Research Article - Neuropsychiatry (2016) Volume 6, Issue 2
The effect of APOE e4 allele on brain perfusion SPECT in late onset Alzheimers disease by an automated program, 3DSRT
- *Corresponding Author:
- Seiju Kobayashi, MD, PhD
Department of Neuropsychiatry, Sapporo Medical University, School of Medicine, Sapporo, Japan
Tel: +81-11-611-2111 (ext.3518)
Fax: +81-11-644-3041
Abstract
Aim: The Apolipoprotein E (APOE) ε4 allele is a risk factor for developing Alzheimer’s disease (AD), however its impact on cerebral functional changes in AD remains controversial. Previous studies reported conflicting results or failed to confirm any association, possibly because they failed to consider either the effect of patient age at onset or that of clinical stage. We performed functional neuroimaging studies using current improved techniques to re-examine the effect of APOE ε4 allele, taking both the age at onset and clinical stage into account.
Methods: 111 late onset AD (LOAD) subjects were divided into 3 subgroups based on Clinical Dementia Rating (CDR). We investigated the influence of ε4 allele on functional cerebral changes in each group of LOAD by using cutting-edge analysis software, 3DSRT.
Results: The APOE ε4 allele was significantly associated with relatively preserved cerebral blood flow in the cerebral cortices. The influence of APOE ε4 allele is not apparent in the mild stage, but is significantly demonstrable in the moderate stage.
Conclusion: Dividing AD subjects into subgroups based on the severity as well as the age at onset is important for precise outcomes. This study may help resolve the uncertainties of previous studies which reported conflicting results or failed to confirm any association. We believe this is the first study focused on the effect of APOE ε4 allele on functional cerebral changes at each stage of AD.
Keywords
Allele, Alzheimer Disease, Single-Photon Emission Computed Tomography, Apolipoprotein E4, 3DSRT
Introduction
The Apolipoprotein E (APOE) ε4 allele is a risk factor for developing Alzheimer’s disease (AD) [1,2].
APOE exists as 3 major alleles (ε2, ε3, and ε4) that translate into three isoforms of the protein (APOE2, E3, and E4). Homozygous persons for the ε4 allele are more likely to develop AD [3]. We previously reported that the allele frequencies of APOE ε4 allele were significantly higher in AD than in controls though the research quoted was mainly about dementia with Lewy bodies (DLB) as well as AD [4,5]. However the impact of the ε4 allele on functional cerebral changes in patients with AD remains controversial. Previous studies had the opposite outcomes to each other or failed to confirm any association. Three studies with Single-Photon Emission Computed Tomography (SPECT) demonstrated that an increase in APOE ε4 alleles is associated with decreased cerebral perfusion in cerebral cortices in clinical AD patients [6-8]. Conversely, two Positron Emission Tomography (PET) studies reported that the APOE ε4 allele was related to relatively preserve regional cerebral glucose metabolism in the frontotemporoparietal association cortices [9,10]. Other PET studies failed to demonstrate any significant differences in cerebral glucose metabolism between AD patients with and without the APOE ε4 allele [11,12]. One possible reason for this controversy is that in previous studies the effect of patient age at symptom onset was ignored. Furthermore, no correction was made for the clinical stage of disease which ranged from mild to severe though the mean group severities were not significantly different. The important background of considering age at onset is as follows. Late onset AD (LOAD) and early onset AD (EOAD) differ in genetic backgrounds [13], rates of progression [14], and levels of impaired verbal and visual cognition [15,16]. The NINCDS/ ADRDA criteria support this notion and state that researchers should be aware of two subtypes; ‘‘less than 65y’’ and ‘‘65y and higher’’ [17]. And the importance of considering severity is based on the great diversity of clinical symptoms of AD depending on the clinical stage. In our previous study [18], we investigated the correlation of regional cerebral blood flow (rCBF) changes and the clinical severity of AD patients and found that the brain portions with decreased rCBF differed depending on the clinical stage. Considering the effect of age at onset, we did not include EOAD (<65y) but focused on LOAD (65y and higher).
Because approximately 90% of the patients were LOAD and about 10% was EOAD in our hospital, the sample size of EOAD was small for the statistical analysis. And considering the effect of clinical severity, we furthermore divided the LOAD group into three subgroups by clinical stages (Clinical Dementia. Rating (CDR) 1: mild, CDR2: moderate, and CDR3: severe). We investigated the influence of ε4 allele on rCBF based on clinical stage. This approach allowed us to determine by what stage the influence of ε4 allele obviously appeared. To the best of our knowledge, there are no existing reports relating clinical stage with the impact of ε4 allele.
