Research Paper - Clinical Investigation (2022) Volume 12, Issue 12
The IMPACT of GROWTH HISTORY on STRESS ROBUSTNESS of Listeria monocytogenes Sharkawy Saleh, Eslam
- Corresponding Author:
- Eslam Saleh
The Technical University of Munich Busching Str.67, 81677 Munich, Germany
E-mail:Eslam.Sharkawy@tum.de
Received: 19-Sep-2022, Manuscript No. fmci- 22-75047; Editor assigned: 20-Sep-2022, PreQC No. fmci- 22- 75047 (PQ); Reviewed: 22-Sep -2022, QC No. fmci- 22-75047 (Q); Revised: 26-Sep -2022, Manuscript No. fmci22-75047 (R); Published: 29-Dec -2022; DOI: 10.37532/2041-6792.2022.12(12).276-284
Abstract
Environmental factors such as oxygen availability have an impact on the adaptive behavior of stress and the robustness of microorganisms. Adaptation to nonoptimal environmental conditions induces molecular changes that help microorganisms develop survival strategies toward more severe stress conditions. Listeria monocytogenes as a foodborne pathogen is regularly exposed to such environmental stresses within its natural habitat, during food processing, and throughout its infection cycle. The ability of Listeria to adapt to such changing environment enhances its ability to survival and growth in food and influences its pathogenic potential.
In this study, we will investigate the impact of oxygen availability on growth and robustness induction toward lethal oxidative and acid stresses for three strains of Listeria monocytogenes, ScottA, EGDe, and F2365. Aerobically grown cells showed higher growth rate and were more resistant to oxidative stress than anaerobically and microaerobically grown cells. However, for acid stress, anaerobically and microaerobically grown cells were more resistant than cells that were cultured aerobically.
Keywords
Listeria monocytogenes • Microaerobically • Blind-source
Introduction
Catalase activity was measured in the exponential phase Listeria cells, cultured under the different atmospheric conditions i.e. aerobic, microaerobic and anaerobic. Induction of catalase activity was found to be correlated with robustness towards lethal oxidative stress with significant correlation only for F2365 strain. This correlation might be associated with intracellular accumulation of Reactive Oxygen Species (ROS) and subsequent induction of catalase activity. Catalase scavenges H2O2 formed during oxidative stress treatment and helps aerobically grown cells to be more resistant to further oxidative stress. Anaerobically grown cells showed a lower level of catalase induction, and hence lower robustness, possibly because of the use of alternative respiration machinery compared to aerobically grown cells, which results in a lower level of ROS formation. That suggests the possibility of using catalase as a biomarker for predicting the robustness of L. monocytogenes towards lethal oxidative stress. However, an inverse correlation was found between catalase activity induction and robustness to lethal acid stress. Therefore, catalase activity could function as an inverse-response biomarker in this condition.
The finding of our study might explain also why L. monocytogenes is a target organism in the minimally processed foods. Modified atmosphere packaging is widely used in ready-to-eat foods. The limited oxygen availability of this condition in particular vacuum packaging can have an impact on the robustness of Listeria to further acid stress treatment used in food industry.
Listeria monocytogenes is the only species among six species currently classified within the genus Listeria that is of concern to human and animal health. The microorganism was first described by Murrey et al. in 1926 as Bacterium monocytogenes as it was found to cause characteristic monocytosis in infected laboratory rabbits. Then, it was renamed Listerella hepatolytica by Pierie in 1927 who isolated the same bacterium from the liver of sick gerbilles (latera lobenquiae) (Pierie, 1927). Due to several outbreaks caused by L. monocytogenes since the 1980s, interest in the organism had grown rapidly with a subsequent increase. Using epidemiological and microbiological investigations, L. monocytogenes was proven to be capable of causing both sporadic cases and outbreaks through contaminated food.
Listeria monocytogenes is a Gram-positive facultative anaerobic rod-shaped bacterium. The cell has peritrichous flagella with a characteristic tumbling motility at 250°C. It can grow in a wide range of temperatures 0°C to 420°C with an optimum growth between 300°C and 350°C. The growth of the bacterium is inhibited at pH 5.5 or lower, with no growth at pH 4.0. However, growth inhibition is dependent on the strain, the growth phase and the acidulant used. Acetic acid was found to be the most effective growth inhibitor among several acids e.g. lactic, citric and hydrochloric acids [1, 2]. Listeria is quite halotolerant and can survive 10% NaCl for an extended period of time.
