Research Article - International Journal of Clinical Rheumatology (2019) Volume 14, Issue 2
The effect of hydroxychloroquine on endothelial dysfunction in patients with rheumatoid arthritis: A doubleblind randomized clinical trial
- Corresponding Author:
- Rudy Hidayat
Department of Internal Medicine
Faculty of Medicine, Universitas Indonesia
Cipto Mangunkusumo Hospital, Jakarta, Indonesia
E-mail: rudy_hid@yahoo.co.id
Abstract
Aim: The aim of this study was to evaluate the effect of hydroxychloroquine on endothelial dysfunction in rheumatoid arthritis patients by measuring soluble vascular cell adhesion molecule-1 (sVCAM-1) and soluble E-Selectin (sE-selectin) level as the biomarkers. Another aim was to assess the role of Homeostatic Model Assessment-Insulin Resistence (HOMA-IR), Free Fatty Acid (FFA), and oxidized Low- Density Lipoprotein (ox-LDL) in endothelial dysfunction improvement. Method: A double-blind randomized clinical trial was conducted on 37 patients with rheumatoid arthritis (with methotrexate treatment) at Rheumatology Outpatient Clinic of Cipto Mangunkusumo Hospital/Faculty of Medicine Universitas Indonesia, Jakarta. Patients with insulin, anti-hypertensive and other treatment which could affect sVCAM-1 and sE-Selectin level, were excluded. Eligible subjects were randomly assigned into two groups, 18 subjects were given hydroxychloroquine 400 mg/day and 19 subjects were given placebo, both given daily for 12 weeks. Serum sVCAM-1, sE-selectin, HOMA-IR, and ox-LDL level were examined using ELISA method, while FFA level using quantification colorimetric technique. Difference level of biomarkers in percentage before and after treatment and correlation between those biomarkers were evaluated. Result: A total of 37 subjects were enrolled in the study and randomized. During the observation, there were 6 drop-out subjects which were evenly distributed in both groups. Thirty one subjects analysed in this study, 15 subjects in the HCQ group and 16 subjects in the placebo group. Serum sVCAM-1 level decreased 15% (median) in HCQ treatment group after 12 weeks, while in placebo group, it increased 9%,7% (median) compared with pre-treatment level (p value <0,05). Serum sE-selectin level in HCQ group had a higher percentage of decrease compared with placebo group, but the difference was not significant. HOMA-IR, FFA, and ox-LDL level showed insignificant changes after HCQ therapy and had no correlation with sVCAM -1 and sE-selectin level. Conclusion: Treatment of 400 mg HCQ for 12 weeks in this study was proven to decrease sVCAM-1 level, but it was not proven to decrease sE-selectin, HOMA-IR, FFA, and ox-LDL level in RA patients. There were also no correlation between each HOMA-IR, FFA, and ox-LDL level with sVCAM-1 and sE-selectin level in this study.
Keywords
rheumatoid arthritis • hydroxychloroquine • endothelial dysfunction • sVCAM-1 • sE-Selectin
Introduction
Increased cardiovascular (CV) risk in patients with Rheumatoid Arthritis (RA) has been demonstrated from various study, including increased risk of stroke [1] coronary artery disease [1,2] and mortality [1,3]. Endothelial dysfunction, which is considered a key factor in atherogenesis, is associated with an increased risk of cardiovascular events [4]. Endothelial dysfunction is the failure of endothelial cells to maintain relaxed vascular tone and low level of oxidative stress by releasing Nitric Oxide (NO) and prostacyclin (PGI2), and also controlling pro-inflammatory and pro-coagulant factors [4].
Endothelial dysfunction in RA occurrs as a result of interaction of many factors especially inflammation and traditional CV risk factors. Treatment with Disease Modifying Anti- Rheumatic Drugs (DMARDs) have been demonstrated to improve endothelial dysfunction [5,6]. On the other hand, some studies demonstrated that endothelial dysfunction still persisted in RA patients in remission state, without traditional CV risk factors [7,8]. Some drugs have been proposed to decrease the CV risk and improve endothelial dysfunction in patients with RA [9].
