Case Report - Diabetes Management (2018) Volume 8, Issue 5
The importance of continuous glucose monitoring when transitioning a patient from insulin detemir to U-500R
- *Corresponding Author:
- Connie A Valdez
University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences
Denver Indian Health and Family Services
Inc. Aurora, Colorado, USA
E-mail: connie.valdez@ucdenver.edu
Abstract
Introduction: Continuous glucose monitoring (CGM) is especially valuable when intensifying insulin in challenging and high risk patients to determine recurring patterns of unrecognized hypoglycemia and excursions of hyperglycemia. In this report, we describe the use of CGM in a patient with severe insulin resistant type 2 diabetes who was transitioned from insulin detemir to U-500R and illustrate how CGM was instrumental in helping find the ideal insulin regimen.
Case study: The patient is a 66 year-old obese Native American woman with a 17- year history of type 2 diabetes mellitus and severe insulin resistance. Despite escalating U-100 insulin detemir up to 230 units per day, her diabetes was still uncontrolled. To fully appreciate the patient’s blood glucose (BG) levels over a 24-hour period, she agreed to wear a CGM for two weeks. The CGM estimated an A1C of 11.8% with a time in goal range (70-180 mg/dl) at only 11%. The patient was transitioned to U-500R with an initial dose of 0.22 mL (110 units) 30 minutes before breakfast and 0.15 mL (75 units) 30 minutes before dinner. Her dose was ultimately titrated to 0.23 mL (115 units) in the morning and 0.15 mL (75 units) in the evening. In order to validate safety of her new U-500R regimen and assess her diabetes control, she scheduled a follow-up visit, which included placement of another CGM. The new estimated A1C was 7.0% and the patient’s BG values were within the goal range during the majority of the day. There were 7 possible episodes of hypoglycemia, all which occurred between 1 am-7 am.
Conclusion: CGM is a valuable tool that can aid in the evaluation of BG values and help formulate safe and effective insulin regimens, especially for patients who have difficulty obtaining adequate glucose control and in patients who are initiated on U-500R.
Keywords
continuous glucose monitoring s, blood glucose, hypoglycemic, insulin
Introduction
Continuous glucose monitoring (CGM) is a valuable tool that can assist the healthcare professional with evaluation of blood glucose (BG) trends and patterns. Although numerous studies have demonstrated the benefits of CGM in adults with type 1 diabetes, CGM has not been well-studied in patients with type 2 diabetes receiving insulin [1-9]. While the additional cost incurred with CGM may be a barrier for widespread use among type 2 patients, there is likely a subset that may benefit greatly from its application. Specifically, CGM may be particularly useful in high risk patients with type 2 diabetes who require treatment intensification with insulin. CGM can be used to determine the presence of unrecognized hypoglycemia and excursions of hyperglycemia. At our clinic, we have implemented the use of CGM in patients who are initiated on concentrated U-500R insulin and in severely insulin resistant patients who fail to achieve optimal BG control with high doses of U-100 insulins. We use CGM in these two populations since patients initiated on U-500R may be at greater risk for hypoglycemia compared to other insulins, which is the major concern of using U-500R in the primary care setting. We also included patients requiring high doses of U-100 insulin who continue to have uncontrolled diabetes, as CGM can be a useful tool to gain a better understanding of their BG values throughout the day and night in order to more accurately adjust their insulin regimen. In this report, we describe the use of CGM in a patient with severe insulin resistant type 2 diabetes mellitus who was transitioned from insulin detemir to U-500R and illustrate how CGM was instrumental in helping find the ideal insulin regimen.