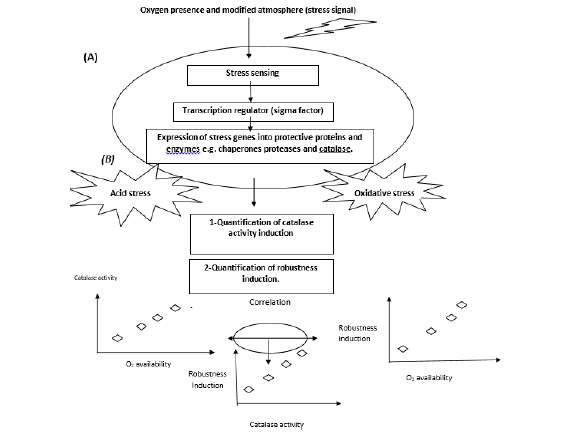
In this study, we are interested in the effect of aerobic, microaerobic and anaerobic conditions on robustness against further lethal oxidative or acid stress treatments. Additionally, we examined the correlation between the induced catalase activity in exponentially growing L. monocytogenes and the robustness to such lethal stresses. If such correlation was proven, that could help to assess whether catalase can function as a biomarker or cellular indicator of the biological state of Listeria that can be further used to predict its robustness level (Figure 1).
Figure 1: Conceptual scheme representing the impact of growth history on stress adaptation of L. monocytogenes: A) molecular mechanisms of stress adaptation include stress sensing, activation of transcription regulator, sigma B, and expression of stress genes, e.g. chaperones, protease, and catalase. B) In our study, catalase activity was quantitatively measured upon growth of L. monocytogenes under three different atmospheric conditions i.e. aerobic, microaerobic, and anaerobic. Also, these cells were exposed to lethal oxidative (H2O2 , 500 mM) and acid (HCl, pH 2.5) to measure the robustness against these stresses.
To evaluate whether catalase activity can predict robustness, At the level, catalase activity was correlated with robustness toward lethal oxidative and acid stresses.
Methods
Bacterial strains culturing
In this study, three strains of Listeria monocytogenes, ScottA and F2365 of serotype 4b and EGDe of serotype 1/2a, were used. The strains were obtained from the culture collection of the Wageningen University Laboratory for Food Microbiology. A stock of each strain was prepared in cryovials containing 0.7 ml of overnight culture, 0.3 ml of 86% glycerol. The cryovials were kept at -25°C. Pure colonies of the three Listeria strains were obtained by streak plating the prepared stock cultures on Brain Heart Infusion Agar medium (BHIA), composed of 37 g/l of BHI (Dickinson, France) and 1.5% w/v of bacteriological agar (Oxoid, UK).Then, the plates were then incubated at 300°C for 24 hours. To prepare an overnight pre-culture, a pure colony was inoculated in 10 ml Brain Heart Infusion (BHI) broth using a sterile loop. Afterwards, the pre-culture was incubated until the stationary phase in 200 rpm shaking incubator (Innova 4335,Netherlands) at 300°C for 17 hours [3].
Growth of L. monocytogenes strains under aerobic, microaerobic, and anaerobic conditions aerobic culture: To prepare an aerobic culture, 50 ml of brain heart infusion broth was inoculated in a 250 ml Erlenmeyer flask with 0.25 ml of overnight preculture (1:200) of each strain in duplicate. The flasks were then incubated in a 200 rpm shaking water bath (Julabo SW23 No. 10101761, Germany) at 300°C for 12 hours.
Micro aerobic culturing: For micro aerobic culture, a 50 ml falcon tube (Greiner Bio-one, Germany) filled to the top with BHI broth was inoculated with 0.25 ml overnight preculture of each strain in duplicate. Then, the falcon tubes were incubated in a 300°C incubator without shaking for 12 hours.
Anaerobic culturing: 50 ml BHI broth in a 100 ml air-tight infusion bottle was inoculated with 0.25 ml overnight pre-culture of each strain in duplicate. Before inoculation, the infusion bottles were sealed with a rubber stopper, sealed with a metal seal, and flushed with sterile nitrogen gas for 6 hours ± 2 hours after being autoclaved at 120°C for 20 minutes. The infusion bottles were then incubated at 300°C without shaking for 12 hours.