Hydroxychloroquine (HCQ) has been widely used as a DMARD for a long time, indicated for mild disease or used as a combination with other DMARDs [10,11]. Interestingly, many studies have reported pleiotropic effects of HCQ, including metabolic profile improvement, antioxidant, anti-aggregation and anti-thrombotic effects [11,12]. HCQ also has been proven to improve endothelial dysfunction in some animal studies with Chronic Kidney Disease (CKD) and Systemic Lupus Erythematosus (SLE) [13-15]. However, information about the effect of HCQ on endothelial dysfunction in RA patients is still limited. Therefore, the aim of this study is to evaluate the effect of HCQ on endothelial dysfunction in RA patients by comparing this treatment with placebo. The endothelial dysfunction was assessed by biomarker of soluble Vascular Cell Adhesion Molecule-1 (sVCAM-1) and soluble E-Selectin (sE-Selectin), which has been shown to correlate with the incidence of endothelial dysfunction in RA cases [16]. This study also assess the role of Homeostatic Model Assessment-Insulin Resistence (HOMAIR), Free Fatty Acid (FFA), and oxidized Low- Density Lipoprotein (ox-LDL) in endothelial dysfunction.
Method
The trial was a double-blind randomized clinical trial to assess the effect of HCQ administration on sVCAM-1 and sE-Selectin level (endothelial dysfunction biomarkers as primary outcome); as well as to HOMA-IR, FFA and ox-LDL level (metabolic change markers as secondary outcome). This study also assessed the correlation between any changes in endothelial dysfunction biomarkers with those various metabolic change markers. This study was conducted at the Rheumatology Outpatient Clinic of Cipto Mangunkusumo Hospital/Faculty of Medicine Universitas Indonesia, Jakarta.
Subjects
Inclusion criteria in this study were RA patients diagnosed by ACR/EULAR 2010 RA classification criteria, who were being or planned to be treated with methotrexate monotherapy. The exclusion criteria included other autoimmune diseases, acute severe infections, acute cardiovascular events, malignancy or other chronic inflammatory diseases, and currently taking medications that influence the endothelial function marker level, such as ACE inhibitor or statin. The patients did not smoke for the last five years.
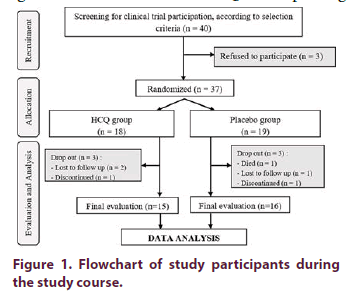
There were 40 RA patients who met the selection criteria and finally 37 patients agreed to join the study. A total of 37 subjects were alocated into two groups using block permuted randomization. There were 18 subjects in treatment group and 19 subjects in placebo group. Intervention code as the result of randomization was concealed in a sealed envelope to avoid knowing the sequence of therapy that was administered to the next patient.
Interventions
Data collection included demographic data, medical history, physical findings, lifestyle information and medication. Blood samples drawn for endothelial dysfunction biomarker examination, and hospital routine blood tests were consecutively. Endothelial dysfunctions in this study were assesed using serum biomarkers of adhesion moleculs (sVCAM-1 and sE-selectin), by applying enzyme-linked immunosorbent assay (ELISA) method before and after treatment (12 weeks). The laboratory kits for sVCAM-1 and sE-Selectin were human sVCAM-1/CD106 Immunoassay and human sE-Selectine/CD62E Immunoassay (kit Quantikine ELISA), from R&D Systems Incorporate Minneapolis, Minnesota, AS. Insulin and ox-LDL level were also examined using ELISA technique, while FFA level using quantification colorimetric technique. The subjects in treatment group were given HCQ 400 mg daily, and the rest received placebo for 12 weeks.