This kind of neuroimaging studies those were common about 15 years ago have benefited by technical advances in diagnostic/analytic methodology, which enable us to re-evaluate previous findings objectively and reproducibly with consideration of the onset age and the clinical stage.
This study may help resolve the differences in past findings especially conflicting results or failure to confirm any association by fathoming the cause of the controversy and improving the methodology.
Our hypotheses in this study were that the APOE ε4 is associated with decreased rCBF in the medial temporal lobe and relatively preserved rCBF in the cerebral cortices. Although the presence of the APOE ε4 allele is recognized to increase the risk of developing AD and lower the mean age of its onset [1,19], we speculated that the APOE ε4 allele does not always have negative effects particularly on the findings of brain perfusion SPECT. The rationale for this hypothesis depends on the many studies of the effect of APOE ε4 on cerebral morphological changes. These studies reported that the medial temporal lobe in patients with APOE ε4 had significantly greater atrophy than patients without APOE ε4, while the whole brain volume loss in patients with APOE ε4 was significantly less than in patients without APOE ε4 [6,20-23]. Our findings may assist judgment of therapeutic effects like whether the ineffectiveness of antidementia medicine is contributing to the progression of decreased rCBF or not while considering the existence of ε4 allele. For example we can decide to switch x-antidementia medicine or add memantine on a cholinesterase inhibitor when we assess the progression of decreased rCBF as well as neuropsychological examination. In this process of assessment, considering the influence of the APOE ε4 allele on rCBF is necessary and helpful, while examining APOE subtype from a blood sample is simple and noninvasive. The involvement of APOE ε4 allele in the rCBF may have therapeutic implications for AD [24], in this regard; our study may have clinical implications as well.
Methods
▪ Subjects
The subjects were originally 129 patients with probable AD according to the NINCDS/ ADRDA criteria [17], successively recruited in Sunagawa City Medical Center. Patients taking anti-Alzheimer drugs which may affect rCBF [25] and patients with vascular lesions were excluded. The subjects for the statistical analysis were 111 LOAD patients because 18 of 129 LOAD patients were taking anti-Alzheimer drugs and were excluded. The 111 LOAD patients were divided into three subgroups by CDR score (CDR1: mild, CDR2: moderate, CDR3: severe) [26]. Of the111 LOAD subjects finally analyzed, 49 patients had one or two APOE ε4 alleles (Table 1). 13 patients of these patients were mildly demented, 24 were at the moderate stage, and 12 were at the severe stage (Table 2).
| ε2/2 | ε2/3 | ε3/3 | ε2/4 | ε3/4 | ε4/4 | E4 carrier | ε4 allele | |
|---|---|---|---|---|---|---|---|---|
| Mild | 0(0) | 1(3.4) | 15(51.7) | 0(0) | 13(44.8) | 0(0) | 44.8% | 22.4% |
| N=29 | ||||||||
| 78.7±5.7 y | ||||||||
| M 9 : F 20 | ||||||||
| Moderate | 0(0) | 2(4.1) | 23(46.9) | 1(2.0) | 18(36.7) | 5(10.2) | 49.0% | 29.6% |
| N=49 | ||||||||
| 79.4 ± 7.8 y | ||||||||
| M 14 : F 35 | ||||||||
| Severe | 0(0) | 2(6.1) | 19(57.6) | 0(0) | 10(30.3) | 2(6.1) | 36.4% | 21.2% |
| N=33 | ||||||||
| 79.8 ± 1.4 y | ||||||||
| M 14 : F 19 | ||||||||
| Total | 62(55.9) | 49(44.1) | 44.1% | 25.2% | ||||
Table 1: APOE4 carrier status and the frequency of the ε4 allele in Mild, Moderate, and Severe LOAD.
| total 111 | 49 APOE ε4(+) | 62 APOE ε4(-) | ||||
| (129-18) | mild | moderate | severe | mild | moderate | severe |
| n | 13 | 24 | 12 | 16 | 25 | 21 |
| sex | 6:7 | 6:18 | 4:8 | 3:13 | 8:17 | 10:11 |
| Age (y) | 77.9±4.0 | 78.0±4.6 | 79.9±5.8 | 79.4±4.6 | 80.1±4.6 | 79.7±5.0 |
| MMSE score | 21.7±1.7 | 15.8±1.8 | 8.0±3.4 | 22.1±2.1 | 16.3±2.0 | 8.8±2.9 |
Table 2: Subjects background in each clinical stage of AD.
The remaining 62 subjects had no APOE ε4 allele (Table 1). 16 of these patients were mildly demented, 25 moderately demented, and 21 severely demented (Table 2). The background characteristics and results of the neuropsychological tests are summarized in (Table 1) and (Table 2). There was no significant difference in age, sex, and Mini-Mental State Examination (MMSE) score between APOE ε4(+) group and APOE ε4(-) group at each stage. We examined the influence of ε4 allele on the rCBF in each LOAD subgroup. Informed written consent was obtained from all subjects and their relatives. This study was approved by the institutional ethical committees at Sunagawa City Medical Center.
