In these cases, the adverse outcome depends not only directly on the PCSK9 protein, but also on other components of the vaccine. Therefore, in this case, it is important to conduct a series of experiments using different types of vaccines: Vector, subunit, peptide, as well as various adjuvants used. In addition to safety, questions related to the immunogenicity of such vaccines also need to be studied. In the studies described in this review, the predominant assessment of immunogenicity was based on the relative level of humoral immunity, while at the same time without a clear analysis of the titers of certain types of antibodies and the dynamics of their changes during the post-vaccination period. The contribution of the cellular immune response elicited by the antigen in the form of PCSK9 should also be determined, which may also help to identify potential cytotoxic effects caused by the use of the vaccine. Since PCSK9 is predominantly localized in the liver, despite secretion into the extracellular space, an important parameter for evaluating the effect of the drug is its bioavailability, the study of which will reveal the optimal dose, regimen, and method of vaccine administration. Finally, it is clear that a data set is required on the therapeutic efficacy of these types of vaccines. It is clear that the more such studies will be carried out, the clearer the picture will become about the appropriateness of this type of treatment for atherosclerosis, as well as other diseases of the cardiovascular system associated with the development of dyslipidemia.
Conclusion
The main function of the PCSK9 enzyme is the degradation of low-density lipoprotein receptors, LDL-R, which, under pathological conditions of atherosclerosis, leads to an increase in the accumulation of cholesterol associated with LDL. In addition, PCSK9 contributes to the progression of atherosclerosis through the induction of lipogenesis in the liver and the development of an inflammatory response in the vessels. The main therapeutic strategy aimed at inhibiting PCSK9 activity in atherosclerosis is the introduction of monoclonal antibodies, which, despite their advantages, have a number of limitations associated with the cost of drugs and side effects. An alternative or complementary therapy targeting PCSK9 binding could be active immunization, which can elicit a strong humoral but not a cellular immune response to avoid a cytotoxic autoimmune response to hepatocytes. To date, there are a number of promising developments of such vaccines with results showing their effectiveness in animal models, but there are no reliable results evaluating the effect of PCSK9 vaccines in clinical studies.
Author Contributions
Writing-original draft preparation, AVB; writing-review and editing, VNS, IIE, IIN,NAG, ANO.
Funding
This work was supported by the Russian Science Foundation (Grant #22-65-00005)
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
Not applicable
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dichgans M, Pulit SL, Rosand J. Stroke genetics: Discovery, biology, and clinical applications. The Lancet Neurology. 18(6): 587-599 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Reiss AB, Grossfeld D, Kasselman LJ, et al. Adenosine and the cardiovascular system. Am J Cardiovasc Drugs. (2019).
[CrossRef] [Google Scholar] [PubMed]
- Catapano AL, Graham I, de Backer G, et al. 2016 ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 253: 281-344 (2016).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Mendis S, Chestnov O. The global burden of cardiovascular diseases: A challenge to improve. Curr Cardiol Rep. 486: 16(5) (2014).
[CrossRef] [Google Scholar] [PubMed]
- Spannella F, Giulietti F, di Pentima C, et al. Prevalence and control of dyslipidemia in patients referred for high blood pressure: The disregarded “Double-Trouble” lipid profile in overweight/obese. Adv Ther. 36(6):1426-1437 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Scherer DJ, Nelson AJ, Psaltis PJ, et al. Targeting low-density lipoprotein cholesterol with PCSK9 inhibitors. Intern Med J. 47(8): 856-865 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of Inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 382(16): 1507-1519 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 379(22): 2097-2107 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Brown M, Ahmed S. Emerging role of proprotein convertase subtilisin/kexin type-9 (PCSK-9) in inflammation and diseases. Toxicol Appl Pharmacol. 370: 170-177 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Gitt AK, Lautsch D, Ferrières J, et al. Cholesterol target value attainment and lipid-lowering therapy in patients with stable or acute coronary heart disease: Results from the Dyslipidemia International Study II. Atherosclerosis. 266: 158-166 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Dicembrini I, Giannini S, Ragghianti B, et al. Effects of PCSK9 inhibitors on LDL cholesterol, cardiovascular morbidity and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. J Endocrinol Invest. (2019).