Ethics
This study was approved by Research Ethic Committee of Faculty of Medicine Universitas Indonesia, Jakarta. This study has been already recorded on www.clinicaltrials.gov with identification number NCT03085940.
Statistical analysis
The changes of all variables before and after intervention were reported as percentage, except DAS28-ESR variable which was the only variable analysed with absolute value changes. For comparisons of continuous independent variables changer between two groups (HCQ vs Placebo), nonparametric tests (Mann-Whitney U test) were applied, since the continuous variables of interest were not normally distributed. P values ≤ 0.05 were considered statistically significant, and all statistical tests were two-sided. Assessment of correlation between sVCAM-1 and sE-Selectin with HOMA-IR, FFA, and ox- LDL used Spearman correlation test.
Result
A total of 37 subjects were enrolled in the study and randomized (Figure 1). During the observation, there were 6 drop-out subjects which were evenly distributed in both groups. In the HCQ group, two subjects were lost to follow up and one discontinued due to pregnancy planning (methotrexate treatment contraindication). While in the placebo group, there were one lost to follow up subject, one discontinued due to pregnancy planning, and one passed away. At the final evaluation, there were 15 subjects in treatment group and 16 subjects in placebo group. The baseline characteristics of subjects of both groups were presented in Table 1.
| Characteristics | HCQ (n=18) | PLACEBO (n=19) | ||
|---|---|---|---|---|
| Age (Years), Mean (SD) | 43,1 | (12,4) | 47,4 | (14,0) |
| Sex, n(%) | ||||
| Female | 17 | -94% | 19 | -100% |
| Male | 1 | -6% | 0 | 0% |
| BMI (kg/m2), mean (SD) | 22,9 | (3,8) | 22,1 | (3,4) |
| Duration of disease (months), median (range) | 54 | (6-132) | 24 | (2-228) |
| History of MTX therapy, n(%) | ||||
| Yes | 18 | -100% | 15 | -79% |
| No | 0 | 0% | 4 | -21% |
| DAS28-ESR, median (range) | 3,9 | (2,01-6,51) | 4,3 | (2,97-8,4) |
| Activity of Disease, n(%) | ||||
| Remission | 2 | -6% | 0 | 0% |
| Low | 3 | -28% | 2 | -5% |
| Moderate | 8 | -44% | 14 | -74% |
| High | 4 | -22% | 4 | -21% |
| Laboratory | ||||
| Positive serology, n (%) | 17 | -89% | 15 | -83% |
| ESR (mm/hour), median (range) | 45 | (12-130) | 45 | (10-122) |
| sVCAM-1(ng/mL), mean (SD) | 632,9 | (176,7) | 585,9 | (162,2) |
| sE-selectin (ng/mL), median (range) | 33,2 | (15,9-80,6) | 28,8 | (15,3-87,8) |
Table 1. Baseline characteristics between two groups.
The data on Table 2 revealed serum sVCAM-1 level decreased 15% (median) in HCQ group, while in placebo group, it increased 9,7% (median) compared with pre-treatment level. The difference in percentage rate change of sVCAM between two groups was significant (p value <0,05). On the other hand, the change of serum E-selectin level in HCQ group was found not significant (p value >0,05) (Table 3 and Table 4).
| Variable | Pre treatment | Post treatment | Difference1 (%) | P value | |
|---|---|---|---|---|---|
| sVCAM-1 (ng/mL) | HCQ | 648.6† (± 186.8) |
552.9† (± 119.8) |
15‡ (-10.7 ; 39.4) |
p=0,006* |
| Placebo | 584.2† (± 154.1) |
665.6† (± 300.6) |
-9,7‡ (-89.6 ; 48.5) |
||
| sE-Selectin (ng/mL) | HCQ | 37.9‡ (15.9-80.6) |
33.3‡ (13.9-62) |
8.9‡ (-40.75 ; 49.7) |
p=0,406* |
| Placebo | 37.8‡ (15.3-87.8) |
38.6‡ (13.3-135.6) |
-7.2‡ (-54.5 ; 36.7) |
Table 2. Mean or median of sVCAM-1 and sE-selectin level percentage difference before and after intervention (12th week).
