Research Article - Diabetes Management (2018) Volume 8, Issue 3
The effect of a combination treatment of biomagnetic therapy and a low glycaemic influenced diet on nonfasting blood glucose levels in type 2 diabetic rats
- *Corresponding Author:
- Williams M
Department of Basic Medical Sciences
Faculty of Medical Sciences
University of the West Indies
Mona Campus, Kingston, Jamaica
E-mail: melisa_williams@live.com
Abstract
This study was done to determine the effects of biomagnetic therapy on non-fasting blood glucose levels in type 2 diabetic (T2D) rats fed with a low glycaemic index influenced (LGI) diet. Both male and female streptozotocin induced T2D sprague dawley rats (4 each per group) weighing 150-250 g were assessed, with and without biomagnetic therapy using 5000 gauss magnetic bracelets. Non-fasting glucose levels were measured in the blood collected from the tail, once weekly for sixteen (16) weeks using a portable glucometer (Glucolab Blood Glucose Monitoring System). Results showed that there was a significant (p<0.05) reduction of non-fasting blood glucose levels (NFBG) over the experimental period for diabetic groups that were under the influence of biomagnetic therapy; fed low GI drink and a combination of both biomagnetic therapy and low GI drink; with average blood glucose levels for the final week being 6.97 ± 1.89 mmol/L, 6.40 ± 0.06 mmol/L and 5.87 ± 0.82 mmol/L respectively as compared to the non-treated diabetic rats (control) which averaged blood glucose levels of 29.40 ± 4.00 mmol/L. Biomagnetic therapy and a low GI influenced diet can facilitate in the maintenance and management of type 2 diabetes by lowering non-fasting blood glucose levels as groups exposed to the biomagnetic therapy showed blood glucose levels trending to that of normo- glycaemic levels of 4.0-7.7 mmol/L.
Introduction
Using magnets for medicinal purposes has recently gain traction as one of the newest trends for various health disorders, even though biomagnetic therapy has been one of the oldest forms of alternative medicine dating back to the 2000 BC. The Chinese used magnetic stones to correct body imbalances, while the Egyptians used magnets as an anti-aging agent as they thought Cleopatra’s youthfulness was due to the magnetic jewellery she wore; and the Greeks, with famous physician Galen, who first used magnets for pain relieve in 200 BC [1]. Presently, magnetic fields are being used to treat various health problems by European and American physicians. In fact, doctors in Russia routinely use magnets to speed healing after surgery as well as to improve circulation, and to mend and strengthen bones. The biomagnetic industry is now a multimillion dollar industry and is steadily increasing in value, and the thought that a single one-time investment can lead to a lifetime of benefit, makes biomagnetic therapy an attractive and cost-effective treatment. With this knowledge, it is important to find out the biochemistry behind how effective biomagnetic therapy truly is, as persons are wearing biomagnetic jewellery hoping that it will help to cure and maintain chronic illnesses, such as diabetes mellitus [1].