[CrossRef] [Google Scholar] [PubMed]
- Proprotein convertase subtilisin/kexin type 9 (PCSK9). Science-Business eXchange. 5(9): 227-227 (2012).
- Cheng JM, Oemrawsingh RM, Garcia-Garcia HM, et al. PCSK9 in relation to coronary plaque inflammation: Results of the ATHEROREMO-IVUS study. Atherosclerosis. 248: 117-122.
[CrossRef] [Google Scholar] [PubMed]
- Tavori H, Giunzioni I, Predazzi IM, et al. Human PCSK9 promotes hepatic lipogenesis and atherosclerosis development via apoE- and LDLR-mediated mechanisms. Cardiovasc Res. 110(2): 268-278 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Sun H, Samarghandi A, Zhang N, et al. Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein b and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor. Arterioscler Thromb Vasc Biol. 32(7): 1585-1595 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Levy E, Ouadda ABD, Spahis S, et al. PCSK9 plays a significant role in cholesterol homeostasis and lipid transport in intestinal epithelial cells. Atherosclerosis. 227(2): 297-306 (2013).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Rashid S, Tavori H, Brown PE, et al. Proprotein convertase subtilisin kexin type 9 promotes intestinal overproduction of triglyceride-rich apolipoprotein B lipoproteins through both low-density lipoprotein receptor-dependent and -independent mechanisms. Circulation. 130(5): 431-441 (2014).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Ding Z, Liu S, Wang X, et al. Cross-talk between PCSK9 and damaged mtDNA in vascular smooth muscle cells: Role in apoptosis. Antioxid Redox Signal. 25(18): 997-1008 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Ding Z, Liu S, Wang X, et al. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid Redox Signal. 22(9): 760-771 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Liu S, Deng X, Zhang P, et al. Blood flow patterns regulate PCSK9 secretion via MyD88 mediated proinflammatory cytokines. Cardiovasc Res. 116(10): 1721-1732 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Luquero A, Badimon L, Borrell-Pages M. PCSK9 functions in atherosclerosis are not limited to plasmatic LDL-cholesterol regulation. Front Cardiovasc Med. 8: 639727 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Guijarro-Muñoz I, Compte M, Álvarez-Cienfuegos A, et al. Lipopolysaccharide activates Toll-Like Receptor 4 (TLR4)-mediated NF-κ B signaling pathway and proinflammatory response in human pericytes. J Biol Chem. 289(4): 2457-2468 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Tang ZH, Peng J, Ren Z, et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κ B pathway. Atherosclerosis. 262: 113-122 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Yurtseven E, Ural D, Baysal K, et al. An update on the role of PCSK9 in atherosclerosis. J Atheroscler Thromb. 27(9): 909-918 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Wu CY, Tang ZH, Jiang L, et al. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol Cell Biochem. 359(1-2): 347-358 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Li J, Liang X, Wang Y, et al. Investigation of highly expressed PCSK9 in atherosclerotic plaques and ox-LDL-induced endothelial cell apoptosis. Mol Med Rep. 16(2): 1817-1825 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Xu B, Li S, Fang Y, et al. Proprotein Convertase Subtilisin/Kexin Type 9 promotes gastric cancer metastasis and suppresses apoptosis by facilitating MAPK signaling pathway through HSP70 up-regulation. Front Oncol. 10: 609663 (2021).
- Katzmann JL, Gouni-Berthold I, Laufs U. PCSK9 inhibition: Insights from clinical trials and future prospects. Front Physiol. 11 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 376(16): 1527-1539 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 385(9965):331-340 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Ray KK, Colhoun HM, Szarek M, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 7(8): 618-628 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Khoshnejad M, Patel A, Wojtak K, et al. Development of novel DNA-encoded PCSK9 monoclonal antibodies as lipid-lowering therapeutics. Mol Ther. 27(1): 188-99 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Liu C, Chen J, Chen H, et al. PCSK9 inhibition: from current advances to evolving future. Cells. 11(19): 2972 (2022).