| Variable | HCQ (n=15) | Plasebo (n=16) | P value |
|---|---|---|---|
| D HOMA-IR (unit) | 32,7† (-296 ; 83,4) |
8,69† (-230 ; 68,6) |
p=0,155* |
| D FFA (nmol/L) | -20† (-500 ; 77,8) |
0† (-300 ; 60) |
p=0,211* |
| D ox-LDL (ng/mL) | 18,6† (-2743,2 ; 96,8) |
-99,7† (-4115,6 ; 97,8) |
p=0,158* |
Table 3. HOMA-IR, FFA, and ox-LDL level changes (percentage decrease) in HCQ and placebo group before and after intervention (12th week).
| Variable | r Value | p Value |
|---|---|---|
| D sVCAM-1 (%) vs D HOMA-IR (%) | 0,038 | 0,839* |
| D sVCAM-1 (%) vs D FFA (%) | -0,119 | 0,524* |
| D sVCAM-1 (%) vs D ox-LDL (%) | 0,05 | 0,789* |
| D sE-Selectin (%) vs D HOMA-IR (%) | -0,191 | 0,304* |
| D sE-Selectin (%) vs D FFA (%) | 0,118 | 0,528* |
| D sE-Selectin (%) vs D ox-LDL (%) | -0,195 | 0,294* |
Table 4. Correlation between Percentage Decrease of sVCAM-1 and sE-Selectin level with HOMA-IR, FFA, and Ox-LDL level Before and After Intervention.
Discussion
To our knowledge, this is the first study to evaluate the effect of HCQ on endothelial dysfunction in patients with RA. The final data of this study revealed a 15% decrease in serum sVCAM-1 level in the HCQ treatment group, and significantly differed from the placebo group. Meanwhile, the other endothelial dyfunction marker, serum sE-selectin level in HCQ group had a higher percentage of decrease compared with placebo group, but the difference was not significant. The role of HCQ in improving endothelial dysfunction was not affected by inflammatory conditions, as the markers of inflammation and disease activity in both groups did not differ significantly. If we compared with other previous studies, using different drug interventions that also have the effect of improving endothelial dysfunction, inconsistent result were also obtained. A systematic review involving 23 studies on endothelial cell cultures showed an inconsistent result of sVCAM-1 and E-Selectin level after statin intervention [17].
In this study, the improvements of endothelial dysfunction was characterized by lowering of sVCAM-1 levels after HCQ therapy. However, these results were not followed by a significant decrease in SE-Selectin level. The cause of this phenomenon was unclear, but there are several possible explanations such as too short treatment period or different molecular mechanisms in the two biomolecules. There was in vitro study which revealed similar result. That study was conducted on endothelial cell cultures which was activated by TNF-α, and subsequently administered mevastatin. The result of the study showed a significant decrease in sVCAM-1 level, but significant increase in sE-selectin level. In that study, mevastatin was proven to inhibit sVCAM-1 and E-selectin mRNA expression. However, mevastatin also inhibited the removal of E-selectin on the surface of endothelial cells. There are two different pathways of E-Selectin removal from the endothelial cell surface : endocytosis and subsequent rapid degradation in lysosomal compartement, and the other pathway is proteolytically cleaved and secreted into extracelluler space (shedding). Mevastatin was able to inhibit endocytosis pathway, and increase shedding process [18]. Another possible explanation is that the duration of HCQ therapy is too short (only 12 weeks), so sE-Selectin level has not decreased yet.