For each atmospheric condition of the previous preparations, i.e. aerobic, micro aerobic, and anaerobic cultures, samples were taken to measure the optical density, pH,, and the corresponding total viable count each 3 hours for 12 hour. To obtain 24 hours monitoring, another set of this experiment was prepared, sequentially incubated overnight under the same conditions, and point measurement was taken between 12 hours to 24 hours of incubation. The Optical Density(OD) was measured by estimating the absorbance value using a spectrophotometer (Pharmacia Biotech) at 600 nm, pH using a pH meter (WTW,N0. 53135009 , Germany) and viable counting by spiral plating of 50 µl culture dilutions in duplicates of BHIA using spiral platter (Eddy jet , No. 279/1700, IUL instruments). The plates were incubated at 300°C for 24 hours.
Control experiment: To ensure adequate anaerobic conditions in all airtight infusion bottles used in the experiments, resazurin was inoculated in a control sterile infusion bottle, containing 50 ml of BHI (0.2 mg/l final concentration).The decolorization of the pink color of resazurin means that the anaerobic atmosphere in the bottles was optimized and the flushing process can be stopped (usually after 4 hours ± 1 hours). A separate control was also prepared for the aerobic and microaerobic conditions by inoculating resazurin in 50 ml BHI in 250 ml Erlenmeyer flasks and 50 ml falcon tubes respectively.
In a control experiment, resazurin solution was used to assess the impact of microbial growth on the dissolved oxygen concentration of cultures under each atmospheric condition. Resazurin decolorizes at a low redox potential of 42 mv, which can be attained at a dissolved oxygen level of 0.02%. Resazurin was inoculated in 50 ml of of of BHI broth (final concentration 0.2 mg/l) in duplicates of 250 ml of Erlenmeyer flasks, 50 ml of Falcon tubes and 100 ml of airtight N2 flushed infusion bottles for aerobic, microaerobic and anaerobic conditions,,, respectively. Afterwards, 0.25 ml overnight culture of each strain was inoculated. The preparations containing resazurin and growing cells were then incubated at 300°C. Resazurin decolonization was observed during microbial growth each 3 hours for 24 hours, along with the point measurement of OD, pH and viable count.
Adapted growth under aerobic, micro aerobic and anaerobic conditions
To obtain aerobically, microaerobically, and anaerobically adapted Listeria cells, cultures were transferred from the stationary phase of the first growth into 50 ml of fresh Brain Heart Infusion broth (BHI) under the same atmospheric conditions. The optical density was then monitored at a point each three hours using the 600 nm spectrophotometer. The volume of the transferred cells was corrected with the ratio between the optical density of the first growth in the stationary phase and the optical density of the overnight pre culture in the stationary phase, i.e., transferred volume OD ratio* 0.25 ml. That correction was made in order to compare the stress adapted exponential growth to the initial exponential growth after being transferred from the pre culture within the same atmospheric condition.
After monitoring the growth curves of the atmospherically adapted L. monocytognes strains under aerobic, micro aerobic and anaerobic conditions, the moment the culture reaches an OD of 0.1, meaning the culture is in the exponential growth phase, was selected to perform lethal acid and oxidative inactivation.
Oxidative inactivation (500 mm H2O2 )
On reaching an OD of 0.1, 1 ml culture of each strain was transferred to 9 ml BHI broth (t=0). At the same time, 20 ml of the culture was transferred to a 250 ml empty sterile Erlenmeyer flask and placed in a 200 rpm shaking water bath at 300°C. The Erlenmeyer flask was used for all the atmospheric conditions to homogenize the heat transfer process. Then 1.2 ml of 30% (v/v) H2O2 stock solution (Merck, Germany) was added to the 20 ml culture. This gives a final concentration of H2O2 during the inactivation process of 500 mm. For each time point, 1 ml sample was taken, serially diluted in 9 ml of BHI broth, and 100 µl selected dilutions were spread in duplicate on BHI agar plates. The plates were finally incubated in 300°C for 48 hours ± 4 hours
In our study, this experiment was applied only one time with sampling after H2 O2 addition each 1.5 minutes for ScottA and EGDe strains and each 2 minutes for F2365 strain. The total time for oxidative inactivation was 12 minutes.
Acid inactivation (HCl, pH=2.5)
20 ml of BHI broth was adjusted at pH of 2.5 using 37% (v/v) hydrochloric acid stock (Merck, Germany). The amount of HCl was added while the pH was simultaneously measured using a pH meter (WTW, Germany). The adjusted BHI broth was then filter sterilized using 0.2 µm microbiological filters (Whatman, Germany) and transferred to a new falcon tube. Once the cultures reached an OD of 0.1, 1 ml of the culture was added to 9 ml of BHI (t=0). At the same time, 200 µl of the culture was added to 20 ml of BHI (1: 100), of which the pH was pre adjusted to 2.5. For each time point, dilutions were made so that 1:5 and 10-1 dilutions were made in BHI broth. However, further dilutions of 10-2 to 10-6 were made in physiological peptone salt solution (PPS, Tritium NL). The selected dilutions were then plated by transferring 0.1 ml of each dilution in duplicate onto BHI agar plates and spreading using a spreader. The plates were finally incubated in 300°C for 48 hours ± 4 hours.