[CrossRef] [Google Scholar ] [PubMed]
- Toth S, Pella D, Fedacko J, et al. Vaccines targeting PSCK9 for the treatment of hyperlipidemia. Cardiol Ther. 9: 323-332 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Branchetti E, Bavaria JE, Grau JB, et al. Circulating soluble receptor for advanced glycation end product identifies patients with bicuspid aortic valve and associated aortopathies. Arterioscler Thromb Vasc Biol. 34(10): 2349-2357 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Poggio P, Songia P, Cavallotti L, et al. PCSK9 involvement in aortic valve calcification. J Am Coll Cardiol. 72(24): 3225-3227 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Whitmire JK, Slifka MK, Grewal IS, et al. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J Virol. 70(12): 8375-8381 (1996).
[CrossRef] [Google Scholar] [PubMed]
- Galabova G, Brunner S, Winsauer G, et al. Peptide-based anti-PCSK9 vaccines-an approach for long-term LDLc management. PLoS One. 9(12): e114469 (2014).
- Kobiyama K, Saigusa R, Ley K, et al. Vaccination against atherosclerosis. Curr Opin Immunol. 59:15-24 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Weisshaar S, Zeitlinger M. Vaccines targeting PCSK9: A promising alternative to passive immunization with monoclonal antibodies in the management of hyperlipidaemia? Drugs. 78: 799-808 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Fattori E, Cappelletti M, Surdo PL, et al. Immunization against proprotein convertase subtilisin-like/kexin type 9 lowers plasma LDL-cholesterol levels in mice. J Lipid Res. 53(8): 1654-61 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Kawakami R, Nozato Y, Nakagami H, et al. Development of vaccine for dyslipidemia targeted to a proprotein convertase subtilisin/kexin type 9 (PCSK9) epitope in mice. PloS one. 13(2): e0191895 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Landlinger C, Pouwer MG, Juno C, et al. The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE* 3Leiden. CETP mice. Eur Heart J. 38(32): 2499-2507 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Wu D, Zhou Y, Pan Y, et al. Vaccine Against PCSK9 Improved Renal Fibrosis by Regulating Fatty Acid β‐Oxidation. J Am Heart Assoc. 9(1): e014358 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Momtazi-Borojeni AA, Jaafari MR, Badiee A, et al. Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system. Atherosclerosis. (2019).
- Momtazi-Borojeni AA, Jaafari MR, Abdollahi E, et al. Impact of PCSK9 immunization on glycemic indices in diabetic rats. J Diabetes Res. 4757170 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Momtazi-Borojeni AA, Jaafari MR, Afshar M, et al. PCSK9 immunization using nanoliposomes: Preventive efficacy against hypercholesterolemia and atherosclerosis. Arch Med Sci. 17(5): 1365-1377 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Chackerian B, Remaley A. Vaccine strategies for lowering LDL by immunization against proprotein convertase subtilisin/kexin type 9. Curr Opin Lipidol. 27(4): 345-350 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Martins IJ. Anti-Aging Genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Advances in Aging Research. 5: 9-26 (2016).
- Martins IJ. Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. J Clin Epigenet. 3(3): 24(2017).
- Martins IJ. Appetite control and core body temperature are linked to autoimmune disease and the global chronic disease epidemic. Enliven: Immunol Immunotechnol. 3(e1): 001-009 (2019).
- Miranda MX, vanTits LJ, Lohmann C, et al. The Sirt 1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur Heart J. 36(1): 51-59 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Miranda MX, Arsiwala T, Obeid S, et al. Circulating Sirt1 Protects Against Atherosclerosis Through Its Modulation of Pcsk9 Activity. Circulation. 134: A19426 (2016).
- Dichgans M, Pulit SL, Rosand J. Stroke genetics: Discovery, biology, and clinical applications. The Lancet Neurology. 18(6): 587-599 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Reiss AB, Grossfeld D, Kasselman LJ, et al. Adenosine and the cardiovascular system. Am J Cardiovasc Drugs. (2019).
[CrossRef] [Google Scholar] [PubMed]
- Catapano AL, Graham I, de Backer G, et al. 2016 ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 253: 281-344 (2016).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Mendis S, Chestnov O. The global burden of cardiovascular diseases: A challenge to improve. Curr Cardiol Rep. 486: 16(5) (2014).