▪Method
We performed APOE genotyping and brain perfusion SPECT (bp-SPECT) in LOAD patients to examine the association between APOE ε4 allele and the findings of bp-SPECT.
▪ APOE genotyping
DNA genotyping of APOE was performed according to the protocol described by Hixson and Vernier et al [27]. For details, refer to our previous study [4].
▪ Brain perfusion SPECT imaging, 3DSRT
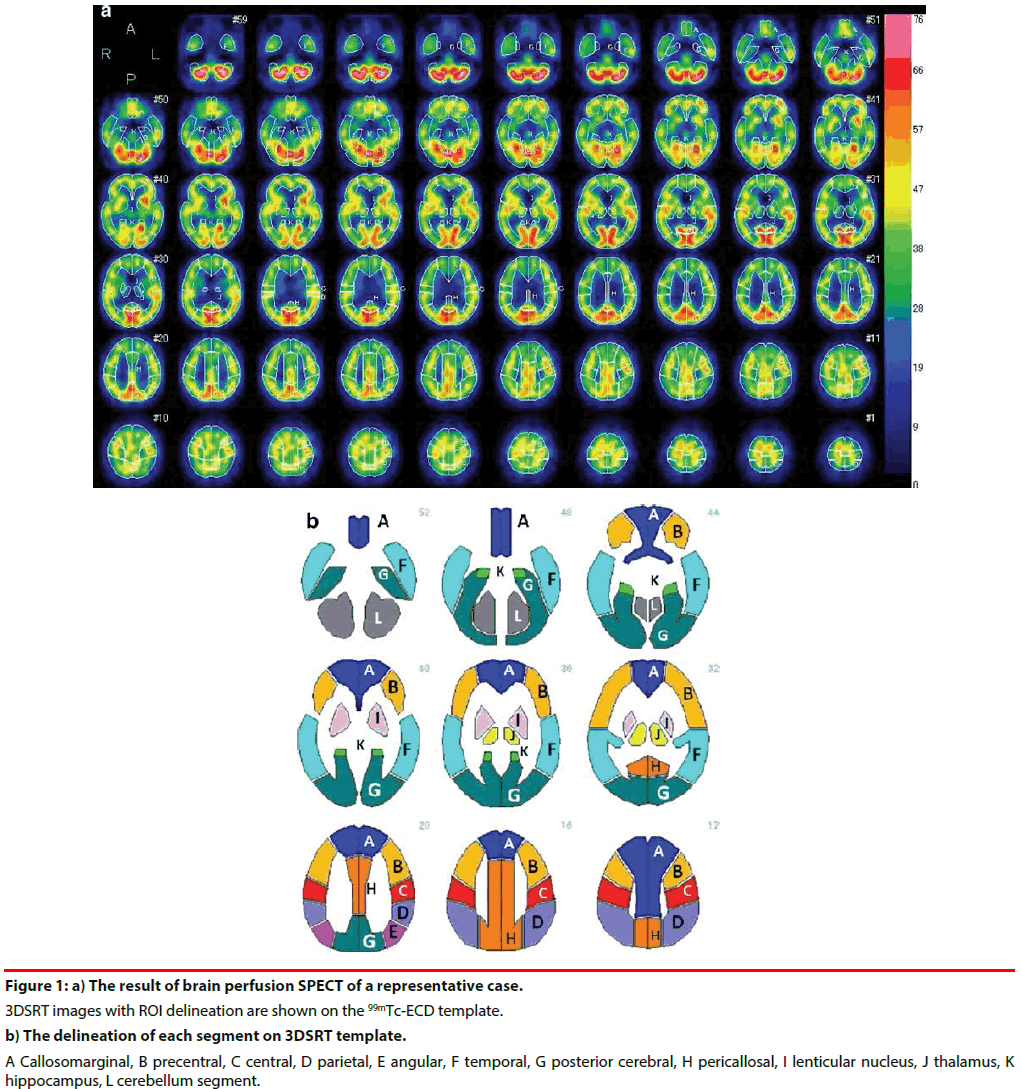
All subjects were positioned supine with their eyes closed. After injecting 600 MBq of 99mTc- Ethyl Cysteinate Dimer (ECD) (Fujifilm RI Pharma Co.,Ltd., Tokyo, Japan), the transit from the heart to the brain was monitored with a rectangular large-field gamma camera (E. Cam Signature, Toshiba Medical, Japan). Ten minutes after the angiography, SPECT images were obtained using a rotating, dualhead gamma camera for 20 min. All images were reconstructed using rampfiltered back-projection and then three-dimensionally smoothed with a Butterworth filter. The reconstructed images were corrected for gamma ray attenuation using the Chang method. Cerebral blood flow (CBF) was quantified by the Patlak plot method [28]. Quantitative flow-mapping images were obtained from the qualitative perfusion images by using Patlak plot graphical analysis and Lassen’s correction [29]. rCBF was quantified using an automated brain perfusion SPECT analyzing program, 3DSRT [18,30,31] (Figure 1). This program includes anatomic standardization of images employing statistical parametric mapping (SPM) [32] only for that purpose, rCBF quantification using a three-dimensional stereotactic ROI template, calculation of CBF, and display of the results. The 636 region of interests (ROIs) in total are categorized into 12 segments in the template (the callosomarginal, precentral, central, parietal, angular, temporal, posterior cerebral, pericallosal, lenticular nucleus, thalamus, hippocampus, and cerebellum segment) (Figure 1). We evaluated absolute value of rCBF in the 12 segments listed above in each group: APOE ε4 (+) vs APOE ε4 (-).