Case Report
A 66-year-old Native American woman (weight 88 kg, height 159 cm, BMI 34.8) with a 17- year history of type 2 diabetes mellitus, diabetic polyneuropathy, hypertension, hyperlipidemia, gastroesophageal reflux disease, osteoarthritis, allergic rhinitis, insomnia, abnormal liver function and history of STEMI and breast cancer receiving care at our clinic. Medications included insulin detemir 115 units subcutaneous twice daily (TDD of 230 units), linagliptin-metformin 2.5/1000 mg orally twice daily, gabapentin 300 mg orally twice daily, lostartan- HCTZ 100/25 mg orally once daily, atorvastatin 40 mg orally once daily, Omega-3 1000 mg orally once daily, Fenofibrate 145 mg orally once daily, omeprazole 20 mg orally once daily, aspirin 81 mg orally once daily, tramadol 50 mg orally every 6 hours as needed, tizanidine 4 mg orally three times daily as needed and trazodone 100 mg orally at bedtime. The patient has an allergy to angiotensin converting enzyme inhibitors (anaphylactic reaction) and is intolerant to hydrocodone and sulfamethoxazole/ trimethoprim. She was also opposed to adding on mealtime insulin. Laboratory findings in February 2017 included an elevated A1C of 10.2%, urinary analysis within normal limits (WNL) except protein which was elevated at 30 mg/dL, microalbumin/Cr ratio WNL, lipid panel WNL except triglycerides which were elevated at 232 mg/dL and HDL which was low at 22 mg/ dL, complete blood count WNL and complete metabolic panel WNL except glucose which was elevated at 240 mg/dL and BUN/SCr ratio which was elevated at 30. The patient reported to be adherent to her medication regimen, was consistent with follow up appointments and was enrolled in the pharmacist-managed rapid insulin titration program. She was able to recognize hypoglycemia and describe how to appropriately manage low blood sugar. Nevertheless, the patient’s diabetes continued to be uncontrolled with A1C values greater than the American Diabetes Association (ADA) goal of 7% or less. She reported difficulty adhering to recommended ADA and Dietary Approaches to Stop Hypertension (DASH) diets. Her ability to exercise was limited by