Perspective - Interventional Cardiology (2012) Volume 4, Issue 2
The intra-aortic balloon pump in high-risk percutaneous coronary intervention: is counterpulsation counterproductive?
- Corresponding Author:
- Aung Myat
King’s College London, The Rayne Institute
4th Floor Lambeth Wing, St Thomas’ Hospital
Westminster Bridge Road, London, SE1 7EH, UK
Tel: +44 207 188 1008
Fax: +44 207 188 0970
E-mail: aung.myat@kcl.ac.uk
Abstract
Keywords
cardiogenic shock, diastolic augmentation, high risk, intra-aortic balloon counterpulsation, intra-aortic balloon pump, left ventricular systolic dysfunction, percutaneous coronary intervention
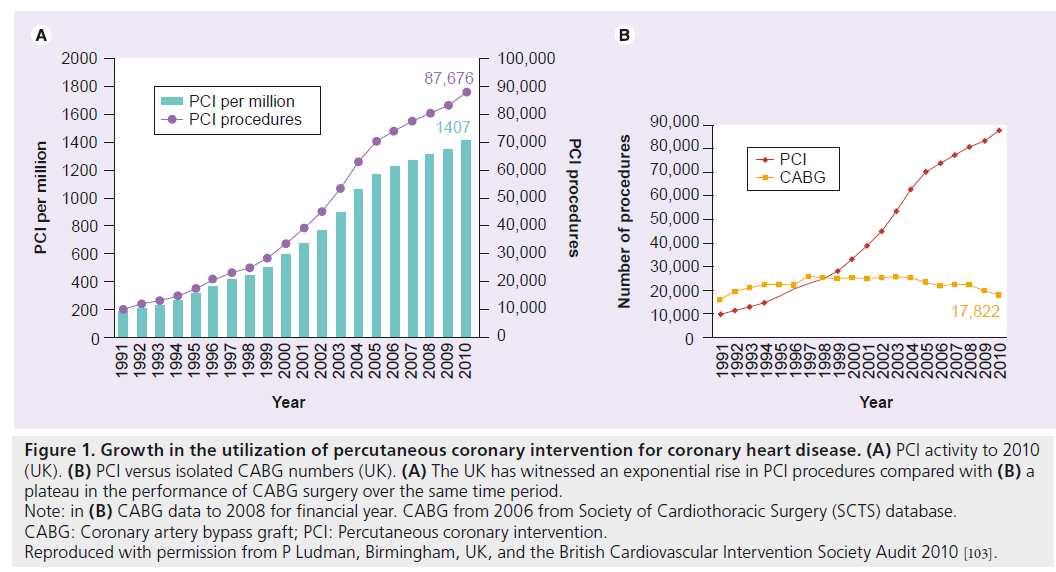
The use of percutaneous coronary intervention (PCI), as a means of arterial recanalization, has witnessed an unprecedented rise over the last three decades and is now one of the most common medical interventions performed today (Figure 1). This expansion has, in part, been borne out of necessity, arising from the increasing incidence and prevalence of coronary heart disease, predominantly due to the aging demographic of ‘industrialized’ nations, the ‘westernization’ of developing countries and the myriad socioeconomic and environmental factors this interplay has created. This is in spite of established primary and secondary coronary heart disease prevention models, public health systems and noninvasive stress testing that facilitate earlier diagnosis, improved access to definitive therapies and the care given by experienced clinicians supported by international guidelines and a robust evidence base. Increased demand and a desire to avoid major adverse cardiac and cerebrovascular events (MACCE) post-PCI have also been the catalyst for advances in stent and balloon technology. These advances include better lesion characterization based on a variety of intracoronary imaging modalities, physiological assessment of flow and objective measures of viability; the deployment of adjunctive mechanical devices and an extensive pharmacotherapeutic armamentarium, components of which have been shown to promote survival, attenuate symptom burden, reduce thrombo-occlusive sequelae and minimize bleeding complications post-PCI, a procedure undertaken electively in stable coronary artery disease (CAD) and emergently for the spectrum of acute coronary syndromes. As a direct result of this development in percutaneous technologies, allied to a population growing older, interventional cardiologists are now attempting revascularization of more complex coronary anatomy, in a high-risk subset of patients demonstrating evidence of reversible ischemia, who would otherwise have been denied access to surgical intervention and, in years gone by, consigned to potentially less effective conservative medical strategies.
Figure 1: Growth in the utilization of percutaneous coronary intervention for coronary heart disease. (A) PCI activity to 2010
(UK). (B) PCI versus isolated CABG numbers (UK). (A) The UK has witnessed an exponential rise in PCI procedures compared with (B) a
plateau in the performance of CABG surgery over the same time period.
Note: in (B) CABG data to 2008 for financial year. CABG from 2006 from Society of Cardiothoracic Surgery (SCTS) database.
CABG: Coronary artery bypass graft; PCI: Percutaneous coronary intervention.
Reproduced with permission from P Ludman, Birmingham, UK, and the British Cardiovascular Intervention Society Audit 2010 [103].