[CrossRef] [Google Scholar] [PubMed]
- Spannella F, Giulietti F, di Pentima C, et al. Prevalence and control of dyslipidemia in patients referred for high blood pressure: The disregarded “Double-Trouble” lipid profile in overweight/obese. Adv Ther. 36(6):1426-1437 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Scherer DJ, Nelson AJ, Psaltis PJ, et al. Targeting low-density lipoprotein cholesterol with PCSK9 inhibitors. Intern Med J. 47(8): 856-865 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of Inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 382(16): 1507-1519 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 379(22): 2097-2107 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Brown M, Ahmed S. Emerging role of proprotein convertase subtilisin/kexin type-9 (PCSK-9) in inflammation and diseases. Toxicol Appl Pharmacol. 370: 170-177 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Gitt AK, Lautsch D, Ferrières J, et al. Cholesterol target value attainment and lipid-lowering therapy in patients with stable or acute coronary heart disease: Results from the Dyslipidemia International Study II. Atherosclerosis. 266: 158-166 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Dicembrini I, Giannini S, Ragghianti B, et al. Effects of PCSK9 inhibitors on LDL cholesterol, cardiovascular morbidity and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. J Endocrinol Invest. (2019).
[CrossRef] [Google Scholar] [PubMed]
- Proprotein convertase subtilisin/kexin type 9 (PCSK9). Science-Business eXchange. 5(9): 227-227 (2012).
- Cheng JM, Oemrawsingh RM, Garcia-Garcia HM, et al. PCSK9 in relation to coronary plaque inflammation: Results of the ATHEROREMO-IVUS study. Atherosclerosis. 248: 117-122.
[CrossRef] [Google Scholar] [PubMed]
- Tavori H, Giunzioni I, Predazzi IM, et al. Human PCSK9 promotes hepatic lipogenesis and atherosclerosis development via apoE- and LDLR-mediated mechanisms. Cardiovasc Res. 110(2): 268-278 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Sun H, Samarghandi A, Zhang N, et al. Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein b and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor. Arterioscler Thromb Vasc Biol. 32(7): 1585-1595 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Levy E, Ouadda ABD, Spahis S, et al. PCSK9 plays a significant role in cholesterol homeostasis and lipid transport in intestinal epithelial cells. Atherosclerosis. 227(2): 297-306 (2013).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Rashid S, Tavori H, Brown PE, et al. Proprotein convertase subtilisin kexin type 9 promotes intestinal overproduction of triglyceride-rich apolipoprotein B lipoproteins through both low-density lipoprotein receptor-dependent and -independent mechanisms. Circulation. 130(5): 431-441 (2014).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Ding Z, Liu S, Wang X, et al. Cross-talk between PCSK9 and damaged mtDNA in vascular smooth muscle cells: Role in apoptosis. Antioxid Redox Signal. 25(18): 997-1008 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Ding Z, Liu S, Wang X, et al. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid Redox Signal. 22(9): 760-771 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Liu S, Deng X, Zhang P, et al. Blood flow patterns regulate PCSK9 secretion via MyD88 mediated proinflammatory cytokines. Cardiovasc Res. 116(10): 1721-1732 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Luquero A, Badimon L, Borrell-Pages M. PCSK9 functions in atherosclerosis are not limited to plasmatic LDL-cholesterol regulation. Front Cardiovasc Med. 8: 639727 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Guijarro-Muñoz I, Compte M, Álvarez-Cienfuegos A, et al. Lipopolysaccharide activates Toll-Like Receptor 4 (TLR4)-mediated NF-κ B signaling pathway and proinflammatory response in human pericytes. J Biol Chem. 289(4): 2457-2468 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Tang ZH, Peng J, Ren Z, et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κ B pathway. Atherosclerosis. 262: 113-122 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Yurtseven E, Ural D, Baysal K, et al. An update on the role of PCSK9 in atherosclerosis. J Atheroscler Thromb. 27(9): 909-918 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Wu CY, Tang ZH, Jiang L, et al. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol Cell Biochem. 359(1-2): 347-358 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Li J, Liang X, Wang Y, et al. Investigation of highly expressed PCSK9 in atherosclerotic plaques and ox-LDL-induced endothelial cell apoptosis. Mol Med Rep. 16(2): 1817-1825 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Xu B, Li S, Fang Y, et al. Proprotein Convertase Subtilisin/Kexin Type 9 promotes gastric cancer metastasis and suppresses apoptosis by facilitating MAPK signaling pathway through HSP70 up-regulation. Front Oncol. 10: 609663 (2021).