Nevertheless, treatment of HCQ for RA patients have a clinical cardiovascular implication due to decrease of sVCAM-1 level. Dessein et al. [19] revealed a correlation between sVCAM level and thickening of tunica intima, tunica media, and plaque formation by evaluating 74 RA patients and 80 non-RA patients. Another study from Jeger et al. [20] found that an increase of 100 ng/ml sVCAM-1 level will increase the risk of cardiovascular event with RR 1.1 (1.05-1.15).
Another data in this study revealed a decrease of HOMA-IR, FFA, and ox-LDL decline but not statistically different between two groups. The role of HCQ to improve insulin resistance is still a matter of controversy. One clinical study showed a decrease in HOMA-IR values, improvement of the Matsuda Insulin Sensitivity Index (ISI), and insulin tolerance after HCQ treatment [21,22]. On the other hand, Solomon et al. [23] revealed the opposite result after three months of HCQ therapy. While the assessment of the effects of HCQ on FFA and ox-LDL, there has been no other study as a comparison. Overall, the result of this study showed that HCQ didn’t proved to affect insulin resistance, FFA, and ox-LDL.
This study also evaluated pathway that affected the improvement of endothelial dysfunction. The result showed no significant correlation between percentage reduction of sVCAM-1 and sESelectin with percentage reduction of HOMAIR, FFA, and ox-LDL. Throughout the author’s best knowledge, there has not been any research assessing the correlation between those factors in the RA population. The results of various in vitro and in vivo studies previously published in non-RA population revealed inconsistent results. In this study also revealed that the role of HCQ to the improvement of endothelial dysfunction is not affected by inflammatory conditions, as the markers of inflammation and disease activity in both groups did not differ significantly.
Our study has several limitations. The biomarkers of endothelial dysfunction that we used is a soluble form which have smaller biological function than the form that is bound to the surface of endothelial cells, besides the fact that the role of these molecules naturally will work more in the cells that produce these molecules, and the surrounding area. Another limitation was related to the number of drop-outs of 16% subjects, which was not analysed in the end of the study. According to Dziura et al. [24] that with 5%-20% drop-outs should be carefully assessed against possible bias, by evaluating randomization, missing data, distribution, missing data types, and also statistical analysis techniques.
Conclusion
Treatment of 400 mg HCQ for 12 weeks in this study was proven to decrease sVCAM-1 level, but it was not proven to decrease sE-selectin, HOMA-IR, FFA, and ox-LDL level in RA patients. There were also no correlation between each HOMA-IR, FFA, and ox-LDL level with sVCAM-1 and sE-selectin level in this study. As a suggestion, further research is needed to asses another pathway that can explain how HCQ can improve endothelial dysfunction in RA patients. Long-term effect of HCQ on Cardiovascular event, associated with morbidity or mortality is also required for follow-up studies with cohort design, clinical trials, or meta-analysis.
Acknowledgments
The funding source of this research is partly derived from Research Department of Cipto Mangunkusumo Hospital, Jakarta. We also acknowledge research assistants, the nurse in Rheumatology Outpatient Clinic of Cipto Mangunkusumo Hospital, and the laboratory staff of Integrated Laboratory, Faculty of Medicine Universitas Indonesia.
References
- Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A metaanalysis of observational studies. Arthritis. Rheum. 59(12), 1690–1697 (2008).
- Levy L, Fautrel B, Barnetche T, et al. Incidence and risk of fatal myocardial infarction and stroke events in rheumatoid arthritis patients: a systematic review of the literature. Clin. Exp. Rheumatol. 26(4), 673–679 (2008).
- Meune C, Touze E, Trinquart L, et al. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and metaanalysis of cohort studies. Rheumatol. 48(10), 1309–1313 (2009).
- Sitia S, Tomasoni L, Atzeni F, et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 9, 830–834 (2010).
- Ursini F, Leporini C, Bene F, et al. Anti-TNF-alpha agents and endothelial function in rheumatoid arthritis: a systematic review and meta-analysis. Sci. Rep. 7, 346 (2017).