▪ How biomagnetic therapy works
It is believed that biomagnetic therapy affects the human body by interacting with three systems of the body: the circulatory, nervous and endocrine systems. There are several claims on which biomagnetism thrives on. Firstly, the electrical conductivity of the blood is said to increase when biomagnets are worn due to the presence of the weak current that runs through the magnetic field that eventually increases the number of ions in the blood. This ionized blood then circulates throughout the body resulting in improved efficiency of blood flow and furthermore a more stable blood pressure [2,3]. Secondly, biomagnetic therapy claims to increase oxygen flow to cells and removes free radicals and wastes from cells as blood is magnetised. The iron in the blood via ferrous haemoglobin is responsible for oxygen and carbon dioxide transport and thus once the blood is magnetised a favourable response is expected [2]. The third feasible explanation revolves around the ability of magnetic fields forming a secondary current which is formed around the flux lines of the cells resulting in an ionized cell protoplasm and energizes the tissue by stimulating cell metabolism. This would then strengthen cell functions as the cell metabolism reacts to the electrical current created by the magnetic field. This current can result in a decrease in muscle spasms, as well as a reduction in inflammation of body tissues. This increase in the metabolism of the cell aids new cell growth, as well as cell regeneration. Negative-pole energy of magnetic fields can interfere with the ability of nerve cells to carry pain impulses to the brain, where the sensation of pain is then registered. When a negative magnetic field is placed near a nerve, the positively charged ions of the nerve impulse are attracted to the negative-pole magnetic field, thus reducing the flow of positively charged ions through the nerves to the brain. Magnetic fields may work to regulate hormone secretions in endocrine glands. One theory, is that the increase in electrical current being created by a magnet forms a net around the glands and ducts. An increase in the concentration of oxygen stimulates production while the net optimizes secretion levels. Normalizing hormone functions in the body impacts conditions caused by a hormonal imbalance. Hormones are an integral factor in regeneration and overall energy levels, while good circulation ensures that hormonal levels are distributed correctly throughout the body [1,2].
▪ Biomagnetic therapy and diabetes
Over the years many diabetics have looked to alternative medicines for treating their conditions as they tend to be more cost effective and compliance is an ease. Energy therapies are one of the more well-known of these methods. However, despite this, very limited in-depth research on diabetes mellitus and biomagnetic therapy have been done, and for those research that exists they dwell solely on opinions on subjective pain relief. A study done by Weintraub (1991) looked at magnetic therapy and peripheral neuropathy. Results showed that 90% of individuals who wore biomagnetic insoles showed significant pain relief. It was conceptualised by Weintraub that the small C-fiber nerves located in the soles of the feet were targeted by the magnetic field created by the insoles, resulting in an ionic flux and electric energy which tranquillises the hyperactive nerves that is caused by this disease. However, it was acknowledged that this theory is unproven. Great potential lies within these theories upon which biomagnetic therapy surrounds, and so by setting specific parameters such as the influence it has on blood glucose levels in diabetics, less subjective and more scientific results can be acquired [4,5].
▪ Low glycaemic index foods and diabetes
The glycaemic index was initially used as a means of classifying different foods based on their effect on postprandial glycaemia [6]. Research done by the Clinical Nutrition Research Unit in Uppsala University, Sweden, showed that a diet characterized by low glycaemic index (LGI) starchy foods lowers the glucose and insulin responses, suggesting a therapeutic potential in diabetes [7]. The consumption of LGI diet lowers blood glucose level and can be used as an inexpensive method of managing diabetes mellitus [8]. By unlocking the relationship biomagnetic therapy has on blood glucose level it is possible that there may be a potential for its use in the treatment and maintenance of type 2 diabetes mellitus (T2D). It has been said that low glycaemic index foods can help the conditions of diabetic patients; and thus, many diabetics are now on a LGI influenced diet [8]. For this reason, the study investigated the effects of a combination treatment of biomagnetic therapy and a low glycaemic influenced diet on non-fasting blood glucose levels in type 2 diabetic rats.
Methods
▪ Induction of T2D in rats
For the induction of T2D in rats a modified version of previously used protocol for fructose-fed streptozotocin-injected type 2 diabetic rat model; Wilson et al., was used [9]. For a gender unbiased study, Male and female Sprague-Dawley rats (150 – 250 kg) were fed rat food and 10% fructose instead of water for the initial 2 weeks after which T2D was induced by intravenously administering 40 mg/kg BW streptozotocin (STZ). Rats are fed with normal water and rat food for remaining of induction. One week later, a non-fasting blood glucose reading is taken and animals with a blood glucose more than 7.8 mmol/L are considered T2D. Type 2 diabetes is brought about when varying levels of insulin is being produced but is insufficient to reduced blood glucose level. This can be accomplished by the destruction of the beta cells found in the pancreas via the use of streptozotocin. Precaution will be taken in handling the Streptozotocin. Chemically resistant gloves, lab coat, eye protection, respirator and a fume hood were employed.