In our study, acid inactivation was reproduced twice at pH of 2.5, differing in the total HCl exposure time and the sampling intervals. In the first reproduction, sampling of 1ml each 3 minutes was applied for 21 minutes. However, in the second reproduction, sampling was performed each 6 minutes for 42 minutes. That is, there were 8 time points in both duplicates including t=0.
Catalase activity assay
Exponentially growing cells of each strain were obtained as described above at OD of 0.1 at 600 nm under aerobic, microaerobic, and anaerobic conditions. The exact OD was determined at this point to be used later during the calculation of catalase activity. 2 ml of the culture was transferred to an Eppendorf tube and centrifuged (Biofuge pico, Germany) at 13000 rpm for 30 seconds. After centrifugation, the supernatant was discarded and the pellet was held by tapping the tube in a clean tissue paper to remove the remnant fluid. 1ml of cold Phosphate Buffer Saline PBS (138 mm NaCl, 2.7 mm KCl, 140 mm Na2 HPO4 , 1.8 mm KH2PO4 ; pH adjusted to 7.4 with HCl) was added to the Eppendorf tube. The tube was then shortly vortexed and cells were kept as a suspension in PBS immediately in ice.
Spectramax (Spectramax plus 384, USA) was used to monitor optical density over time. The spectramax program was set at kinetics of 240 nm, 300°C and reading for 20 minutes every 10 seconds, i.e. 121 readings. The reference was first established using a cuvette that contains 895 µl PBS. An amount of 4.5 µl of 37% hydrogen peroxide (Merck, Germany) and 100 µl of the prepared suspension was then added to the reference cuvette. After wards, the cuvette was covered by a sterile para film and shaked slowly upside down to homogenize the sample. Finally, the cuvette was inserted in the apparatus and catalase activity was measured. Avoiding formation of bubbles during shaking and pipetting as much as possible is important to get more accurate measurement of catalase activity. The cuvette can be gently tapped with a finger for a few seconds to release the bubbles formed. Reproduction of this experiment was performed on different dates. One unit of catalase activity can be defined as 'decreases in one unit of absorbance at 240 nm per minute, corrected for the concentration factor (2) and the dilution factor (×10).
Correlation The robustness of L. monocytogenes towards lethal oxidative and acid stress was correlated with the induced catalase activity upon growth under aerobic, micro aerobic and anaerobic conditions. Using SPSS software (IBM, version 20), average catalase activity of each duplicate measurement was correlated with the average robustness measured as log Nt /N0 . The robustness level used for this correlation was selected at the time point that resulted in the most remarkable inactivation difference between atmospheric conditions. When P<0.05 the correlation between both tested parameters is statistically significant, ie, the slope of the linear correlation is significantly different from zero.
Results and Finding
Oxidative inactivation
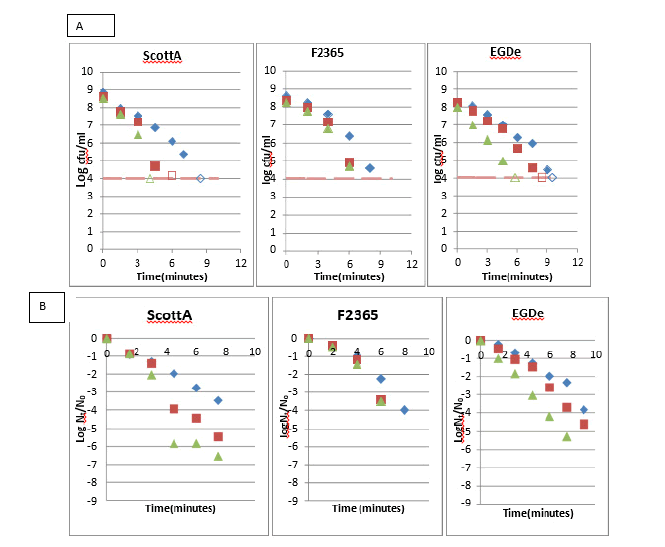
The kinetics of oxidative inactivation and robustness of Listeria strains have been estimated, as indicated in Figure 2. The inactivation kinetics have shown strains variation with the viable count of ScottA, EGDe and F2365 anaerobic cells falling below the detection limit within the first 4 minutes, 6 minutes and 7 minutes respectively. After 6 minutes of exposure to H2O2 , the viable count of aerobically grown cells from ScottA, F2365 and EGDe strains was reduced by 2.8 log cfu/ml, 2.2 log cfu/ml and 1.9 log cfu/ml, respectively. Therefore, the F2365 strain was more resistant to oxidative stress than the ScottA strain when compared to the most resistant culture type for their aerobic cells. However, EGDe strain was the most robust strain with the highest survival rate.
