Research Article - Diabetes Management (2024) Volume 14, Issue 5
The impact of insulin resistance evaluated by 20/ (fasting blood glucose × fasting c-peptide) on cognitive declined Japanese type 2 diabetes patient
- Corresponding Author:
- Takahiro Nonaka
Department of Physical Therapy, International University of Health and Welfare, Narita,
Japan
E-mail: 22s1123@g.iuhw.ac.jp
Received: 27-Sep-2024, Manuscript No. FMDM-24-148916; Editor assigned: 30-Sep-2024, PreQC No. FMDM-24-148916 (PQ); Reviewed: 14-Oct-2024, QC No. FMDM-24-148916; Revised: 21-Oct-2024, Manuscript No. FMDM-24-148916 (R); Published: 28-Oct-2024, DOI: 10.37532/1758- 1907.2024.14(5).650-656.
Abstract
This study investigates the mechanisms by which type 2 diabetes affects brain parenchyma and impairs higher brain functions, focusing on insulin resistance. The analysis included 20 participants with an average age of 72.5 years and an average diabetes duration of 12 years. The study found a significant positive correlation between insulin resistance C-Peptide Immuno Reactivity-Insulin Resistance (CPR-IR) and general cognitive function Montreal Cognitive Assessment (MoCA-J), but no significant correlations with executive function Frontal Assessment Battery (FAB) or attention function (TMT-A and TMT-B) (TMT-Trail Making Test). These findings suggest that insulin resistance contributes to cognitive decline in diabetic patients, likely due to impaired insulin signaling in the brain. This study highlights the importance of evaluating and addressing both insulin resistance and secretion capacity in diabetes treatment, particularly for Japanese patients who often have lower insulin secretion. The results underscore the potential benefits of exercise therapy in improving cognitive functions and suggest that designing treatment programs to enhance behavioral modifications could be beneficial for diabetes management. Future research should involve larger sample sizes and more detailed evaluation items to further explore these relationships and improve therapeutic strategies for cognitive decline in diabetic patients.
Keywords
• Type 2 diabetes, • cognitive function, • insulin resistance
Introduction
The 2019 National Health and Nutrition Survey by Japan’s Ministry of Health, Labor, and Welfare reported a diabetes prevalence of 19.7% in men and 10.8% in women, with higher rates in older age groups [1]. Diabetes causes persistent hyperglycemia, leading to arteriosclerosis and microvascular complications such as diabetic retinopathy, nephropathy, and neuropathy, which significantly reduce patients’ Activities of Daily Living (ADL) and Quality of Life (QOL) [2].
Poor glycemic control, prevalent in 45.2% to 93% of diabetes patients worldwide, is primarily due to lifestyle factors such as overnutrition and lack of physical activity [3]. Self-management of eating habits, exercise, and medication is crucial for diabetes treatment [2]. Studies have shown that interventions focusing on behavioral changes in diet and exercise can significantly reduce HbA1c levels and weight [4].
High-level cognitive functions, such as memory, attention, and executive functions, are essential for behavioral changes in exercise and dietary habits [5]. Factors hindering behavioral change include knowledge, skills, self-efficacy, motivation, and environmental constraints.
Recent studies indicate that metabolic disorders linked to insulin resistance affect not only peripheral organs but also brain parenchyma [6]. Patients with type 2 diabetes exhibit impairments in memory, information processing, and attention [7]. Insulin resistance, characterized by reduced insulin sensitivity, affects the central nervous system, impairing synaptic plasticity and leading to cognitive decline [8].
Insulin resistance disrupts metabolic processes in brain tissue, affecting neuronal survival, plasticity, and higher brain functions [9]. Factors such as lipotoxicity, glucotoxicity, inflammation, and oxidative stress lead to impaired insulin signaling in the brain, resulting in neuronal apoptosis and cognitive decline [10]. Furthermore, insulin secretion also affects cognitive decline because insulin has a significant function in storing memory [11].