Figure 1: a) The result of brain perfusion SPECT of a representative case. 3DSRT images with ROI delineation are shown on the 99mTc-ECD template. b) The delineation of each segment on 3DSRT template. A Callosomarginal, B precentral, C central, D parietal, E angular, F temporal, G posterior cerebral, H pericallosal, I lenticular nucleus, J thalamus, K hippocampus, L cerebellum segment.
▪ Statistical analysis
Statistical analyses were performed using JMP version 12 and SAS version 9.4 (SAS Institute Inc., Cary, NC). Data obtained by 3DSRT were divided into 6 groups by the stage of AD and the existence of APOE ε4 allele: [mild and ε4 (+)], [mild and ε4 (-)], [moderate and ε4 (+)], [moderate and ε4 (-)], [severe and ε4 (+)], and [severe and ε4 (-)]. After performing an analysis of variance (ANOVA) among the 6 groups, p-values were adjusted for multiple comparisons by the False Discovery Rate (FDR) method. The brain areas which had significances (p<0.05) were focused on. Subsequently in the areas, the ROIs which had significant differences in rCBF between the APOE ε4 (+) group and APOE ε4 (-) group were detected using chi-squared tests with contrast statistics in each stage: mild, moderate, and severe, respectively. Statistical significance was set at p<0.05 (significant) or p<0.01 (highly significant).
Results
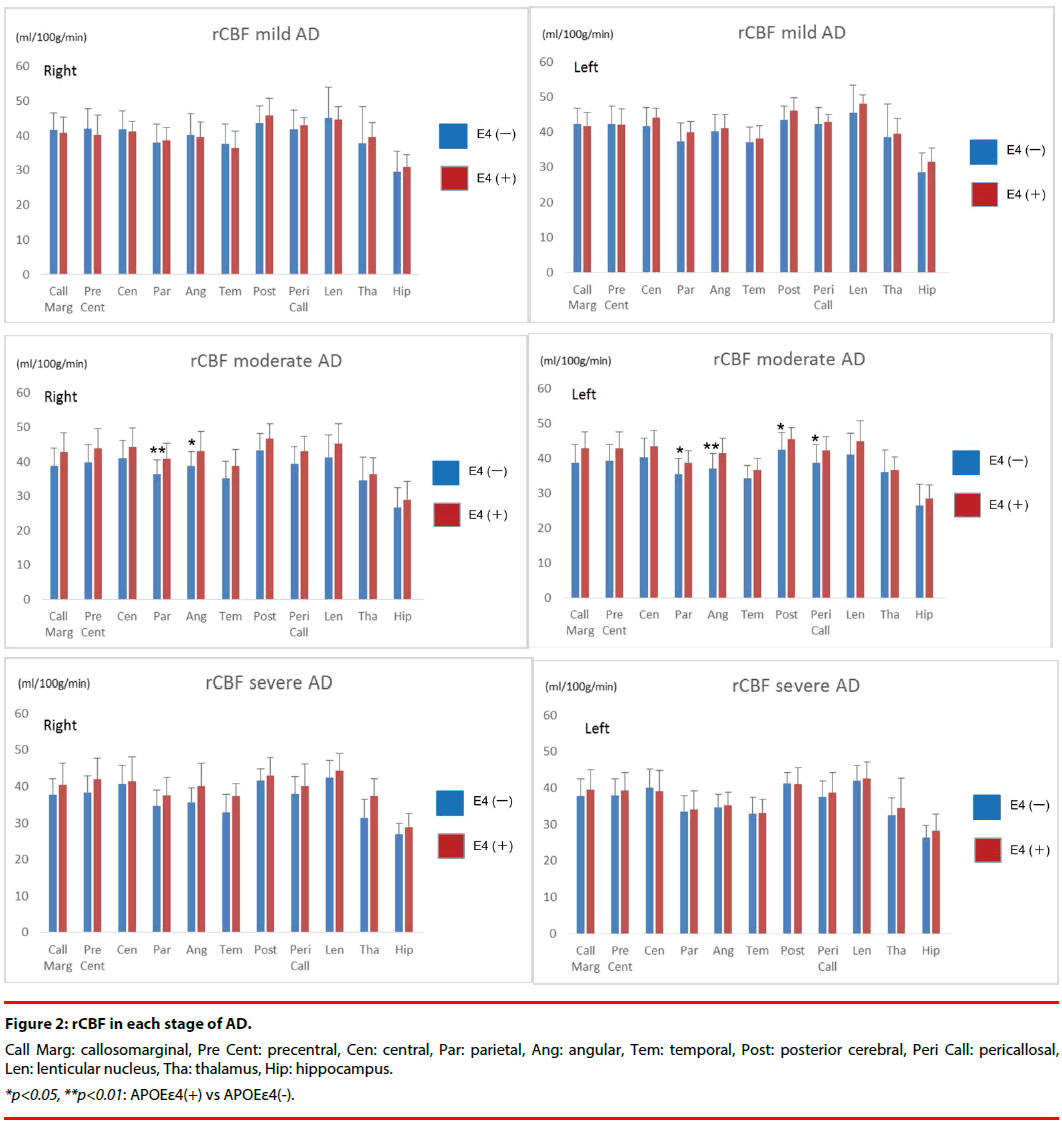
Results of quantification of rCBF in all groups are shown in (Figure 2).
In the mild stage, there were no significant differences between APOE ε4 (+) group and APOE ε4 (-) group in any region.
In the moderate stage, all segments had higher rCBF in APOE ε4 (+) group than in APOE ε4 (-) group. rCBF in the bilateral parietal and angular, left posterior cerebral, and left pericallosal segments was significantly higher in the APOE ε4 (+) group than in the APOE ε4 (-) group. The rCBF (ml/100g/min) of the bilateral parietal in APOE ε4 (+) group was 40.9 ± 4.5 (Right) and 38.7 ± 3.5 (Left), whereas that in APOE ε4 (-) group was 36.4 ± 4.2 (Right) and 35.4 ± 4.6 (Left). The rCBF of the bilateral angular in APOE ε4 (+) group was 43.0 ± 5.8 (Right) and 41.4 ± 4.5 (Left), whereas that in APOE ε4 (-) group was 38.8 ± 4.1 (Right) and 37.0 ± 4.4 (Left). The rCBF of left posterior cerebral in APOE ε4 (+) group was 45.6 ± 3.2, whereas that in APOE ε4 (-) group was 42.6 ± 4.8.
The rCBF of left pericallosal in APOE ε4 (+) group was 42.4 ± 3.8, whereas that in APOE ε4 (-) group was 38.7 ± 5.3.
In the severe stage, the same tendency as the moderate stage was shown in all segments. However no significant differences in rCBF were identified between the APOE ε4 (+) and APOE ε4 (-) groups.
Discussion
The aim of this paper was to investigate the association between the APOE genotype and functional cerebral changes. Although the presence of the APOE ε4 allele is recognized to increase the risk of developing AD and lower the mean age of its onset [1,19], the impact of the ε4 allele on clinical manifestations in patients with AD remains controversial. Though the effect of APOE ε4 allele on cerebral morphological changes had also been controversial, we have possibly recently reached a consensus with a majority of recent studies reporting that APOE ε4 is significantly associated with pronounced atrophy in the medial temporal lobe