osteoarthritis and knee pain. Despite increasing doses of insulin detemir to 115 units subcutaneous twice daily (TDD of 230 units), the patient continued to have elevated BG values. In order to improve our understanding of the patient’s BG trends throughout the day and night, she agreed to wear a continuous glucose monitor for two weeks and then return to the clinic for removal and interpretation of the data.
▪ CGM placement to assess BG values with insulin detemir
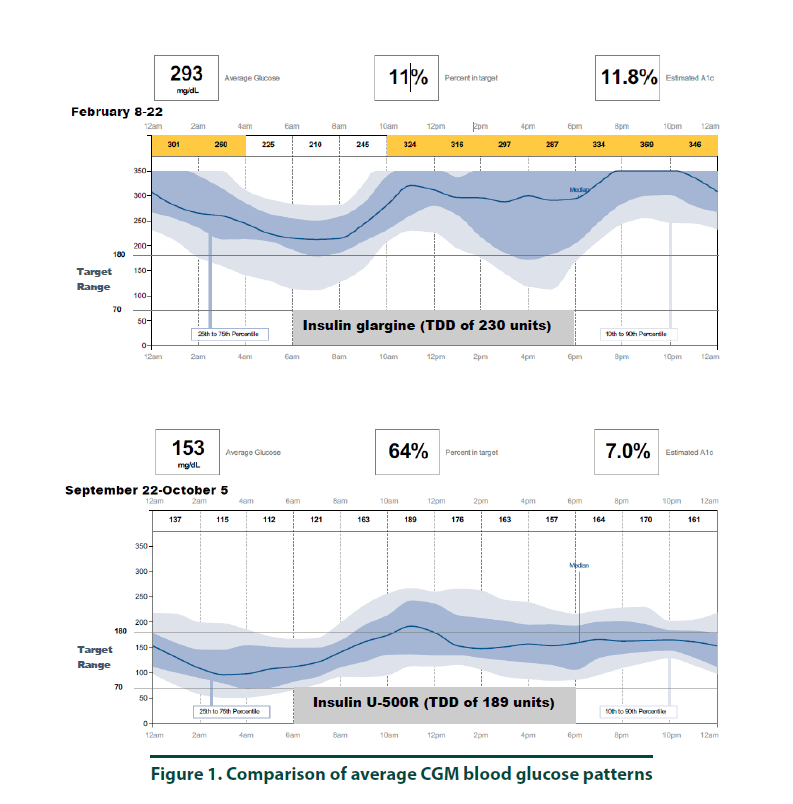
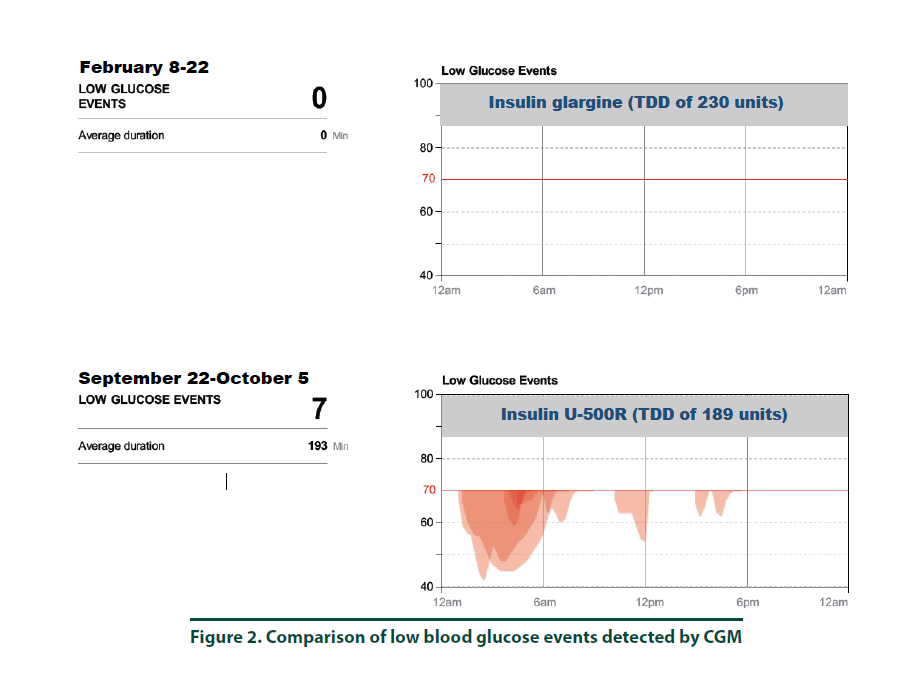
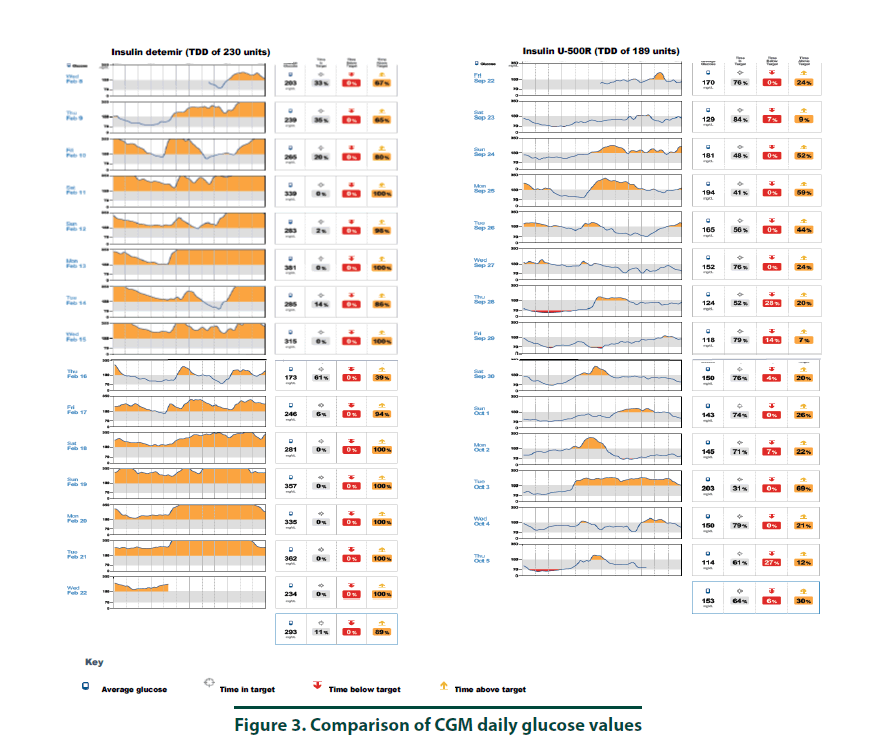
According to manufacturer’s instructions, a FreeStyle Libre ProTM sensor was attached to the back of the patient’s arm [10]. The CGM monitor was in place for two weeks while the patient maintained her usual medication regimen. In addition, the patient was asked to keep a food, physical activity, and insulin log. Upon return to the clinic, the CGM was removed and all data collected was downloaded using the LibreView cloud-based diabetes management system. The CGM report demonstrated the current dose of insulin detemir resulted in an average 2-week BG of 293 mg/dl (Figure 1, top panel and Figure 3, left panel). The estimated A1C was 11.8% and the time in goal range (70-180 mg/ dl) was 11% whereas the majority of the time (89%), the patient’s BG values were above goal (see Figure 1, top panel). The CGM report did not detect any hypoglycemic events (BG less than 70 mg/dL, Figure 2). Since the patient was unhappy with her current insulin regimen, which consisted of splitting her morning and evening doses of 115 units into four injections [57 units and 58 units in the morning and again in the evening] and knowing that additional insulin would now be required to improve her BG control, she was receptive to considering a transition to U-500R.
▪ Transition from U-100 insulin to U-500R