Japanese diabetes patients typically have lower insulin secretion capacity compared to Western patients, leading to inflammatory endothelial dysfunction. This affects memory formation and cognitive function, which are significant for learning and behavioral change [9].
Japanese diabetic patients often have low insulin secretion capacity, making it essential to consider both insulin resistance and secretion capacity when evaluating higher brain functions. The CPR-IR indicator, which correlates with the glucose tolerance test, is used to evaluate insulin resistance in Japanese patients [12].
Still a treatable mechanisms of diabetes related cognitive decline is yet to be known [9]. This study aims to investigate the mechanisms by which type 2 diabetes pathology affects brain parenchyma and impairs higher brain functions. We hypothesize that insulin resistance, which can be improved through exercise therapy, contributes to cognitive decline in diabetic patients.
Materials and Methods
• Participant selection and study design participants
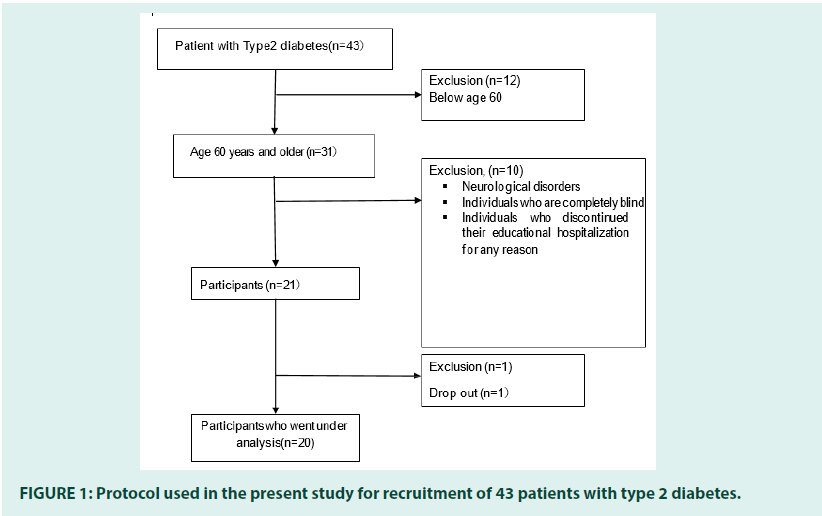
The participants of this study were selected from type 2 diabetes patients admitted for educational purposes related to glycemic control at the International University of Health and Welfare Narita Hospital, who consented to participate in the study. Informed consent was confirmed to all patients.
The inclusion criteria were: Age 60 years or older.
The exclusion criteria included:
1. Individuals with neurological disorders such as stroke.
2. Individuals who are completely blind in both eyes due to diabetic retinopathy or other causes.
3. Individuals who discontinued their educational hospitalization for any reason (FIGURE 1).
• Study design
A cross-sectional study design was employed. Basic demographic information, physical function, insulin resistance, and higher brain functions (cognitive function, executive function, attention) were evaluated in all participants.
• Procedures
Informed consent: All participants were informed about the study in both written and oral formats, and their consent to participate was obtained.
Ethical considerations: The study was conducted in accordance with the Declaration of Helsinki and the current ethical code. The study was conducted with the approval of the Research Ethics Committee of the International University of Health and Welfare (Ethics number: 22-Ig- 257). All methods were performed in accordance with this guideline.
•Methods
1. Investigation of patient attributes: The following patient attributes were extracted from electronic medical records: Age, gender, medical history, educational background, medication status, Body Mass Index (BMI), diabetes duration, visceral fat, creatinine levels. Physical functions were also extracted, including knee joint extension strength, grip strength, basal metabolism, body composition, Short Physical Performance Battery (SPPB), and the 6-Minute Walk Test (6MWT).