and relatively preserved atrophy in the whole brain. However the debate continues about the impact of the ε4 allele on cerebral blood flow in AD. Three studies with SPECT demonstrated that increased APOE ε4 alleles are associated with decreased cerebral perfusion in the left occipital cortex [6], the bilateral parietal and occipital cortices [7], and the right frontal and left occipital lobes [8] in clinical AD patients. Conversely, two PET studies reported that the APOE ε4 allele was related to relatively preserve regional cerebral glucose metabolism in the frontal [9] and frontotemporoparietal association cortices [10]. Other PET studies failed to demonstrate any significant differences in cerebral glucose metabolism between AD patients with and without the APOE ε4 allele [11,12]. Although FDG PET is reported to be superior to blood flow SPECT, bp-SPECT has enough high sensitivity and specificity for dementia/no-dementia: over 70% [33]. The clinical usage of SPECT is much more common than that of PET and since there are disagreements even among PET studies as well, they are unlikely to be due to equipment differences (SPECT or PET). One possible explanation for this reported difference is that previous studies failed to consider the effect of patient age at symptom onset. LOAD and EOAD differ in genetic backgrounds [13], rates of progression [14], and levels of impaired verbal and visual cognition [15,16]. The NINCDS/ ADRDA criteria support this notion and states that researchers should be aware of two subtypes; ‘‘less than 65y’’ and ‘‘65y and higher’’ [17]. We did not include EOAD but focused on LOAD. Another possible explanation for the conflicting findings is that previous studies did not correct for the impact of the clinical disease stage. Even though APOE ε4 (+) group and APOE ε4 (-) group were matched for the mean MMSE, each group includes a spectrum of patients varying from mild to severe. The clinical symptoms of AD vary greatly depending on the clinical stage. Consequently, we divided our subjects into three subgroups based on the CDR score: mild, moderate, and severe stages. To the best of our knowledge, there are no neuroimaging studies regarding APOE ε4 allele in which AD subjects are divided into 3 groups on the basis of severity. Of previous studies, the one by Hirono et al. [34] strictly separated the subjects into two groups based on the age at onset. The mean MMSE scores in APOE ε4 (+) and APOE ε4 (-) groups were 19.5 ± 5.1 and 20.1 ± 4.0, respectively. Although the subjects were mainly mild AD patients, background factors including MMSE were also well controlled. They found no significant differences in the regional cerebral glucose metabolism pattern between APOE ε4 (+) and APOE ε4 (-) groups. We support their result because our results showed the same outcomes in the mild stage. However we also found that the differences appeared from the moderate stage. At what stages APOE ε4 (+) are significantly influential to rCBF is a novel and valuable point. Crucially, our report in which the outcomes differ depending on the stages stresses the importance of including the stage classification as well as the age at onset. Our results suggest that though the difference doesn’t appear in the mild stage, it becomes most significant in