Criteria for initiation into the U-500R insulin program in our clinic includes patients who have minimal or no mental cognition impairment, are receiving 200 units or more of insulin daily, are able to recognize and appropriately manage hypoglycemic episodes and are adherent with clinic visits. Per clinic protocol, all patients initiated on U-500R will have a 20% total daily insulin dosage reduction to ensure safety and reduce the likelihood of hypoglycemia. Patients are transitioned to twice daily dosing of U-500R with 60% of the total daily dose administered before breakfast and 40% before dinner. In February 2017, the patient was transitioned to U-500R per protocol and initiated on a dose of 0.22 mL (110 units) 30 minutes before breakfast and 0.15 mL (75 units) 30 minutes before dinner. Based on her elevated post-prandial BG breakfast and lunch values, her U-500R dose was titrated to 0.23 mL (115 units) in the morning and 0.15 mL (75 units) in the evening which then adequately controlled her diabetes. She remained on this dose until her next diabetes follow-up visit with her primary care provider in September 2017. In September 2017, laboratory findings included A1C and fasting BG (FBG) values that were much improved and indicated controlled diabetes with an A1C of 6.5% and FBG of 103 mg/dL, respectively. The urinary analysis was WNL with protein no longer present. Her lipid panel remained similar to previous values with the exception of reduced triglycerides (168 mg/ dL). The patient’s weight was slightly lower at 87 kg with a BMI 34.4.
▪ CGM placement to assess BG values with U-500R
To ensure safety of the current U-500R regimen of 0.23 mL (115 units) in the morning and 0.15 mL (75 units) in the evening, a CGM was placed during the September 2017 visit. Results of that CGM are shown in Figure 1 (bottom panel) and demonstrate an average 2-week BG of 153 mg/dl (Figure 1, bottom panel and Figure 3, right panel). The estimated A1C was 7.0% and BG values during the majority of the day were within the goal range of 70-180 (Figure 3, right panel). However, the CGM report also identified that seven possible hypoglycemic events (BG less than 70 mg/dL) occurred between 1 am-7 am (Figure 2). Specifically, the patient was below 70 mg/dL six percent of the time during the 14-day monitoring period (Figures 2 and 3). Based on CGM trends, we decreased her evening dose and increased her morning U-500R dose.