2. Insulin resistance: Insulin resistance was assessed using the CPR-IR index, calculated as CPR-IR=20/( fasting blood glucose × fasting C-peptide). The CPR-IR index has been reported to correlate with the glucose tolerance test results, considered the most reliable indicator of insulin resistance, and is more reliable than the Homeostasis Model Assessment of Insulin Resistance (HOMA- IR) for patients, including Japanese patients, who have begun insulin therapy. Fasting blood glucose and fasting blood C-peptide levels on the day of admission were collected from the electronic medical records, and CPR-IR was calculated using the defined formula. Additionally, the fasting blood glucose level used in calculating CPR-IR was also employed as an indicator of immediate blood glucose control.
3. Higher brain functions: Higher brain functions were evaluated using the following neuropsychological tests, administered according to their respective manuals. Evaluations were conducted in a quiet room in the rehabilitation unit of the International University of Health and Welfare Narita Hospital, avoiding times when patients might experience decreased alertness.
4. General cognitive function: The Montreal Cognitive Assessment-Japan (MoCA-J) was used for general cognitive function. MoCA-J is reported to be more reliable than the Mini-Mental State Examination (MMSE) for screening mild cognitive impairment. MoCA-J has a maximum score of 30 points and includes subtests for executive functions (trail making, word fluency, abstraction: 4 points), visuospatial ability (cube copying, clock drawing: 4 points), language (naming, repetition tasks: 5 points), attention (target tapping, serial 7s: 6 points), memory (delayed recall: 5 points), and orientation (6 points). Total scores and subtest scores were calculated.
5. Executive function: The Japanese version of the Frontal Assessment Battery (FAB) was used to evaluate executive function. FAB consists of subtests for conceptualization, mental flexibility, motor programming, sensitivity to interference, inhibitory control, and environmental autonomy, with a maximum score of 18 points. Higher scores indicate better frontal lobe function.
6. Attention function: The Japanese version of the Trail Making Test (TMT) was used to assess attention. TMT-A evaluates selective attention by measuring the time required to sequentially connect numbers from 1 to 25. TMT-B evaluates alternating and divided attention, as well as concentration, by measuring the time required to alternately connect numbers (1 to 13) and hiragana characters (あ to し). Faster times indicate better performance.
•Statistical analysis
For each attribute, blood data, body composition, physical function, insulin resistance, and higher brain function indicators, means and standard deviations were calculated. The normality of the variables was verified using the Kolmogorov- Smirnov test. Spearmanʼs correlation coefficients were calculated to examine the relationships between insulin resistance, and general cognitive function, executive function, and sustained/ divided attention functions. A significance level of less than 5% was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics Version 25.
•Data availability
All data generated or analyzed during this study are included in this published article.
Results
• Characteristics of the participants
The analysis included 20 participants. The results of the measurements of participant attributes are shown in TABLE 1. The participants had an average age of 72.5 (25th percentile=68.5,75th percentile=79.0) years, an average duration of bdiabetes of 12.0 (6.0,28.5) years, and an average educational background of 12.0 (12.0,13.8) years.