▪ The animal study
The animal study involved Sprague-Dawley rats weighing approximately 150 g - 250 g rat at 90 days of age obtained from the Animal House of The University of the West Indies. Animals were housed in stainless steel cages, which were cleaned daily and under a 12/12-hour light/ dark cycles with rats having free access to food and water. Rats were divided into four groups each having 4 males and 4 females; D- Diabetic rats fed normal rat diet; DM- Diabetic rats on biomagnetic therapy and fed normal rat diet; DL- Diabetic rats on low glycaemic index drink; DML- Diabetic rats on both biomagnetic therapy and low glycaemic index drink; N- Non- Diabetic rats fed normal rat diet; NM- Non-diabetic rats on biomagnetic therapy and fed normal rat diet; NL- Non-Diabetic rats fed low glycaemic index drink; NML- Non-Diabetic rats on both biomagnetic therapy and fed low glycaemic index drink.. Based on the daily requirements for known diabetic drink, each rat was given 12 ml of the LGI drink daily in groups DML and NML which was drank in its entirety after which, the normal diet of Formulab Diet rat food (purchased from OK Feed Store, Miami, FL., U.S.A) and water were resumed. Magnetic bracelets of 5000 gauss in strength were purchased from Billy the Tree, New York, U.S.A., and worn on specific rat groups around the torso region for a period of three (3) months. The duration experimentation was 3 months and 1 month for induction, during this period blood samples were taken weekly after which animals were sacrificed.
▪ Non-fasting blood glucose
Non-fasting blood glucose levels were measured in the blood collected from the tail, once weekly for twelve (12) weeks using a portable glucometer (Glucolab Blood Glucose Monitoring System). Animals were sacrificed after experimental period using Sodium Pentobarbital 100 or > mg/kg IV as euthanasia agent and blood and organ samples collected for further testing.
▪ Ethical approval
Animals were maintained in accordance to the rules and regulations of the University of the West Indies, Mona Animal Ethics Committee (Ethical approval number: AN 17,13/14).
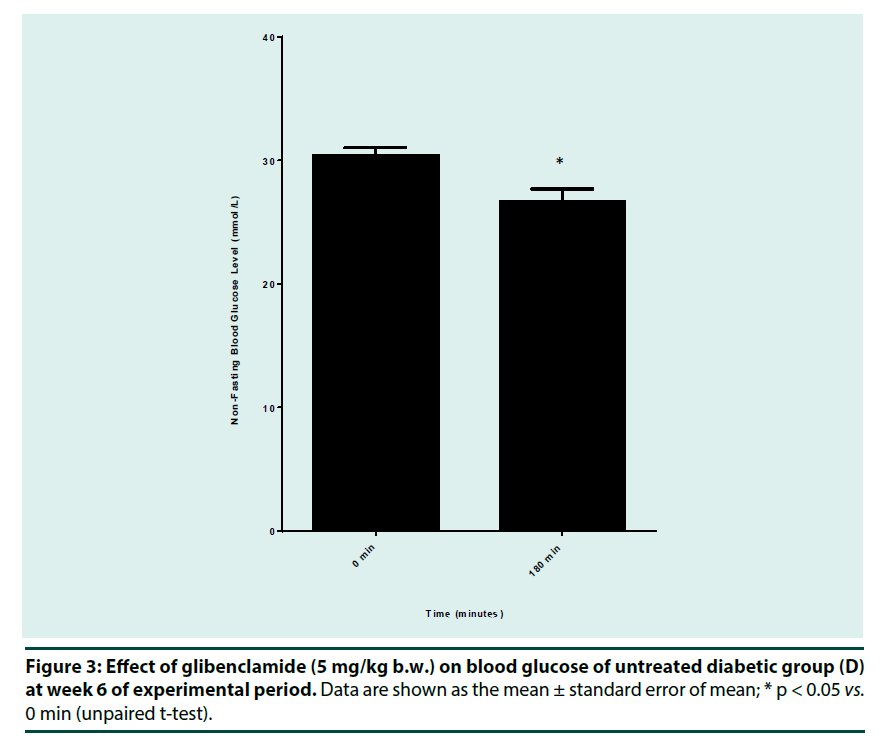
▪ Anti-diabetic drug response test
Anti-diabetic drug response test was performed on week 6 by using glibenclamide (sulfonylurea). Animals in group D (untreated diabetic rats) were fasted for 3 h before receiving a single oral dose of glibenclamide (5 mg/kg b.w.) dissolved in 1% sodium carboxymethyl cellulose (Na-CMC). Blood glucose concentration was measured at 0 and 180 min post oral administration of the drug.