In conclusion, upon exposure to hydrogen peroxide (H2O2 , 500 mm), the aerobically grown cells of ScottA, EGDe and F2365 strains were more resistant than micro aerobically grown cells. However, anaerobically grown cells were the most sensitive.
The shape of the H2O2 exposed cell colonies on brain heart infusion agar showed serrated outlines and a degenerate appearance. The exposed colonies needed longer incubation time 30 hours/300°C to 48 hours/300°C than the unexposed ones which usually takes 24 hours till being visible. That indicates that oxidative cellular damage was repaired after exposed cells were plated (Figure 2) [4].
Figure 2: Effect of oxygen availability on oxidative stress survival of L. monocytogenes strains ScottA, EGDe and F2365. The aerobically (blue diamond’s), microaerobically (red squares) and anaerobically (green triangle) grown cells were exposed to 500 mm H2O2 . Samples were taken each 1.5 minutes (ScottA and EGDe) and each 2 minutes (F2365). The number of colony-forming units per ml was counted and expressed as log cfu/ml (A) and log Nt /N0 (B), representing log reductions over time. Number of repetitions (n=1). The open symbols indicate points below the detection limit.
Acid inactivation
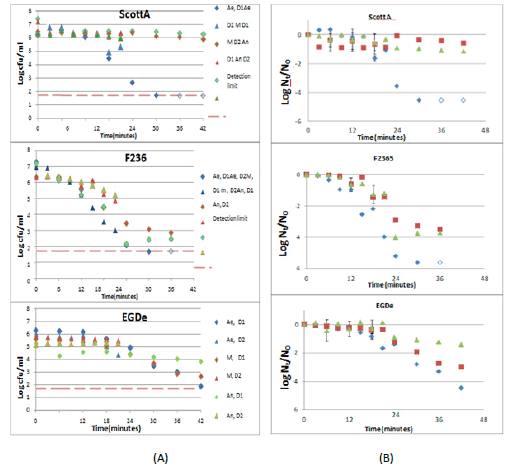
Exponentially grown cells at OD of 0.1 were exposed to HCl at pH of 2.5. The experiment was reproduced twice with the acid-exposed cultures sampled each 3 minutes and 6 minutes during total times of 21 minutes and 42 minutes of each duplicate, respectively (Figure 3).
Figure 3: Effect of oxygen availability on acid stress survival of L. monocytogenes strains ScottA, EGDe, and F2365. The experiment was carried out in duplicates; duplicate1 (D1) with HCl exposed cells sampled every 6 minutes for 42 minutes and dublicate 2(D2) with HCl exposed cells sampled each 3 minutes for 21 minutes. Cells grown aerobically (Ae, blue diamonds), microaerobically (M, red squares) and anaerobically (An, green triangles) grown cells were exposed to HCl at pH of 2.5. The viable count of both duplicates was calculated and represented as log cfu/ml (A) and average log Nt /N0 (B). The open symbols indicate points below the detection limit
Aerobic, micro aerobic and anaerobic cultures that were exposed to HCl for 21 minutes showed a high inactivation rate for only aerobically grown cells of F2365, EGDe and ScottA strains. The aerobic culture of the F2365 strain was the most sensitive, with its viable count reduced by 4 log cfu/ml after 21 min of exposure to HCl. However, aerobic cultures of EGDe and ScottA were less sensitive and their viable counts were reduced by 1.6 and 1 log cfu/ml respectively [5].