| n | 25th percentile | Median | 75th percentile |
|---|---|---|---|
| Age (Years) | 68.5 | 72.5 | 79 |
| Years of education (Years) | 12 | 12 | 13.8 |
| Duration of diabetes (Years) | 6 | 13 | 28.5 |
| Height (cm) | 151.8 | 159.8 | 166.3 |
| Weight (kg) | 52.5 | 60 | 67.7 |
| BMI (kg/m2) | 20.37 | 23.2 | 24.4 |
| Basal | 1189 | 1390 | 1806 |
| Metabolism (kcal/day) | |||
| Muscle Mass (kg) | 33.3 | 40.7 | 47 |
| Body fat percentage (%) | 17.3 | 25.6 | 34.8 |
| SMI | 5.6 | 6.9 | 7.3 |
| HDL-C (mg/dl) | 42 | 51 | 61 |
| LDL-C (mg/dl) | 84.3 | 105 | 117.5 |
| Creatinine (mg/dl) | 0.6 | 0.7 | 0.9 |
| eGFR (60ml/min/1.73 m2) | 60.98 | 71.8 | 83.88 |
| Fasting blood glucose level (mg/dl) | 152 | 190.5 | 257 |
| HbA1c (%) | 8 | 8.75 | 10.1 |
| CPR (pmol/L) | 1.11 | 1.65 | 2.77 |
| CPR-IR | 0.04 | 0.06 | 0.11 |
| Visceral fat area (cm2) | 51.56 | 90.49 | 153.8 |
| Grip Strength ratio (%) | 20.7 | 31.2 | 39 |
| Bodyweight normalized | 0.4 | 0.5 | 0.6 |
| Knee extension force | |||
| SPPB (score) | 10 | 12 | 12 |
| 6MWT (m) | 371.5 | 442 | 529 |
| FAB (Score) | 14.3 | 16 | 16 |
| MoCA (Score) | 20.3 | 24 | 25 |
| TMT-A (sec) | 48.8 | 59.5 | 81.5 |
| TMT-B (sec) | 77.3 | 99.5 | 140 |
Note: Data are 25th percentile, Median, 75th percentile, or n (%). CPR-IR:C-Peptide Immuno Reactivity- Insulin Resistance, MoCA: Montreal Cognitive Assessment, TMT-A: Trail Making Test A, TMT-B: Trail Making Test B, FAB Frontal Assessment Battery.
Table 1: Characteristics of the participants.
•Association between insulin resistance and general cognitive function, executive function and attention function
As shown in TABLE 2, there was a significant positive correlation between insulin resistance (CPR-IR) and general cognitive function (MoCA-J) (r=0.49, p=0.030). Also in TABLE 2, there was no significant correlation between insulin resistance and executive function (FAB) (r=-0.03, p=0.901). Finally, in TABLE 2, there was no significant positive correlation between insulin resistance and sustained attention (TMT-A) (r=-0.09, p=0.67). Additionally, there was no significant correlation between insulin resistance and divided attention (TMT-B) (r=0.12, p=0.616).
| CPR-IR | P | |
|---|---|---|
| N | 20 | |
| MoCA | 0.485 | 0.030* |
| FAB | -0.03 | 0.901 |
| TMT-A | -0.099 | 0.678 |
| TMT-B | 0.12 | 0.616 |
Note: CPR-IR:C-Peptide Immuno Reactivity- Insulin Resistance, MoCA: Montreal Cognitive Assessment, TMT-A: Trail Making Test A, TMT-B: Trail Making Test B, FAB Frontal Assessment Battery.
Table 2: Association between insulin resistance and general cognitive function.
Discussion
A significant positive correlation was found between insulin resistance and general cognitive function as measured by the total MoCA score, consistent with previous studies [13]. Recent studies have indicated that chronic hyperglycemia and insulin resistance impact cognitive function in patients with type 2 diabetes, particularly affecting elderly patients [14]. Umegaki et al., reported that insulin resistance specifically affects memory function [14].
Moreover, diabetes patients are prone to physical function decline and sarcopenia [10]. Considering the shared factors between physical and cognitive decline, Umegaki et al., also suggested that chronic inflammation induced by insulin resistance might be the cause of general cognitive decline [14]. Previous research has highlighted the importance of higher brain functions for maintaining self-care and autonomy in daily life, such as planning meals [15]. General cognitive functions are significant for acquiring exercise adherence and appropriate dietary habits, thus controlling blood glucose levels. Thera are also findings support an abundance of insulin receptors were identified in the brain, it is now appreciated that insulin signaling specifically modulates brain function with diverse metabolic or cognitive outcomes, affecting memory, olfactory perception, emotional regulation, eating behavior, and peripheral metabolisms [16]. These findings also indicate the connection between insulin resistance, cognitive function and behavioral modification.