- Katzmann JL, Gouni-Berthold I, Laufs U. PCSK9 inhibition: Insights from clinical trials and future prospects. Front Physiol. 11 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 376(16): 1527-1539 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 385(9965):331-340 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Ray KK, Colhoun HM, Szarek M, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 7(8): 618-628 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Khoshnejad M, Patel A, Wojtak K, et al. Development of novel DNA-encoded PCSK9 monoclonal antibodies as lipid-lowering therapeutics. Mol Ther. 27(1): 188-99 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Liu C, Chen J, Chen H, et al. PCSK9 inhibition: from current advances to evolving future. Cells. 11(19): 2972 (2022).
[CrossRef] [Google Scholar ] [PubMed]
- Toth S, Pella D, Fedacko J, et al. Vaccines targeting PSCK9 for the treatment of hyperlipidemia. Cardiol Ther. 9: 323-332 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Branchetti E, Bavaria JE, Grau JB, et al. Circulating soluble receptor for advanced glycation end product identifies patients with bicuspid aortic valve and associated aortopathies. Arterioscler Thromb Vasc Biol. 34(10): 2349-2357 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Poggio P, Songia P, Cavallotti L, et al. PCSK9 involvement in aortic valve calcification. J Am Coll Cardiol. 72(24): 3225-3227 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Whitmire JK, Slifka MK, Grewal IS, et al. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J Virol. 70(12): 8375-8381 (1996).
[CrossRef] [Google Scholar] [PubMed]
- Galabova G, Brunner S, Winsauer G, et al. Peptide-based anti-PCSK9 vaccines-an approach for long-term LDLc management. PLoS One. 9(12): e114469 (2014).
- Kobiyama K, Saigusa R, Ley K, et al. Vaccination against atherosclerosis. Curr Opin Immunol. 59:15-24 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Weisshaar S, Zeitlinger M. Vaccines targeting PCSK9: A promising alternative to passive immunization with monoclonal antibodies in the management of hyperlipidaemia? Drugs. 78: 799-808 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Fattori E, Cappelletti M, Surdo PL, et al. Immunization against proprotein convertase subtilisin-like/kexin type 9 lowers plasma LDL-cholesterol levels in mice. J Lipid Res. 53(8): 1654-61 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Kawakami R, Nozato Y, Nakagami H, et al. Development of vaccine for dyslipidemia targeted to a proprotein convertase subtilisin/kexin type 9 (PCSK9) epitope in mice. PloS one. 13(2): e0191895 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Landlinger C, Pouwer MG, Juno C, et al. The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE* 3Leiden. CETP mice. Eur Heart J. 38(32): 2499-2507 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Wu D, Zhou Y, Pan Y, et al. Vaccine Against PCSK9 Improved Renal Fibrosis by Regulating Fatty Acid β‐Oxidation. J Am Heart Assoc. 9(1): e014358 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Momtazi-Borojeni AA, Jaafari MR, Badiee A, et al. Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system. Atherosclerosis. (2019).
- Momtazi-Borojeni AA, Jaafari MR, Abdollahi E, et al. Impact of PCSK9 immunization on glycemic indices in diabetic rats. J Diabetes Res. 4757170 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Momtazi-Borojeni AA, Jaafari MR, Afshar M, et al. PCSK9 immunization using nanoliposomes: Preventive efficacy against hypercholesterolemia and atherosclerosis. Arch Med Sci. 17(5): 1365-1377 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Chackerian B, Remaley A. Vaccine strategies for lowering LDL by immunization against proprotein convertase subtilisin/kexin type 9. Curr Opin Lipidol. 27(4): 345-350 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Martins IJ. Anti-Aging Genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Advances in Aging Research. 5: 9-26 (2016).
- Martins IJ. Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. J Clin Epigenet. 3(3): 24(2017).
- Martins IJ. Appetite control and core body temperature are linked to autoimmune disease and the global chronic disease epidemic. Enliven: Immunol Immunotechnol. 3(e1): 001-009 (2019).
- Miranda MX, vanTits LJ, Lohmann C, et al. The Sirt 1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur Heart J. 36(1): 51-59 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Miranda MX, Arsiwala T, Obeid S, et al. Circulating Sirt1 Protects Against Atherosclerosis Through Its Modulation of Pcsk9 Activity. Circulation. 134: A19426 (2016).