Research Article - Pharmaceutical Bioprocessing (2018) Volume 6, Issue 2
The effect of ulinastatin combined with bone marrow mesenchymal stem cells on directional repairing of liver ischemia and reperfusion injury in rats
- *Corresponding Author:
- Weixing Wang
Department of Hepatobiliary and Laparoscopic Surgery
Wuhan University Renmin Hospital
99 East Ziyang Road, Wuhan, Hubei 430072, China
E-mail: heharwuxeaxea@163.com
Abstract
Objective: To investigate repairing effect of ulinastatin combined with bone marrow mesenchymal stem cells (MSCs) on liver ischemia and reperfusion injury in rats.
Methods: A total of 54 healthy rats were randomly divided into sham operation group, model group and treatment group with 18 rats included in each group. The Pringle ‘s method was adopted to establish ischemia-reperfusion rat model and in each group a quantity of 3 mL blood was extracted from abdominal aorta respectively at 1, 2, 3 and 24 hours after reperfusion (6 rats were taken at each point) followed by measurement of level changes of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), malondialdehyde (MDA), nitric oxide (NO) and endothelin-1 (ET-1); The specimen of ischemic lobes of liver was taken to detect the protein expression of Toll like receptor 4 (TLR4).
Results: The expression level of ALT, AST and MDA at 1, 2, 3 and 24-hour after reperfusion was significantly increased in model group and treatment group than those in the sham-operation group (P<0.05). Compared with the sham-operation group, the NO level in other two groups (it was higher in the treatment group than in the model group) was significantly lower (P<0.05) and the ET-1 level in other two groups (it was higher in the model group than in the treatment group) turned out to be notably higher (P<0.05); At 24 hours after reperfusion, TLR4 protein was detected in all three groups and its level was higher in the model group and the treatment group(it was higher in the model group than in the treatment group) than those in the sham operation group.
Conclusion: Pretreatment of ulinastatin combined with bone marrow mesenchymal stem cells changes serum ALT, AST, MDA, NO and ET-1 level after ischemia reperfusion injury, inhibits the expression of liver cell TLR4 and then has a protective effect on the ischemia reperfusion injury.
Keywords
ulinastatin, bone marrow mesenchymal stem cells, rat, liver ischemia, reperfusion injury
Introduction
The decrease of blood perfusion in tissues and organs is the most important factor causing ischemic damage to cells. Therefore, it is one of the most fundamental ways to restore the blood flow of tissues and organs as soon as possible in the treatment of ischemic injury. However, after the recovery of blood flow and reperfusion in tissues and organs, the dysfunction of cell function and the function of the structure and animal organs of some patients deteriorate further.
Liver ischemia and reperfusion injury is a common pathologic damage in the process of liver diseases in which metabolism disorder of hepatocyte and the destruction of liver structure are aggravated, causing damages to liver tissue and even failure of ischemic organs when serious [1,2]. Therefore, it is important to strengthen the study on mechanism and effect of liver ischemia and reperfusion injury. Related studies have shown that ulinastatin can protect coagulation mechanism and platelet function. Bone marrow mesenchymal stem cells (MSCs) may differentiate into various tissue cells like liver cells and the research has proved that they have a protective effect on liver ischemia and reperfusion injury in rats [3,4]. However, the effect of ulinastatin combined with bone marrow mesenchymal stem cells in this regard has not been reported yet. In this study, we used hepatic ischemia reperfusion rat to study the effect of ulinastatin and bone marrow mesenchymal stem cells (MSCs) on liver ischemia and reperfusion injury in rats and explore its possible molecular mechanism.
Materials and methods
Experimental animals and groups
A total of 54 healthy male rats were selected from the experimental animal center of our hospital. They weighed in at 185-232g with a mean weight of (198.3±12.2) g 54 rats and were randomly divided into sham operation group, model group and treatment group with 18 rats in each group.
The establishment of animal model
Rats in each group needed to be fasted 12 hours but they can drink freely before surgery. Hepatic ischemic rat model was established with Pringle’s method as follow: the livers of rats were given mobilization in which the portal vein and the hepatic artery and common bile duct were separated followed by occlusion with noninvasive micro vessel clamp and at 30 min after ischemia the vessel clamp was released to produce reperfusion.
Sham operation group: the hepatic blood flow was separated by simple anaesthesia yet with no clamp of blood vessels in the liver.
Model group: the rats were intravenously injected with sterile saline (10 mL/kg BW) from their dorsum penis at 30 min prior to hepatic blood occlusion.
Treatment group: the rats were injected with ulinastatin (5 mL/kg) and bone marrow mesenchymal stem cell suspension (5 mL/kg) the same way as the model group.