There was no significant correlation between insulin resistance and executive function as assessed by the FAB, sustained/divided attention function as measured by TMT-A and TMT-B. The small sample size in our study might have contributed to this lack of significant correlation. Previous studies have validated the use of FAB, TMT-A, and TMT-B to assess executive function and ability of attention in elderly individuals with MCI and early Alzheimer’s disease [17,18]. Additionally, brain activity assessed by SPECT in MCI elderly showed significant decreases in brain blood flow in the low FAB and TMT score group compared to the high score group [19]. For patients with type 2 diabetes, previous studies have validated the use of other outcomes for assessing executive function and attention [19]. It may be necessary to consider using more sensitive tasks to evaluate these outcomes in diabetic patients [20].
The present study has several limitations. Firstly, the insufficient sample size is a major limitation. In the master’s research, the relationship between insulin resistance and higher brain function was examined with 20 cases. Although the sample size for this study was calculated to be 32 cases using G-power 3.1.9.4, the study was conducted with only 20 cases. This was due to the restrictions on research activities due to the spread of COVID-19 at Narita Hospital, International University of Health and Welfare, an increase in hospitalizations of middle-aged patients with type 2 diabetes for blood glucose control, and a decrease in hospitalizations of elderly patients. In the future, it will be necessary to increase the number of cases and examine the relationship with a statistically appropriate sample size.
Additionally, during the research planning, more detailed and reliable evaluation items should have been selected to evaluate the relationship between insulin resistance and executive function. Considering the temporal burden on patients, FAB, which can evaluate executive function simply and quickly, was chosen, but tests such as the Stroop Test should also have been considered.
Conclusion
This study examined the correlation between insulin resistance and higher brain function. Results suggested a correlation between insulin resistance and general cognitive function. This implies that in physical therapy for diabetic patients, evaluating behavioral modification improvements and designing treatment programs in exercise guidance could be beneficial.
Author contributions
Takahiro Nonaka wrote the main manuscript. Kenichi Kono, Hideaki Ishii, Yusuke Nishida helped the analysis and advised Takahiro Nonaka. Takahiro Nonaka, Go Owari, Yuto Watabe collected the data. Minoru Takemoto was responsible of the patients in the hospital. All authors reviewed the manuscript.
Conflict of interest
There are no conflicts of interest to declare.
Ethics declarations
All study participants provided informed consent, the study design was approved by the appropriate ethics review boards, and our study complies with the provisions of the declaration of Helsinki as revised in 2004.
References
- Ministry of Health, Labour and Welfare. Results of the National Health and Nutrition Survey (2019).
- Japan Diabetes Society. Evidence-Based Practice Guideline for the Treatment for Diabetes. Tokyo, Nankodo (2019).
- Rakhis Sr SA, AlDuwayhis NM, Aleid N, et al. Glycemic Control for Type 2 Diabetes Mellitus Patients: A Systematic Review. Cureus. 14(6): e26180 (2022).
[Crossref] [Google Scholar] [Pubmed]
- Cradock KA, ÓLaighin G, Finucane FM, et al. Behaviour Change Techniques Targeting Both Diet and Physical Activity in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int J Behav Nutr Phys Act. 14(1):1-7 (2017).
[Crossref] [Google Scholar] [Pubmed]
- Cane J, O’Connor D, Michie S, et al. Validation of the Theoretical Domains Framework for Use in Behaviour Change and Implementation Research. Implement Sci. 7:1-7 (2012).
[Crossref] [Google Scholar] [Pubmed]
- Maciejczyk M, Żebrowska E, Chabowski A, et al. Insulin Resistance and Oxidative Stress in the Brain: What’s New?. Int J Mol Sci. 20(4):874 (2019).
[Crossref] [Google Scholar] [Pubmed]
- Gunstad J, Lhotsky A, Wendell CR, et al. Longitudinal Examination of Obesity and Cognitive Function: Results from the Baltimore Longitudinal Study of Aging. Neuroepidemiology. 34(4):222-229 (2010).