▪ Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6. Data obtained from the experiments are expressed as mean ± SE. Values of p≤0.05 were considered significant using One Way ANOVA test.
Results
Table 1 shows the non-fasting blood glucose levels of rats in various experimental groups at the initial and final weeks of the experiment. Results showed that there was a significant (p<0.05) reduction of non-fasting blood glucose levels (NFBG) over the experimental period for diabetic groups that were under the influence of biomagnetic therapy; fed low GI drink and a combination of both biomagnetic therapy and low GI drink; with average blood glucose levels for the final week being 6.97 ± 1.89 mmol/L, 6.40 ± 0.06 mmol/L and 5.87 ± 0.82 mmol/L respectively as compared to the non-treated diabetic rats (control) which averaged blood glucose levels of 29.40 ± 4.00 mmol/L.
| Groups | Initial Non-Fasting Blood Glucose (mmol/L) |
Final Non-Fasting Blood Glucose (mmol/L) |
|---|---|---|
| D | 23.62 ± 1.19 a | 29.40 ± 4.00 a |
| DM | 22.57 ± 0.38 a | 6.97 ± 1.89 b |
| DL | 25.63 ± 4.20 a | 6.40 ± 0.06 b |
| DML | 22.20 ± 4.68 a | 5.87 ± 0.82 b |
| N | 5.73 ± 0.56 b | 4.93 ± 0.44 b |
| NM | 6.43 ± 0.09 b | 4.10 ± 0.53 b |
| NL | 5.67 ± 0.28 b | 4.17 ± 0.35 b |
| NML | 5.40 ± 0.35 b | 4.87 ± 0.24 b |
D- Diabetic rats fed normal rat diet; DM- Diabetic rats on biomagnetic therapy and fed normal rat diet; DL- Diabetic rats on low glycaemic index drink; DML- Diabetic rats on both biomagnetic therapy and low glycaemic index drink; N- Non-Diabetic rats fed normal rat diet; NM- Non-diabetic rats on biomagnetic therapy and fed normal rat diet; NL- Non-Diabetic rats fed low glycaemic index drink; NML- Non-Diabetic rats on both biomagnetic therapy and fed low glycaemic index drink. Data are shown as the mean ± standard error of the mean. Figures in vertical columns with different superscripts are significantly different; p < 0.05 One Way ANOVA test.
Table 1: Initial non-fasting blood glucose and final non-fasting blood glucose for rats treated with/without biomagnetic therapy and low glycaemic index drink.