Detection index
5 mL blood in each group a quantity of was extracted from abdominal aorta respectively at 1, 2, 3 and 24-hour after reperfusion (6 rats were taken at each point) followed by measurement of level changes of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), malondialdehyde (MDA), nitric oxide (NO) and endothelin -1 (ET-1);The specimen of ischemic lobes of liver was taken to detect the protein expression of Toll like receptor 4 (TLR4).
Detection of ALT, AST and MDA
The concentrations of ALT, AST and MDA were detected by automatic biochemical analyzer.
Detection of NO and ET-1
NO level was measured by nitrate reductase method and serum ET-1 level radioimmunoassay according to instructions of related kits, which were purchased from Beijing Furui Runze Biological Technology Co. ltd.
Western blot analysis
100 mg liver specimen was taken followed by being grounded with the addition of RIPA buffer and phenylmethylsulfonyl fluoride, centrifuged with high speed at 4°C for 30 min. The supernatant was collected and stored at -20°C with the protein quantified by BCA method. Protein extracts were electrophoresed through 10% or 15% polyacrylamide gels and transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were then blocked in 5% BSA for 2 hours and were incubated overnight at 4°C with primary antibodies for TLR4 and GAPDH. After the membranes were washed 3 times for 10 min with TBST (10 mM Tris, pH 7.5, 150 mM NaCl, and 0.05% Tween 20), the membranes were incubated with HRP-conjugated secondary antibody at room temperature for 90 min. The specific bands were detected by chemiluminescence. The band intensity was detected and quantified using the ChemiDoc XRS Imaging System.
Statistical methods
SPSS 21 statistical software was used for single factor analysis of variance. The significant differences among each group were analyzed by SNK test, P<0.05, suggested there was statistical significance.
Results
Comparison of serum level of ALT, AST and MDA levels in three groups
At 1,2,3 and 24 hours after reperfusion, the expression level of ALT, AST and MDA was moderately low in the sham operation group, and in the model group it significantly increased in comparison with the treatment group(P<0.05) as shown in Table 1.
| Groups | n | ALT (U/L) | AST (U/L) | MDA (mmol/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1h | 12h | 24h | 1h | 12h | 24h | 1h | 12h | 24h | ||
| Sham operation group | 6 | 35.65 ± 5.57 | 36.05 ± 2.35 | 37.07 ± 3.07 | 122.35 ± 10.23 | 124.45 ± 6.89 | 126.33 ± 4.52 | 1.04 ± 0.22 | 1.05 ± 0.65 | 1.06 ± 0.45 |
| Model group | 6 | 64.78 ± 3.01 | 70.36 ± 4.52 | 78.94 ± 6.01 | 178.92 ± 9.84 | 185.62 ± 7.31 | 197.01 ± 3.65 | 6.72 ± 0.55 | 8.94 ± 0.71 | 9.02 ± 0.86 |
| Treatment group | 6 | 45.13 ± 2.33 | 47.02 ± 1.58 | 49.14 ± 2.35 | 141.26 ± 3.45 | 152.26 ± 2.07 | 159.07 ± 3.15 | 3.52 ± 0.41 | 3.97 ± 0.36 | 4.15 ± 0.57 |
| F | - | 7.605 | 6.723 | 8.182 | 6.036 | 6.952 | 7.802 | 6.935 | 6.002 | 7.106 |
| P | - | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
Table 1. Comparison of serum level of ALT, AST and MDA levels in three groups
Comparison of serum level of NO and ET-1 in three groups
Compared with the sham operation group, the NO level in other two groups (it was higher in the treatment group than in the model group) was significantly lower (P<0.05) and the ET-1 level in other two groups (it was higher in the model group than in the treatment group) turned out to be notably higher (P<0.05). The level of NO and ET-1 in serum reached the peak at 12 h after reperfusion as shown in Table 2.