the moderate stage and indistinguishable in the severe stage. We speculated the reason why there were no significant differences between APOE ε4 (+) group and APOE ε4 (-) group in the severe stage though the same trend as the moderate stage was shown in all segments. Because severe AD patients usually reveal a diffuse decrease of rCBF throughout the cerebral cortices [18], the influence of the severity strengthen and that of APOE ε4 may be relatively weakened and be indistinguishable. This may be due to a maximal effect on decreasing rCBF in the severe stage of AD. Additionally the adjustment of p-values by FDR method for multiple comparisons might make it more difficult to have significant differences in some regions compared to focusing exclusively on the severe stage of AD.
One of our hypotheses, which is that APOE ε4 is associated with relatively preserved rCBF in the whole brain, was proven. However another hypothesis that the APOE ε4 was associated with decreased rCBF in the medial temporal lobe wasn’t found. It may be because the medial temporal lobe such as hippocampus may have functional compensatory mechanism [35] though entorhinal and hippocampal atrophy are the earliest and most specific Magnetic Resonance Imaging (MRI) predictors of future dementia.
Before concluding this discussion, we briefly review the technical advances in neuroimaging methodology, 3DSRT. Objectivity when observing the region of interest (ROI) is critical. As long as we employ manual settings for the ROI, the reproducibility and objectivity of the results will be jeopardized, because the ROIs must be consistently placed on SPECT referential slices for every subject. Takeuchi et al. developed fully automated rCBF quantification software, 3DSRT, which allows objective assessment of rCBF by setting the ROIs identically on anatomically standardized SPECT images by SPM [31,36]. Most published neuroimaging studies employed conventional manual ROI settings. In the present study, we used the fully automated ROI analysis software 3DSRT, which compensates for the weaknesses of former quantitative analyses and allows us to obtain universal ROI free of subjective errors. The usefulness and validity of 3DSRT have been already reported in many papers [18,37-40].
This novel technique also seemed to contribute to more precise outcomes.
Limitations
We acknowledge some limitations to our study. The number of subjects was small, because we had to divide the 111 LOAD patients into three subgroups based on severity and also compared rCBF in APOE ε4 (+) vs APOE ε4 (-) group, respectively. However we think this division is essential for accurate outcomes as we have repeated. Also we have not confirmed the diagnosis through neuropathological means. However, we strongly believe that a detailed history combined with a careful physical, neuro-psychological cognitive test, and neuroimaging tools (MRI and bp-SPECT) can significantly increase the precision of clinical diagnosis. Since the members of our memory clinic include psychiatrists, a neurologist, a neurosurgeon, a clinical psychologist and radiological technicians, this team conference also contributes to providing accurate diagnoses. A greater number of large and wellcontrolled studies are required to further assess this approach.