On reproducing the experiment with increasing HCl exposure time to 42 minutes, aerobically grown cells also showed the highest acid sensitivity in the three strains. Aerobic F2365 remained highly sensitive,with the viable count falling below the detection limit after 24 minutes of exposure. However, it took the aerobic cells of ScottA and EGDe strains around 30 minutes and 42 minutes respectively to get below the detection limit (Figure 3). In addition, the inactivation kinetics showed that the F2365 strain was the most sensitive, with the viable count of its anaerobic cells being reduced by 3.7 log cfu /ml after 30 minutes of exposure to HCl. However, EGDe and ScottA showed comparable level of sensitivity and higher robustness than F2365 strain with the viable counts of their anaerobic cells got reduced by around 1 log cfu/ml within the same exposure time.
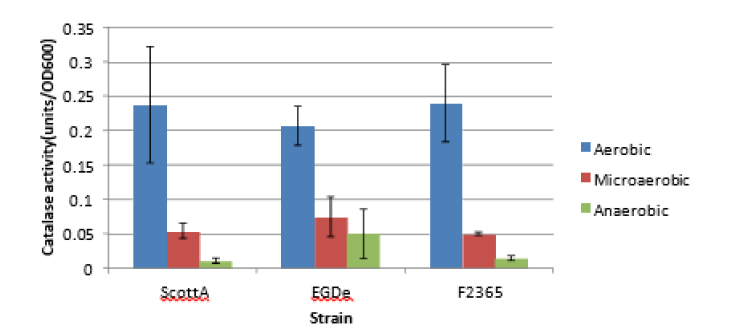
Catalase activity response
Induction of catalase enzyme was found to be clearly affected by changes in oxygen availability. The activity of catalase enzyme of exponentially growing cultures of ScottA, EGDe and F2365 adapted under aerobic, micro aerobic and anaerobic conditions was estimated as shown in Figure 4. Enzyme activity was measured at the same point of exponential growth (OD=0.1) at which cells were exposed to lethal oxidative and acid stresses [6].
Figure 4: Effect of oxygen availability on catalase activity induction of L. monocytogenes strains ScottA, EGDe andF2365. Catalase enzyme of the stress adapted listeria growing aerobically (blue column), microaerobically (red column) and anaerobically (green column) was measured at OD600 of 0.1. The error bars represent the standard deviation of a duplicate experiment.
Correlation
The impact of oxygen availability on robustness induction towards lethal acid and oxidative stresses, as well as catalase activity, has been investigated through previous experiments.
Correlation between the two outcomes can help verify whether catalase induction is initiated, upon The adaptation of L. monocytogenes growth under different atmospheric conditions can predict robustness toward further exposure to lethal stress (Table 1).
Table 1. Two-sided t-test showing P values between the average catalase activity of L. monocytogenes ScottA, EGDe and F2365 strains at different atmospheric conditions. Average catalase acitivity wasn’t significantly different between L. monocytogenes strains (P>0.05).
| Strains | Aerobic | Microaerobic | Anaerobic |
|---|---|---|---|
| EGDe/F2365 | 0.561588 | 0.43496883 | 0.398134 |
| F2365/ScottA | 0.978593 | 0.66554224 | 0.389519 |
| EGDe/ScottA | 0.701747 | 0.49157498 | 0.366158 |
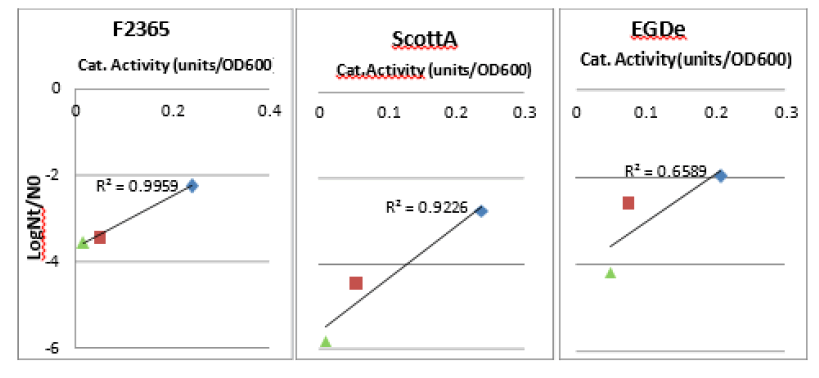
Robustness towards lethal oxidative stress was correlated with catalase activity induction (Figure 5). This correlation was statistically significant for F2365 strain with P value below 0.05 but not significant for the two other strains (Table 2). Aerobically grown L. monocytogenes ScottA, EGDe and F2365 strains, which showed the greatest catalase induction, were the most resistant and showed the highest survival upon exposure to 500 mm H2O2 . However,anaerobically grown cells, with the lowest catalase induction level, were the most sensitive towards oxidative stress and showed the lowest robustness. Micro aerobic cells showed moderate robustness and catalase induction level in between aerobic and anaerobic conditions [7].