| Groups | n | NO level (μmol/L) |
ET-1level (ng/L) |
||||
|---|---|---|---|---|---|---|---|
| 1h | 12h | 24h | 1h | 12h | 24h | ||
| Sham operation group | 6 | 96.32 ± 6.32 | 103.16 ± 1.65 | 97.75 ± 5.44 | 41.23 ± 1.95 | 45.28 ± 3.35 | 41.06 ± 3.02 |
| Model group | 6 | 49.85 ± 5.74 | 54.33 ± 4.06 | 51.06 ± 2.17 | 126.71 ± 6.03 | 158.82 ± 4.03 | 106.67 ± 5.66 |
| Treatment group | 6 | 68.05 ± 2.05 | 70.38 ± 3.24 | 64.95 ± 3.22 | 91.44 ± 2.25 | 115.02 ± 5.01 | 86.83 ± 2.35 |
| F | - | 7.043 | 9.361 | 6.061 | 6.552 | 9.247 | 6.805 |
| P | - | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
Table 2. Comparison of serum level of NO and ET-1 in three groups
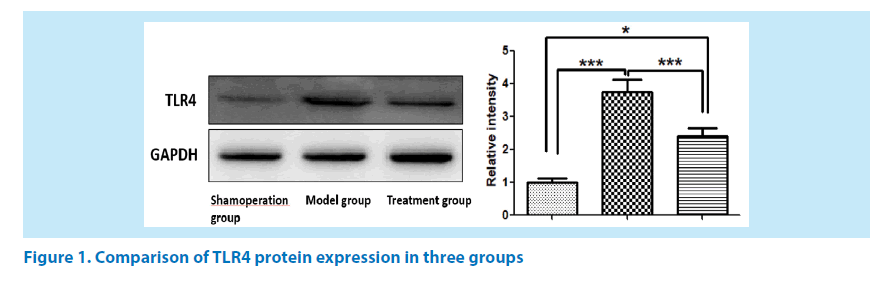
Comparison of TLR4 protein expression in three groups
At 24 hours after reperfusion, TLR4 protein was detected in all three groups and its level was higher in the model group and the treatment group (it was higher in the model group than in the treatment group) than in the sham operation group as shown in Figure 1 (GAPDH used as a reference for TLR4).
Discussion
Hepatic ischemia and reperfusion injury often occurs in liver transplantation and hepatectomy, which will affect graft function after the transplantation and give rise to acute and chronic rejection [5,6]. Studies have also shown that [7], it will restrict the utilization of organs in liver edge and reduce the utilization efficiency of the donor. Therefore, it is very important to enhance the tolerance of liver tissue to ischemia reperfusion, reduce intraoperative bleeding and protect liver function in the patients.
Ulinastatin, a urinary trypsin inhibitor, is an acid glycoprotein [8] released from the liver of the body. Relevant studies have shown that [9] it plays an important role in countershock and anti-operation stimulation. But, in recent years, its protective effect on hepatic ischemia reperfusion injury has also attracted more and more attention. Some scholars have found by animal experiments that [10] ulinastatin can inhibit the activity of oxygen free radicals and then protect the tissues from ischemia and hypoxia.
It has been reported abroad that [11] cell factors can induce homing of bone marrow hemopoietic stem cells in infarct area followed by further differentiation into cardiomyocytes and then improve myocardial function. Previous animal experiments showed that [12,13] the number of transplanted bone marrow mesenchymal stem cells which were established themselves in rats with liver damage was significantly higher than that in healthy rats, suggesting the homing of transplanted bone marrow mesenchymal stem cells to injured liver tissues.
The abnormal levels of ALT, AST and MDA were closely related to liver function [14]. In this study, we examined ALT, AST and MDA level in each group and the results showed that at 1, 2, 3 and 24-hour after reperfusion, the expression level of ALT, AST and MDA was moderately low in the sham operation group, and in the model group it significantly increased in comparison with the treatment group (P<0.05). The reason may be that ulinastatin combined with bone marrow mesenchymal stem cells can effectively stabilize the function of liver cell membrane, then reduce the level of ALT, AST as well as MDA and alleviate pathological damage to liver tissues.
NO and ET-1 are vaso-active substances with complementary effect, both of which can modulate the dynamic equilibrium in the body through negative feedback. Studies have shown that [15] NO can diastolic blood vessels in the body, inhibit the contraction effect of ET-1 on vessels and then promote the normal function of vasomotoricity with relative maintenance of dynamic equilibrium in between; But under the stress condition, there would be disorder of dynamic balance between NO and ET-1, thereby fastening the incidence and development of many diseases. On the condition of ischemia reperfusion injury, liver cells will be seriously injured with the release of many ET-1 and ETA receptors mediates, which then induces intensified contraction of liver blood vessels with worse congestion, ischemia and hypoxia in liver and releases a large number of various inflammatory factors. NO can be generated in liver cells and take part in liver biotransformation with an important role in maintenance of microcirculation. The results of this study show that compared with the sham operation group, the NO level in other two groups (it was higher in the treatment group than in the model group) was significantly lower(P<0.05) and the ET-1 level in other two groups (it was higher in the model group than in the treatment group) turned out to be notably higher (P<0.05). It is suggested that the treatment of ulinastatin combined with bone marrow mesenchymal stem cells is to play its protective role in hepatic ischemiareperfusion injury by increasing NO level and decreasing ET-1 level in serum through a certain way.