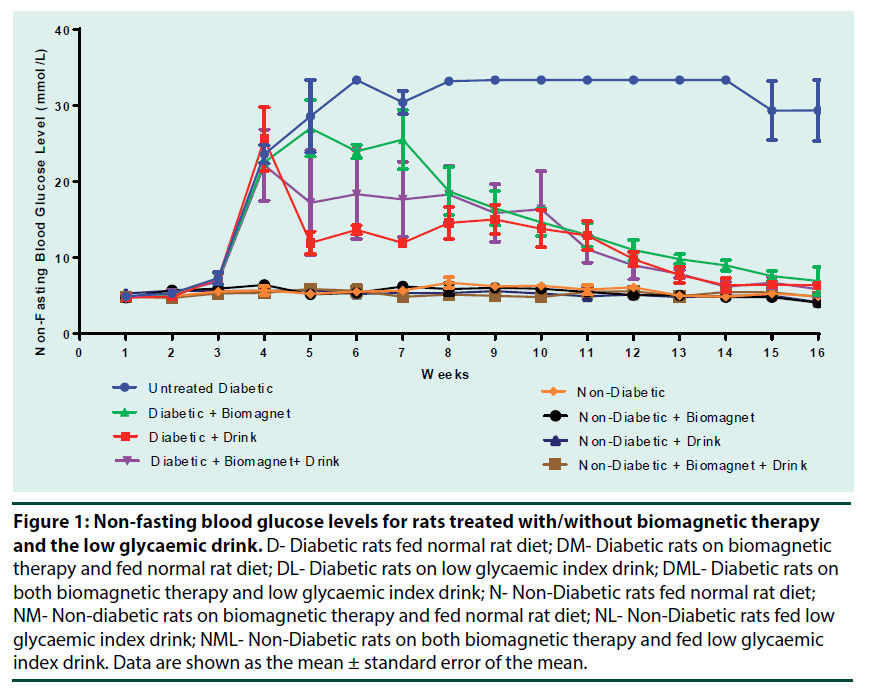
Figure 1 shows the non-fasting blood glucose levels for all groups over the experimental period. There were not any significant differences (p>0.05) in NFBG levels over the experimental period for thee non-diabetic groups and they were all in speculated range, with non-fasting blood glucose levels of 4.0-7.7 mmol/L. Slight fluctuations were seen for all diabetic groups, however there was a greater degree of inconsistency in blood glucose levels for the groups that were on individual therapy (groups DM and DL), as oppose to the group the was on both biomagnetic therapy and the low GI influenced diet (group DML). From weeks 7- 14, untreated diabetic rats (group D) had very high NFBG levels of >33 mmol/L, however, from weeks 15-16 the NFBG levels started to decrease (Figure 1). Blood glucose levels for untreated diabetic group (group D) was significantly different from all treated diabetic groups (groups DM, DL and DML) at the end of the experiment.
D- Diabetic rats fed normal rat diet; DM- Diabetic rats on biomagnetic therapy and fed normal rat diet; DL- Diabetic rats on low glycaemic index drink; DML- Diabetic rats on both biomagnetic therapy and low glycaemic index drink; N- Non-Diabetic rats fed normal rat diet; NM- Non-diabetic rats on biomagnetic therapy and fed normal rat diet; NL- Non-Diabetic rats fed low glycaemic index drink; NML- Non-Diabetic rats on both biomagnetic therapy and fed low glycaemic index drink. Data are shown as the mean ± standard error of the mean.
Figure 1: Non-fasting blood glucose levels for rats treated with/without biomagnetic therapy and the low glycaemic drink.
Table 2 shows the initial body weight and final body weight for rats treated with/without biomagnetic therapy and fed normal and low glycaemic index diet throughout the experimental period. There were no significant differences (p ≥ 0.05) in initial body weights among all groups, however, significant differences (p≤0.05) were observed in their final weights. There was a significant difference in final body weight between untreated diabetic rats and diabetic rats fed the low GI drink (groups D and DL) among all other groups. No significant differences were observed among groups DM, DML, NML and NL. A significant difference was seen between the untreated non-diabetic and non-diabetic group treated with biomagnetic therapy (groups N and NM) which were heavier in weight against all other groups except for group the non-diabetics on both biomagnetic therapy and on the low GI diet (group NML).