Figure 5: Correlation between induction of catalase activity and robustness toward oxidative stress. Robustness of aerobically (blue diamond), microaerobically (red square) and anaerobically (green diamond) grown L. monocytogenes towards 500 mm H2 02 was expressed as log Nt / N0 . Catalase activity was corrected for OD600 0.1.
Table 2. Correlation between catalase activity and robustness induction towards 500 mM H2O2 inactivation. The strain F2365 strain showed a significant correlation (P < 0.05). ScottA and EGDe strains showed insignificant correlation (P>0.05)
| Listeriastrains | R2 | P |
|---|---|---|
| ScottA | 0.92 | 0.18 |
| F2365 | 0.1 | 0.04x |
| EGDe | 0.66 | 0.4 |
Positive correlation between catalase induction and lethal oxidative robustness was higher in F2365 strain than ScottA strain i.e. the squared coefficient of determination (R2 ) closer to 1. However, it was the lowest in the EGDe strain. This could be due to the relatively higher catalase activity level in anaerobic EGDe than in anaerobic F2365 and ScottA (Figure 5).
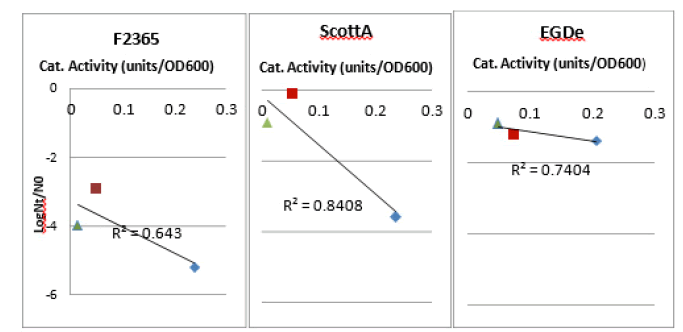
For acid inactivation, there was an inverse correlation between catalase activity induction and robustness to acid stress at pH 2.5 (Figure 6). Aerobically grown cells, which showed the highest catalase activity, were the most sensitive. Whereas, the microaerobically and anaerobically grown cells which showed relatively lower induction level of catalase were the most robust. However, this correlation seemed to be insignificant in the three strains with P>0.05 (Table 3)
Figure 6: Correlation between induction of catalase activity and robustness towards lethal acid stress. Robustness of aerobically (blue diamond), microaerobically (red diamond) and anaerobically (green diamond) grown L. monocytogenes towards H2 02 500 mm was expressed as log Nt / N0 . Catalase activity was corrected for OD600.
Table 3. Correlation between catalase activity and induction of robustness toward acid inactivation (HCl) pH=2.5. P>0.05 showing an insignificant correlation for the three strains of the virus L. monocytogenes.
| Strains | R2 | P |
|---|---|---|
| ScottA | 0.84 | 0.26 |
| F2365 | 0.64 | 0.41 |
| EGDe | 0.19 | 0.34 |
Discussion
In this study, the influence of oxygen presence and modified atmosphere on growth and survival of L. monocytogenes strains ScottA, F2365and EGDe strains upon exposure to lethal oxidative and acid stresses were investigated. The absence of oxygen greatly affected the growth of L. monocytogenes strains and resulted in lower yield and growth rate. The growth under micro aerobic condition was not clearly different from the anaerobic one. The very limited oxygen availability of this condition and the absence of oxygen influx into fully filled culture tubes reduced the growth of Listeria while oxygen was gradually depleted by bacterial metabolism. The level of dissolved oxygen concentration in the cultures was roughly estimated in the control experiments by using resazurin as indicator. The enhanced growth of Listeria in the aerobic condition was accompanied with rapid decolorization of resazurin which takes place at a threshold level of -42 mv i.e. 0.02% dissolved oxygen [8].
Such threshold was exceeded faster in the aerobic condition than the micro aerobic one. However, the anaerobic cultures were colorless before inoculation, indicating optimized N2 flushing of the anaerobic infusion bottles.