Western-blot assay results showed that at 24 hours after reperfusion, TLR4 protein was detected in all three groups and its level was higher in the model group and the treatment group(it was higher in the model group than in the treatment group) than in the sham operation group, which suggests that ulinastatin combined with bone marrow mesenchymal stem cells may exert strong antioxidant and anti-inflammatory effects by inhibiting TLR-4 signal pathway, thus protecting the liver from reperfusion injury [16].
To sum up, the pretreatment of ulinastatin combined with bone marrow mesenchymal stem cells changes serum ALT, AST, MDA, NO and ET-1 level after ischemia reperfusion injury, inhibits the expression of liver cell TLR4 and then has a protective effect on the ischemia reperfusion injury.
References
- Liu SQ, Cheng MX, Gong JP et al. Effects of silencing TSG-6 gene modified mesenchymal cells transplantation on hepatic ischemia reperfusion injury in rats. J. Zhongshan. University. 35(1), 47-53 (2014).
- Zheng S, Yang J, Zhang F et al. Effects of allogeneic bone marrow mesenchymal stem cells transplantation on hepatic ischemia-reperfusion injury in rats. World. Chinese. J. Digestol. 24(11), 1639-1648 (2016).
- Yang L, Shen ZY, Wang RR et al. Effects of heme oxygenase-1-modified bone marrow mesenchymal stem cells on microcirculation and energy metabolism following liver transplantation. World. J. Gastroenterol. 23(19), 3449-3467 (2017).
- Zhang Tao, Li Xiaojian, Deng Zhongyuan et al. The impact of ulinastatinon coagulation function and systemic inflammatory reaction on patients with burn injury. Med. J. West. China. 28(2), 201-203 (2016).
- Kanazawa H, Fujimoto Y, Teratani T et al. Bone Marrow-Derived Mesenchymal Stem Cells Ameliorate Hepatic Ischemia Reperfusion Injury in a Rat Model. Plos. One. 6(4), e19195 (2011).
- Li X, Su L, Zhang X, et al. Ulinastatin downregulates TLR4 and NF-kB expression and protects mouse brains against ischemia/reperfusion injury. Neurological Research, 2017, 39(4), 367-373.
- Bao C, Wang Y, Min H et al. Combination of Ginsenoside Rg1 and bone marrow mesenchymal stem cell transplantation in the treatment of cerebral ischemia reperfusion injury in rats. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 37(3), 901-910 (2015).
- Cao LJ, Wang J, Hao PP et al. Effects of ulinastatin, a urinary trypsin inhibitor, on synaptic plasticity and spatial memory in a rat model of cerebral ischemia/reperfusion injury. Chinese. J. Physiol. 54(6), 435-437 (2011).
- Wang X, Zhuang X, Wei R et al. Protective effects of Acanthopanax vs. Ulinastatin against severe acute pancreatitis-induced brain injury in rats. Int. Immunopharmacol. 24(2), 285-298 (2015).
- Jin G, Qiu G, Wu D et al. Allogeneic bone marrow-derived mesenchymal stem cells attenuate hepatic ischemia-reperfusion injury by suppressing oxidative stress and inhibiting apoptosis in rats. Int. J. Mol. Med. 31(6), 1395-1401 (2013).
- Lu G, Huang S, Chen Y et al. Umbilical cord mesenchymal stem cell transplantation ameliorates burn-induced acute kidney injury in rats. Int. J. Lower. Extremity. Wounds. 12(3), 205-211(2013).
- Pan GZ, Yang Y, Zhang J et al. Bone marrow mesenchymal stem cells ameliorate hepatic ischemia/reperfusion injuries via inactivation of the MEK/ERK signaling pathway in rats. J. Surgical. Res. 178(2), 935-948 (2012).
- Gu SJ, Chen YH, Zhao H et al. Effect of different transplantation approaches of human umbilical cord menchymal stem cells on repairing hepatic ischemia-reperfusion injury in rat models. Organ. Transplantation. 8(3), 190-194 (2017).
- Zhou JH, Fang LM. The effect of alcohol on improvement of liver function, glutathione peroxidase and malondialdehyde in patients with alcoholic liver disease. Modern. Practical. Med. 25(8), 886-888.
- Song YM, Lian CH, Cheng-Song WU et al. Effects of bone marrow-derived mesenchymal stem cells transplanted via the portal vein or tail vein on liver injury in rats with liver cirrhosis. Exp. Ther. Med. 9(4), 1292-1298 (2015).
- Li X, Su L, Zhang X et al. Ulinastatin downregulates TLR4 and NF-kB expression and protects mouse brains against ischemia/reperfusion injury. Neuro. Res. 39(4), 367-373 (2017).