| Groups | Initial Body Weight (g) |
Final Body Weight (g) |
|---|---|---|
| D | 213.33 ± 3.33 a | 96.67 ± 3.33 a |
| DM | 228.00 ± 2.00 a | 257.00 ± 18.68 b |
| DL | 223.33 ± 3.33 a | 163.33 ± 8.82 a |
| DML | 225.00 ± 8.47 a | 258.33 ± 10.14 b |
| N | 243.75 ± 14.75 a | 385.00 ± 17.73 c |
| NM | 235.71 ± 9.72 a | 342.82 ± 7.47 c |
| NL | 237.14 ± 7.17 a | 247.14 ± 9.69 b |
| NML | 207.86 ± 3.76 a | 300.00 ± 3.78 cb |
D- Diabetic rats fed normal rat diet; DM-Diabetic rats on biomagnetic therapy and fed normal rat diet; DL- Diabetic rats on low glycaemic index drink; DML- Diabetic rats on both biomagnetic therapy and low glycaemic index drink; N- Non-Diabetic rats fed normal rat diet; NM- Non-diabetic rats on biomagnetic therapy and fed normal rat diet; NL-Non- Diabetic rats fed low glycaemic index drink; NML- Non-Diabetic rats on both biomagnetic therapy and fed low glycaemic index drink. Data are shown as the mean ± standard error of the mean. Figures in vertical columns with different superscripts are significantly different; p < 0.05 One Way ANOVA test.
Table 2: Initial body weight and final body weight for rats treated with/without biomagnetic therapy and fed normal and low glycaemic index diet.
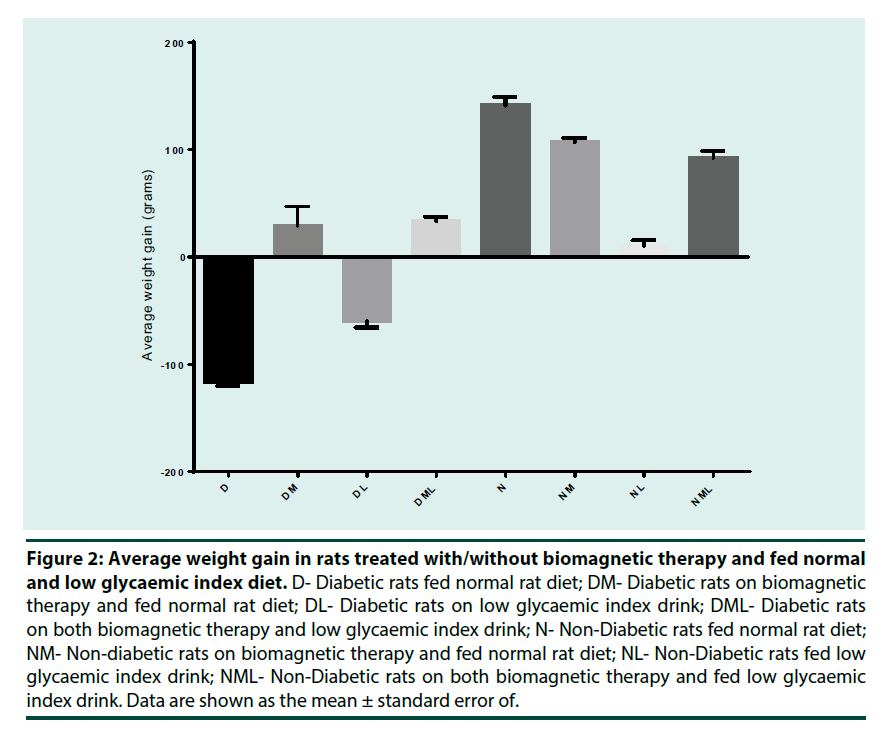
Figure 2 shows the average weight gain in rats over the experimental period. There was a weight gain for all groups except for groups D and DL which experience a weight loss.
D- Diabetic rats fed normal rat diet; DM- Diabetic rats on biomagnetic therapy and fed normal rat diet; DL- Diabetic rats on low glycaemic index drink; DML- Diabetic rats on both biomagnetic therapy and low glycaemic index drink; N- Non-Diabetic rats fed normal rat diet; NM- Non-diabetic rats on biomagnetic therapy and fed normal rat diet; NL- Non-Diabetic rats fed low glycaemic index drink; NML- Non-Diabetic rats on both biomagnetic therapy and fed low glycaemic index drink. Data are shown as the mean ± standard error of.