- Deyab G, Hokstad I, Whist JE, et al. Methotrexate and anti-tumor necrosis factor treatment improves endothelial function in patients with inflammatory arthritis. Arthritis. Res. Ther. 19, 232 (2017).
- Vaudo G, Marchesi S, Gerli R, et al. Endothelial dysfunction in young patients with rheumatoid arthritis and low diseases activity. Ann. Rheum. Dis. 63, 31–35 (2004).
- Mondal M, Sarkar RN, Chakroborty A, et al. Atherosclerosis in an Indian cohort of rheumatoid arthritis with low disease activity and its correlation with inflammatory markers. Indian. J. Rheumatol. 6(2), 61–67 (2011).
- van den Oever IAM, van Sijl AM, Nurmohamed MT. Management of cardiovascular risk in patients with rheumatoid arthritis: evidence and expert opinion. Ther. Adv. Musculoskel. Dis. 5(4), 166–181 (2013).
- Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 1–18 (2017).
- Olsen NJ, Schleich MA, Karp DR. Multifaceted effects of hydroxychloroquine in human disease. Semin. Arthritis. Rheum. 43(2), 264–272 (2013).
- Rainsford KD, Parke AN, Clifford-Rashotte M, et al. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 23(5), 231–269 (2015).
- Shukla AM, Bose C, Karaduta OK, et al. Impact of hydroxychloroquine on atherosclerosis and vascular stiffness in the presence of chronic kidney disease. PLoS One. 10(9), e0139226 (2015).
- Mosca, Marta, Tani, et al. Effects of in vivo treatment with hydroxychloroquine on endothelial function in a murine model of systemic lupus erythematosus [abstract]. Arthritis Rheum. 65(Suppl 10), 55 (2013).
- Gomez-Guzman M, Jimenez R, Zarzuelo MJ, et al. Chronic hydroxychloroquine improves endothelial dysfunction and protects kidney in a mouse model of systemic lupus erythematosus. Hypertension. 64(2), 330–337 (2014).
- Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction testing and clinical relevance. Circulation. 115, 1285–1295 (2007).
- Greenwood J, Mason JC. Statins and the vascular endothelial inflammatory response. Trends. Immunol. 28(2), 89–98 (2006).
- Rasmussen LM, Hansen PR, Nabipour MT, et al. Diverse effects of inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase on the expression of VCAM-1 and E-selectin in endothelial cells. Biochem. J. 360, 363–70 (2001).
- Dessein PH, Joffe BI, Singh S. Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis. Res. Ther. 7, R634–R643 (2005).
- Jager A, van Hinsbergh VW, Kostense PJ, et al. Increased levels of soluble vascular cell adhesion molecule 1 are associated with risk of cardiovascular mortality in type 2 diabetes: the Hoorn study. Diabetes. 49(3), 485–491 (2000).
- Mercer E, Rekedal L, Garg R, et al. Hydroxychloroquine improves insulin sensitivity in obese non diabetic individuals. Arthritis. Res. Ther. 14(3), R135 (2012).
- Araiza-Casillas R, Diaz-Molina R, González-Ortiz M, et al. Effects of hydroxychloroquine on insulin sensitivity and lipid profile in patients with rheumatoid arthritis. Rev. Med. Chil. 141(8), 1019–1025 (2013)
- Solomon DH, Garg R, Lu B, et al. Effect of hydroxychloroquine on insulin sensitivity and lipid parameters in rheumatoid arthritis patients without diabetes mellitus: a randomized, blinded crossover trial. Arthritis. Care. Res. (Hoboken). 66(8), 1246–1251 (2014).
- Dziura JD, Post LA, Zhao Q, et al. Strategies for dealing with missing data in clinical trials: From design to analysis. Yale. J. Biol. Med. 86(3), 343–358 (2013).