Acknowledgments
We are indebted to Dr. Peter M Olley for his English revision on our manuscript and Mr. Ryo Susukida for his technical support. This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Young Scientists (B) 26860937).
Author Contributions
SK: Principal investigator, manuscript draft preparation, design or conceptualization.
TI: Design or conceptualization, analysis and interpretation.
MT: Manuscript draft preparation, design or conceptualization.
HS: Acquisition and collection of data, analysis and interpretation.
YK: Acquisition and collection of data, analysis and interpretation.
TI: Manuscript draft preparation.
KF: Analysis and interpretation.
HT: Acquisition and collection of data, analysis and interpretation.
HM: Acquisition and collection of data, analysis and interpretation.
WU: Study supervision.
EH: Study supervision.
KU: Study supervision, acquisition and collection of data, design or conceptualization.
CK: Study supervision.
References
- Corder EH, Saunders AM, Strittmatter WJ, et al.Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261(5123), 921-923(1993).
- Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology 43(8), 1467-1472(1993).
- Strittmatter WJ, Roses AD. ApolipoproteinE and Alzheimer's disease. Proc. Natl. Acad. Sci. U S A 92(11), 4725-4727 (1995).
- Kobayashi S, Tateno M, Park TW, et al.Apolipoprotein E4 frequencies in a japanese population with Alzheimer's disease and dementia with lewy bodies. PLoS. One 6(4), e18569(2011).
- Kawanishi C, Suzuki K, Odawara T, et al.Neuropathological evaluation and apolipoprotein e gene polymorphism analysis in diffuse Lewy body disease. J. Neurol. Sci. 136(1-2), 140-142(1996).
- Lehtovirta M, Soininen H, Laakso MP, et al. SPECT and MRI analysis in Alzheimer's disease: Relation to apolipoprotein E epsilon 4 allele. J.Neurol.Neurosurg. Psychiatry 60(6), 644-649(1996).
- Lehtovirta M, Kuikka J, Helisalmi S, et al.Longitudinal SPECT study in Alzheimer's disease: Relation to apolipoprotein E polymorphism. J. Neurol.Neurosurg. Psychiatry 64(6), 742-746(1998).
- Hogh P, Knudsen GM, Kjaer KH, et al.Single photon emission computed tomography and apolipoprotein E in Alzheimer's disease: Impact of the epsilon4 allele on regional cerebral blood flow. J.Geriatr. Psychiatry.Neurol 14(1), 42-51(2001).
- Higuchi M, Arai H, Nakagawa T, et al. Regional cerebral glucose utilization is modulated by the dosage of apolipoprotein E type 4 allele and alpha1-antichymotrypsin type A allele in Alzheimer's disease. Neuroreport 8(12), 2639-2643(1997).
- Mielke R, Zerres K, Uhlhaas S, et al. Apolipoprotein E polymorphism influences the cerebral metabolic pattern in Alzheimer's disease. Neurosci.Lett 254(1), 49-52(1998).
- Corder EH, Jelic V, Basun H, et al.No difference in cerebral glucose metabolism in patients with Alzheimer disease and differing apolipoprotein E genotypes. Arc. Neurol54(3), 273-277(1997).
- Hirono N, Mori E, Yasuda M, et al.Lack of association of apolipoprotein E epsilon4 allele dose with cerebral glucose metabolism in Alzheimer disease.Alzheimer. Dis. Assoc.Disord 12(4), 362-367(1998).
- Cummings JL. Cognitive and behavioral heterogeneity in Alzheimer's disease: Seeking the neurobiological basis. Neurobiol. Aging 21(6), 845-861(2000).
- Jacobs D, Sano M, Marder K,et al.Age at onset of Alzheimer's disease: Relation to pattern of cognitive dysfunction and rate of decline. Neurology 44(7), 1215-1220(1994).
- Fujimori M, Imamura T, Yamashita H, et al. Age at onset and visuocognitive disturbances in Alzheimer disease.Alzheimer. Dis. Assoc.Disord 12(3), 163-166(1998).
- Imamura T, Takatsuki Y, Fujimori M, et al.Age at onset and language disturbances in Alzheimer's disease. Neuropsychologia 36(9), 945-949(1998).
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology 34(7), 939-944(1984).
- Kobayashi S, Tateno M, Utsumi K, et al.Quantitative analysis of brain perfusion spect in Alzheimer's disease using a fully automated regional cerebral blood flow quantification software, 3DSRT. J. Neurol.Sci 264(1-2), 27-33(2008).
- Blacker D, Haines JL, Rodes L, et al. ApoE-4 and age at onset of Alzheimer's disease: The NIMH genetics initiative. Neurology 48(1), 139-147(1997).