Figure 2: Average weight gain in rats treated with/without biomagnetic therapy and fed normal and low glycaemic index diet.
Discussion
There was a significant (p<0.05) reduction of non-fasting blood glucose levels (NFBG) over the experimental period for diabetic groups that were under the influence of biomagnetic therapy; fed low GI drink and a combination of both biomagnetic therapy and low GI drink; with average blood glucose levels for the final week being 6.97 ± 1.89 mmol/L, 6.40 ± 0.06 mmol/L and 5.87 ± 0.82 mmol/L respectively as compared to the non-treated diabetic rats (control) which averaged blood glucose levels of 29.40 ± 4.00 mmol/L (Table 1 and Figure 1). The decrease in NFBG levels seen in treated diabetic rats can be due exposure of the animals to the biomagnetic field as well as the consumption of the low GI drink daily. Biomagnetic therapy claims to attract charged particulates in the body thus increasing the blood flow; increase the flow of oxygen to cells, decrease fatty deposits on artery walls, alter nerve impulses, and even increase alkalinity of bodily fluids and moves ions [10]. It was also claimed that the diameter of blood vessels increases with biomagnetic therapy. This would allow more blood to flow through carrying oxygen and essential nutrients to the injured tissues and in return leave with the various toxins such as relative oxygen species, decreasing inflammation and aiding in cell restoration; however, there were no significant evidence for these claims [1,10,11]. The significant (p<0.05) reduction of the NFBG levels for the diabetic rats under biomagnetic therapy over the experimental period can indicate that there is a possibility that inflammation associated with organ damage from diabetes was reduced, helping to gain evidence that biomagnetic therapy can reduce tissue inflammation. Research previously done suggests that inflammation participates in the pathogenesis of type 2 diabetes [12,13]. Therefore, if inflammation is reduced by biomagnetic therapy then it can aid in the control and maintenance of diabetes by preventing further complications and preserving organ integrity. Additionally, the low GI drink also aided in the maintenance of a fairly consistent blood glucose level (Figure 1). There was an immediate decrease of blood glucose level for rats that consumed the low GI. This sharp decrease was also accompanied by a reduction in weight (Figure 2). This was expected as a low GI diet release glucose slower than medium and high GI foods thus aiding in weight loss for obese individuals. Carbohydrates that are broken down and absorbed quickly during digestion and release glucose rapidly into the bloodstream are referred to as high glycaemic index while those that break down more slowly and release glucose moderately into the bloodstream have a low glycaemic index and that which releases glucose at an intermediate rate into the blood stream is considered to have a medium glycaemic index [14,15]. However, this rapid weight loss encouraged premature death in these animals as well as hypoglycaemia which was observed in this group. The low GI drink aided in maintaining a specific weight as little weight gain was seen for non-diabetic rats fed the low GI drink. Groups exposed to just the biomagnetic therapy, both diabetic and non-diabetic rats, had weight gain similar to the untreated non-diabetic (group N), this would indicate that biomagnetic therapy did not affect the normal weight gaining process of the subjected rats. On the other hand, not only did the combination of both biomagnetic therapy and a low GI diet aided in a gradual blood glucose reduction and limited the fluctuations which were observed when animals were only exposed to individual treatments; but was fair weight gain as opposed to the unhealthy weight loss seen by both the untreated diabetic and diabetic fed the low GI drink (Figure 2). Non-fasting blood glucose (NFBG) levels for non-diabetic rats on the combination of biomagnetic therapy and low GI drink (NML) had less fluctuations and an overall lower NFBG than that of the untreated, non-diabetic rats (N group). However, there were not any significant differences (p>0.05) in NFBG levels over the experimental period for these control groups and they were all in speculated range for non-diabetic, non-fasting blood glucose levels of 4.0-7.7 mmol/L. These results would suggest that biomagnetic therapy along with the low GI drink can help to reduce slight fluctuations in NFBG levels in non-diabetics indicating that the body’s sensitivity to insulin is maintained and thus reducing the future onset of type 2 diabetes. From weeks 7- 14, untreated diabetic rats (group D) had very high NFBG levels of >33 mmol/L, however, from weeks 15-16 the NFBG levels started to decrease (Figure 3). It can be assumed that this reduction of blood glucose levels in the final weeks was due to serious complications and a sign that rats were under a significant amount of stress and were possibly about to die due to the severity of the condition.