[Crossref] [Google Scholar] [Pubmed]
- Dutta BJ, Singh S, Seksaria S, et al. Inside the Diabetic Brain: Insulin Resistance and Molecular Mechanism Associated with Cognitive Impairment and its Possible Therapeutic Strategies. Pharmacol Res. 182:106358 (2022).
[Crossref] [Google Scholar] [Pubmed]
- Ferris JK, Inglis JT, Madden KM, et al. Brain and Body: A Review of Central Nervous System Contributions to Movement Impairments in Diabetes. Diabetes. 69(1):3-11 (2020).
[Crossref] [Google Scholar] [Pubmed]
- Kalyani RR, Corriere M, Ferrucci L, et al. Age-Related and Disease-Related Muscle Loss: The Effect of Diabetes, Obesity, and Other Diseases. Lancet Diabetes Endocrinol. 2(10):819-829 (2014).
[Crossref] [Google Scholar] [Pubmed]
- Tsai CL, Huang TH, Tsai MC, et al. Neurocognitive Performances of Visuospatial Attention and the Correlations with Metabolic and Inflammatory Biomarkers in Adults with Obesity. Exp Physiol. 102(12):1683-1699 (2017).
[Crossref] [Google Scholar] [Pubmed]
- Ohkura T, Shiochi H, Fujioka Y, et al. 20/(Fasting C-Peptide × Fasting Plasma Glucose) is a Simple and Effective Index of Insulin Resistance in Patients with Type 2 Diabetes Mellitus: A Preliminary Report. Cardiovasc Diabetol. 12:1-8 (2013).
[Crossref] [Google Scholar] [Pubmed]
- Alagiakrishnan K, Zhao N, Mereu L, et al. Montreal Cognitive Assessment is Superior to Standardized Mini‐Mental Status Exam in Detecting Mild Cognitive Impairment in the Middle‐Aged and Elderly Patients with Type 2 Diabetes Mellitus. Biomed Res Int. 2013(1):186106 (2013).
[Crossref] [Google Scholar] [Pubmed]
- Umegaki H, Makino T, Uemura K, et al. The Associations among Insulin Resistance, Hyperglycemia, Physical Performance, Diabetes Mellitus, and Cognitive Function in Relatively Healthy Older Adults with Subtle Cognitive Dysfunction. Front Aging Neurosci. 9:72 (2017).
[Crossref] [Google Scholar] [Pubmed]
- Palta P, Carlson MC, Crum RM, et al. Diabetes and Cognitive Decline in Older Adults: The Ginkgo Evaluation of Memory Study. J Gerontol A Biol Sci Med Sci. 73(1):123-130 (2018).
[Crossref] [Google Scholar] [Pubmed]
- Kullmann S, Kleinridders A, Small DM, et al. Central Nervous Pathways of Insulin Action in the Control of Metabolism and Food Intake. Lancet Diabetes Endocrinol. 8(6):524-534 (2020).
[Crossref] [Google Scholar] [Pubmed]
- D Tashiro, M Nakahara, K Tanaka, et al. Comparison of Cognitive Function Based on MMSE/MoCA-J Score among Different Age groups of Eldery Community Residents. Physiotherapy Sci. 34(9):331-335 (2019).
[Crossref]
- T Kubota, H Sakamoto, Y Matukura, et al. Relationship Between Frontal Lobe Function and Occuupational Performance in Community-Dwelling Elderly. Int J Exercise. 3(1):5-12 (2020).
[Crossref]
- Yatani M, Uejo K, Otao H, et al. Relationship Between Cognitive Function, Physical Function, and Attention Function in Community-Dwelling Elderly-Comparison Between Normal Cognitive Function Group and Suspected Mild Cognitive Impairment Group. J Phy Therapy Saga. 4(1):13-18 (2018).
- Sanz-Cánovas J, López-Sampalo A, Cobos-Palacios L, et al. Management of Type 2 Diabetes Mellitus in Elderly Patients with Frailty and/or Sarcopenia. Int J Environ Res Public Health. 19(14):8677 (2022).
[Crossref] [Google Scholar] [Pubmed]