Discussion
We describe a patient with type 2 diabetes mellitus and severe insulin resistance who was treated with U-100 insulin detemir and transitioned to U-500R. While receiving U-100 insulin detemir, CGM was used to gain a better understanding of the patient’s BG values throughout the day and night to accurately adjust her insulin regimen. CGM was used again after the patient was transitioned and stabilized on U-500R. The initial CGM estimated an A1C of 11.8% with an average BG value of 293 mg/ dl. This data provided clear evidence that an increase in basal insulin as well as the addition of mealtime insulin was required to achieve better BG control. The patient was transitioned to U-500R per clinic protocol, where the dose of U-500R was initiated at 80% (184 units) of the TDD of U-100 insulin. To assess safety and dosage adjustment needs with U-500R, a second CGM was placed on the patient to evaluate BG values over a 2 week period. The CGM resulted in an estimated A1C of 7.0% with an average BG value of 153. Although the patient was within goal range during the majority of time, she was below goal 6% and above goal 30% of the time, suggesting a need to reduce her evening dose and increase her morning dose. The CGM was important in the identification of these trends as all of the possible hypoglycemic events were occurring during the early morning hours prior to her awakening. These findings were consistent with a previous case report which also found how CGM was critical in identifying hypoglycemia in a patient stabilized on U-500R who was also unaware he was having nocturnal hypoglycemic episodes [11]. Although hypoglycemia is a primary concern, risk appears to decline with stabilization. A study by Dailey et al. demonstrated a slight increase in risk for mild hypoglycemia within the first few months of treatment with U-500R [12]. For this reason, CGM is appears to be most useful during the first few months following U-500R initiation to assess safety as well as serve as a tool to more accurately adjust insulin doses, especially while stabilizing therapy. The FreeStyle Libre ProTM is approved for professional use. However, similar to other clinical trials, data used to approve this device was based primarily from patients with Type 1 diabetes mellitus and only a small percentage of patients with Type 2 diabetes mellitus. The average BMI of patients enrolled in the approval study was 28.3%, indicating that most patients were overweight, not obese [13]. Since most patients with severe insulin resistance are obese (as observed in our patient with a BMI of 34.8), the accuracy of CGM in these subjects may be in question. Although the CGM report in our patient reproduced A1C values similar to the laboratory A1C values, we acknowledge that application of this tool in obese type 2 patients with severe insulin resistance is not well studied. We did notice, however, the values from CGM compared to laboratory values were slightly higher in February and again in September. This variance is likely representative of the direct measurement of glycosylated hemoglobin obtained by laboratory analysis versus a calculated A1C based on BG trends from the CGM. For this reason, results from the CGM were interpreted to only identify trends of BG values to ensure safety and efficacy and not as absolute values. Our patient’s laboratory A1C values were reduced by 3.7%, from 10.2% to 6.5%, over a 6-month period which is far more than what is reported in the literature. A study by Eby et al. evaluated 445 patients on U-500R and found an A1C reduction of 0.68% compared to baseline (P<0.0001) [14]. In addition, Hood et al. evaluated 163 obese patients receiving twice daily U-500R and reported a reduction in A1C of 1.22% (P<0.001) [15]. The combination of using more concentrated insulin, U-500R, along with CGM could possibly explain the robust 3.7% A1C decrease in our patient as multiple studies have found that CGM can also reduce A1C1-9. Although the majority of data has been demonstrated in type 1 diabetes, few studies exist demonstrating similar results in type 2 diabetes. A study by Beck et al. found a correlation of A1C reduction with CGM in patients with type 2 diabetes who were using multiple daily insulin injections. In this study, patients who used CGM had a significant improvement at 24 weeks without a pharmacologic change in therapy compared to patients who used a blood glucose monitor (P=0.022) [16]. Another study by Vigorsky et al. evaluated patients with type 2 diabetes who were receiving insulin (mealtime only), also found a significant improvement in A1C after 12 weeks using real-time CGM intermittently compared with those who used a blood glucose monitor. These patients had sustained A1C improvement during the 40 week follow-up period without CGM (P=0.04) [17]. The authors note that periodic use of real time CGM every few months may be beneficial to sustain A1C improvement in type 2 diabetes management. These results suggest that CGM may improve patient awareness, which could provide an additional management method for patients with type 2 diabetes. The large variance in A1C reduction in our patient compared to the literature may have also possibly been the result of significant lifestyle changes. The patient was enrolled in the pharmacist-managed rapid insulin titration program which provides establishment and accountability of weekly SMART goals related to diet and exercise. Although she was enrolled in the pharmacy management program while using both insulin detemir and U-500R, the impact of this intervention is unknown.