Conclusion
Biomagnetic therapy and a low GI influenced diet seems to facilitate the maintenance and management of type 2 diabetes by lowering non-fasting blood glucose levels as groups exposed to the biomagnetic therapy showed blood glucose levels trending to that of normo- glycaemic levels of 4.0-7.7 mmol/L. Biomagnetic therapy combined with a low GI influenced diet can also help to in the maintenance of a healthy weight in diabetics. These results suggest that the combined effect of having a LGI influence diet plus wearing biomagnets can aid in the maintenance and management of type 2 diabetes.
Future work
Ongoing investigations are being done on morphological, physiological, histological and biochemical changes associated with biomagnetic therapy influence by a LGI diet.
Acknowledgement
The authors acknowledge the Scientific Research Council (SRC) for the production of the low GI drink. We also acknowledge funding from National Health Fund (NHF) given to SRC and UWI (Project # HPP83 National Health Fund of Jamaica).
Melisa Williams wishes to acknowledge the Principal Investigator and Group Leader, Professor Helen N. Asemota, for conceptualization and direction of the research, as well as to Professor Elivra Williams for the physical and magnetic theories, and to Professor Mitko Voutchkov for advice on the magnetic strength required. She also acknowledges Dr. Donovan McGrowder for chemo-pathological aspects; Dr. Chukuemeka Nwokocha for aiding in physiological inter-pretations and Ryan Francis for laboratory aid throughout the study.
References
- http://www.magnetic-therapy-guide.com/
- Null G (1998). Healing with magnets. New York, Carroll & Graf.
- Williams E. The Application of Magnetism in Complementary & Alternative Medicine. PowerPoint presentation to Morgan State University’s School of Community Health & Policy’s, Complementary & Alternative Medicine Research & Training Program, Raleigh, NC, USA (2008).
- Weintraub M, Wolfe G, Barohn R et al. Static magnetic field therapy for symptomatic diabetic neuropathy: a randomized, double-blind, placebo-controlled trial. Arch. Phys. Med. Rehabil. 84(5), 736–746 (2003).
- Guthrie D, Gamble M. Energy therapies and diabetes mellitus. Diabetes. Spectr. 14 (3), 149–153 (2001).
- Brouns F, Bjorck I, Frayn K et al. Glycemic index methodology. Nutr. Res. Rev. 18(1), 145–171 (2005).
- Jarvi A, Karlstrom B, Granfeldt Y. Improved glycemic control and lipid profile and normalized fibrinolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes. Care. 22(1), 10–18 (1999).
- http://www.ncbi.nlm.nih.gov/pubmed/11988062
- Wilson R, Islam S. Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol. Rep. 64(1), 129–139 (2012).
- Livingston J. Magnetic therapy: plausible attraction? Skeptical Inquirer 22, 25–25 (1998).
- https://biomagscience.net/live-cell-microscopy-case-studies/
- Donath M, Shoelson S. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11 (2), 98–107 (2011).
- Fasanmade O, Odeniyi I, Ogber A. Diabetic ketoacidosis: diagnosis and management. Afr. J. Med. Med. Sci. 37 (2), 99–105 (2008).
- Brand-Miller J, Holt S, Pawlak D et al. Glycemic Index and Obesity. Am. J. Clin. Nutr. 76, 281–285 (2002).
- Raffetto M. The glycaemic index diet for dummies. (2010).