In the normal physiological processes, L. monocytogenes develops several defense mechanisms to scavenge such free radicals or repair the damage they cause. One of these mechanisms includes induction of catalase and superoxide dismutase enzymes. In our study, we have quantified the level of catalase activity upon growth of L. monocytogenes under aerobic, micro aerobic and anaerobic conditions. Catalase activity was found to be 4 times to 18 times more induced in aerobically growing cells than microaerobically and anaerobically grown cells respectively. This may reflect the role catalase plays in scavenging and alleviating ROS toxicity formed upon aerobic respiration. However, anaerobically growing cells use fermentation enzymes or other alternative respiration machinery compared to the aerobic ones. This resulted in less ROS formation and subsequently less catalase activity induction [9]
Upon exposure of L. monocytogenes strains to lethal oxidative stress, aerobically grown cells were more resistant than microaerobically and anaerobically grown cells. The high level of induction of catalase activity in aerobic cells elicited an enhanced robustness response of the bacterial stem.
L. monocytogenes toward further lethal oxidative stress treatment. This was also reflected through the positive correlation between catalase response and robustness induction.
In contrast, catalase seemed to have a minor protective role upon exposure of Listeria to the acid stress at pH 2.5. Although exposure of L. monocytogenes to acid stress leads to secondary oxidative damage that also results in ROS formation. The cells responded to such stimuli mainly through more specific acid tolerance response mechanisms e.g. ATPase and GAD system [10].
Conclusion
Cell death due to continuous acid exposure takes place as a result of increased concentration of intracellular protons, depletion of cellular energy to retain homeostasis and damage to the phospholipids’ structure of cell membrane. The role of catalase here seems to be relatively insignificant in enhancing the tolerance to acid stress. That was in fact reflected through the inverse correlation between catalase response and robustness. Aerobically grown cells that showed the highest catalase activity induction were the most sensitive to acid stress. Whereas, microaerobic and anaerobic cells which had relatively lower catalase activity level were more robust than aerobic cells. However, more research is needed to elucidate the results and the pronounced mechanism of cell death due to lethal acid stress exposure.
It can be conclusively demonstrated through the current study that oxygen availability elicited an initial environmental stimulus that triggered L. monocytogenes to adapt differently upon further exposure to other lethal stimuli. Therefore, oxygen availability was found to be relevant for investigation as a growth history condition of L. monocytogenes.
Acknowledgement
Thanks to the whole members of The Food Microbiology Department at Wageningen University and Research Center in the Netherlands for their help, technical guidance and cooperation for making this work completed successfully particularly Eng. Gerieke van Middendor, Dr. Heidy den Besten, Dr. Tjakoo Abee and Prof. Marcel Zwietering. Thanks as well to the generous support from the Qalaa Holding Scholarship Foundation (formerly Citadel Capital Foundation) in Egypt for financing this research project as a part of my MSc study at Wageningen University in The Netherlands.
References
- Aarts HJ, Hakemulder LE, Van AM. Genomic typing of Listeria monocytogenes strains by automated laser fluorescence analysis of amplified fragment length polymorphism fingerprint patterns. Int j food microbiol. 49:95-102(1999).
[Google Scholar] [Crossref]
- Ahamad N, and Marth EH. Behavior of Listeria monocytogenes at 7, 13, 21, and 35°C in tryptose broth acidified with acetic, citric or lactic acid. J food prot. 52:688-95(1989).
- Aldsworth TG, Sharman RL, Dodd CE. Bacterial suicide through stress. Cell mol life sci. 56:378–83(1999).
[Google Scholar] [Crossref]
- Mitchell A, Romano GH, Groisman B, et al. Adaptive prediction of environmental changes by microorganisms. Nature. 460:220–24(2009).
[Google Scholar] [Crossref]
- Audia JP, Webb CC, and Foster JW. Breaking through the acid barrier:An orchestrated response to proton stress by enteric bacteria. Int j med microbiol. 291:97–106(2001).
[Google Scholar] [Crossref]
- Begley M, Sleator RD, Gahan CG, et al. Contribution of three bile associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infect immun. 73:894–904(2005).
- Begley M, Gahan CG, Hill C. Bile stress response in Listeria monocytogenes LO28: Adaptation, cross-protection, and identification of genetic loci involved in bile resistance. Appl environ microbiol. 68:6005–12(2002).
- Bischoff M, Dunman P, Kormanec J, et al. Microarray-based analysis of the Staphylococcus aureus sigma B. Int j food microbiol. 186:4085–99(2004).
- Gray ML, Killinger AH. Listeria monocytogenes and listeric infections. Bacteriol rev. 30(2):309-82(1966).
[Google Scholar] [Crossref]
- Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol. 4(6):423-34(2006).
[Google Scholar] [Crossref]