- Mori E, Lee K, Yasuda M, et al. Accelerated hippocampal atrophy in Alzheimer's disease with apolipoprotein E epsilon4 allele. Ann.Neurol 51(2), 209-214(2002).
- Yasuda M, Mori E, Kitagaki H, et al.Apolipoprotein E epsilon 4 allele and whole brain atrophy in late-onset Alzheimer's disease. Am. J. Psychiatry 155(6), 779-784(1998).
- Hashimoto M, Yasuda M, Tanimukai S, et al.Apolipoprotein E epsilon 4 and the pattern of regional brain atrophy in Alzheimer's disease. Neurology 57(8), 1461-1466(2001).
- Geroldi C, Pihlajamaki M, Laakso MP, et al. ApoE-epsilon4 is associated with less frontal and more medial temporal lobe atrophy in AD. Neurology 53(8), 1825-1832(1999).
- Suwa A, Nishida K, Utsunomiya K, et al.Neuropsychological evaluation and cerebral blood flow effects of apolipoprotein E4 in Alzheimer's disease patients after one year of treatment: An exploratory study. Dement.Geriatr.Cogn. Dis. Extra 5(3), 414-423(2015).
- Tateno M, Kobayashi S, Utsumi K, et al.Quantitative analysis of the effects of donepezil on regional cerebral blood flow in Alzheimer's disease by using an automated program, 3DSRT. Neuroradiology 50(8), 723-727(2008).
- Morris JC. The clinical dementia rating (cdr): Current version and scoring rules. Neurology 43(11), 2412-2414(1993).
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid. Res 31(3), 545-548(1990).
- Matsuda H, Yagishita A, Tsuji S, et al. A quantitative approach to technetium-99m ethyl cysteinate dimer: A comparison with technetium-99m hexamethylpropylene amine oxime. Eur. J.Nucl. Med22(7), 633-637(1995).
- Lassen NA, Andersen AR, Friberg L, et al. The retention of [99mTc]-d,l-HM-PAO in the human brain after intracarotid bolus injection: A kinetic analysis. J.Cereb. Blood. Flow.Metab8(6), S13-22(1988).
- Tateno M, Utsumi K, Kobayashi S, et al. Usefulness of a blood flow analyzing program 3DSRT to detect occipital hypoperfusion in dementia with Lewy bodies. Prog.Neuropsychopharmacol. Biol. Psychiatry 32(5), 1206-1209(2008).
- Takeuchi R, Matsuda H, Yoshioka K, et al.Cerebral blood flow SPET in transient global amnesia with automated ROI analysis by 3DSRT. Eur. J.Nucl. Med. Mol. Imaging 31(4), 578-589(2004).
- Acton PD, Friston KJ. Statistical parametric mapping in functional neuroimaging: Beyond PET and fMRI activation studies. Eur. J.Nucl. Med25(7), 663-667(1998).
- O'Brien JT, Firbank MJ, Davison C, et al.18F-FDG PET and perfusion SPECT in the diagnosis of Alzheimer and Lewy body dementias. J.Nucl. Med 55(12), 1959-1965(2014).
- Hirono N, Hashimoto M, Yasuda M, et al.The effect of APOE epsilon4 allele on cerebral glucose metabolism in AD is a function of age at onset. Neurology 58(5), 743-750(2002).
- Guedj E, Barbeau EJ, Didic M, et al. Effects of medial temporal lobe degeneration on brain perfusion in amnestic MCI of AD type: Deafferentation and functional compensation?Eur. J.Nucl. Med. Mol. Imaging 36(7), 1101-1112(2009).
- Takeuchi R, Yonekura Y, Takeda SK, et al.Fully automated quantification of regional cerebral blood flow with three-dimensional stereotaxic region of interest template: Validation using magnetic resonance imaging-technical note. Neurologia medico-chirurgica 43(3), 153-162(2003).
- Li X, Shimizu S, Jibiki I, et al.Correlations between z-scores of VSRAD and regional cerebral blood flow of SPECT in patients with Alzheimer's disease and mild cognitive impairment. Psychiatry.Clin.Neurosci. 64(3), 284-292(2010).
- Tateno M, Honma T, Kobayashi S, et al. Decreased blood perfusion in right thalamus after transient global amnesia demonstrated by an automated program, 3DSRT. Psychiatry.Clin.Neurosci 62(1), 244(2008).
- Tateno M, Kobayashi S, Shirasaka T, et al.Comparison of the usefulness of brain perfusion SPECT and MIBG myocardial scintigraphy for the diagnosis of dementia with Lewy bodies.Dement.Geriatr.Cogn.Disord26(5), 453-457(2008).
- Tateno M, Kobayashi S, Saito T. Imaging improves diagnosis of dementia with Lewy bodies. Psychiatry.Investig 6(4), 233-240(2009).