We suspect that tighter monitoring may have occurred after the initiation of U-500R since the patient was assumed to be at greater risk for hypoglycemia as lifestyle changes intensified. She additionally received education from our diabetes prevention team while using both insulin detemir and U-500R. For this reason, it is possible that education may have also positively impacted her dietary and exercise habits, resulting in better results with the U-500R than what is reported in the literature.
Conclusion
This case report described how CGM can be a valuable tool in the evaluation of BG values and help formulate safe and effective insulin regimens for patients who are difficult to control and in patients who are initiated on U-500R. CGM was particularly helpful in the identification of silent/nocturnal hypoglycemic events during the stabilization phase of this patient’s therapy.
References
- Battelino T, Phillip M, Bratina N, et al. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes. Care. 34(4),795–800 (2011).
- Bergenstal R, Tamborlane W, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N. Engl. J. Med. 363(4), 311–320 (2010).
- Fonseca V, Grunberger G, Anhalt H, et al. Consensus conference writing committee continuous glucose monitoring: A consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr. Pract. 22(8), 1008–1021 (2016).
- Gandhi G, Kovalaske M, Kudva Y, et al. Efficacy of continuous glucose monitoring in improving glycemic control and reducing hypoglycemia: a systematic review and meta-analysis of randomized trials. J. Diabetes. Sci. Technol. 5(4), 952–965 (2011).
- Hommel E, Olsen B, Battelino T, et al. Group Impact of continuous glucose monitoring on quality of life, treatment satisfaction, and use of medical care resources: Analyses from the SWITCH study. Acta. Diabetol. 51(5), 845–851(2014).
- Tamborlane W, Beck R, Bode B, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl. J. Med. 359(14), 1464–476 (2008).
- Beck R, HirschI B, Laffel L, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes. Care. 32(8), 1378–1383 (2009).
- Weiss R, Garg S, Bode B, et al. Hypoglycemia reduction and changes in hemoglobin A1c in the ASPIRE In-Home study. Diabetes. Technol. Ther. 17(8),542–547 (2015).
- Yeh H, Brown T, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann. Intern. Med. 157(5), 336–347 (2012).
- The Free Style Libre System [Het Free Style Libre System].Available from http://www.freestylelibrepro.us/safety-information.html. Accessed August 2, 2017.
- Valdez C, Fitzgerald L, Sirherl Hormachea S. Adjusting doses of U-500R insulin: The importance of using continuous blood glucose monitoring. Diabetes. Manag. 7(5),355–362 (2017).
- Dailey A, Williams S, Taneja D, et al. Clinical efficacy and patient satisfaction with U-500 insulin use. Diabetes Res. Clin. Pract. 88(3),259–264 (2010).
- U.S. Food and Drug Administration. IDE Review Memorandum: Summary of Safety and Effectiveness Data (SSED). Eby E, Curtis B, Gelwicks S, et al. Initiation of human regular U-500 insulin use. BMJ. Open. Diabetes. Res. Care. 3,e000074 (2015).
- Hood R, Arakaki R, Wysham C, et al. Two treatment approaches for human regular U-500 insulin in patients with type 2 diabetes not achieving adequate glycemic control on high-dose U-100 insulin therapy with or without oral agents: a randomized, titration-to-target clinical trial. Endocr. Pract. 22(7),782–793(2015).
- Beck R, Riddlesworth T, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily ınsulin ınjections: A randomized trial. Ann. Intern. Med. 167(6), 365–374 (2017).
- Vigersky R, Fonda S, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes. Care. 35(1), 32–38 (2012).