In this article we review the current status quo with regard to the incorporation of intra-aortic balloon counterpulsation (IABC) in high-risk PCI, analyze the evidence for its role as either an ‘elective/prophylactic’, ‘standby/provisional’ or ‘rescue/bailout’ method of percutaneous circulatory assistance and make inferences on which mode of use can provide optimal procedural success and outcomes. We will present a clinical physiologist’s perspective on IABC, detailing tips and tricks alongside the advantages and pitfalls of using this technology in a real world setting. Finally, we will explore the future role of IABC and its evolution in the context of a rapidly expanding percutaneous circulatory assist device (PCAD) arena in which the Impella® Recover LP (Abiomed, Aachen, Germany/Danvers, MA, USA) and the TandemHeart® (Cardiac Assist, Pittsburgh, PA, USA) devices have emerged as potential rivals.
How do we define high-risk PCI?
Somewhat surprisingly, there is no universally accepted definition of what constitutes highrisk PCI. Akin to the controversy generated by the attempt to compare rates of stent thrombosis (ST) and/or bleeding in trials of therapeutic strategies for acute coronary syndromes/PCI, prior to general acceptance of the Academic Research Consortium definition of ST [1] and the recent Bleeding Academic Research Consortium (BARC) categorization of bleeding events [2], respectively, it is difficult to gauge how robust the outcomes are between trials of high-risk PCI when the patient cohort is significantly heterogeneous and lacks standardization. Box 1 lists several putative clinical, anatomical and hemodynamic criteria that have been used by various investigators to denote ‘high risk’ [3].
One way of assessing or assigning risk is to use a ‘myocardium at risk’ score. These scores help to systematically calculate the severity of CAD based on angiographic findings and also give an indication of the relative importance of each of the three major epicardial coronary arteries on an individual patient basis. This is clearly a preferred method of ascertaining risk over that of simply demonstrating the number of diseased major vessels. Moreover, they also provide prognostic information.
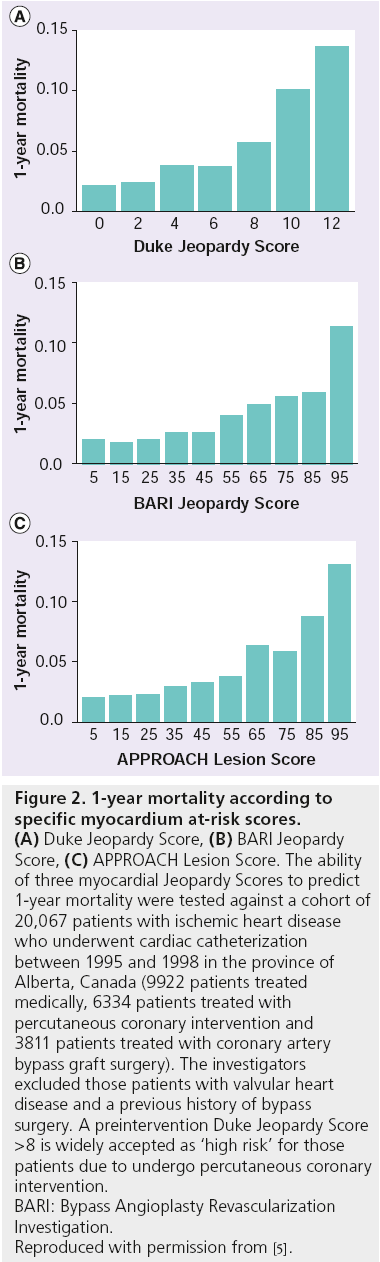
One of the most commonly adopted is the Jeopardy Score from Duke University (NC, USA), which divides the coronary tree into six segments (left anterior descending, diagonal branch, circumflex, obtuse marginal, right coronary and posterior descending arteries) with all segments distal to a ≥70% stenosis considered as being at risk [4]. Each segment receives two points if affected, plus two further points if the lesion affects two of the six downstream myocardial territories, giving rise to a maximum score of 12. The Alberta Provincial Project for Outcome Assessment in Coronary Heart (APPROACH) disease investigators assessed and validated the prognostic value of their own Lesion Score with that of the Duke Jeopardy Score and the Bypass Angioplasty Revascularization Investigation (BARI) Myocardial Jeopardy Index in >20,000 patients undergoing coronary catheterization for ischemic heart disease and found all three scores to be predictive of 1-year mortality (see Figure 2) [5].
Figure 2: 1-year mortality according to
specific myocardium at-risk scores. (A) Duke Jeopardy Score, (B) BARI Jeopardy
Score, (C) APPROACH Lesion Score. The ability
of three myocardial Jeopardy Scores to predict
1-year mortality were tested against a cohort of
20,067 patients with ischemic heart disease
who underwent cardiac catheterization
between 1995 and 1998 in the province of
Alberta, Canada (9922 patients treated
medically, 6334 patients treated with
percutaneous coronary intervention and
3811 patients treated with coronary artery
bypass graft surgery). The investigators
excluded those patients with valvular heart
disease and a previous history of bypass
surgery. A preintervention Duke Jeopardy Score
>8 is widely accepted as ‘high risk’ for those
patients due to undergo percutaneous coronary
intervention.
BARI: Bypass Angioplasty Revascularization
Investigation.
Reproduced with permission from [5].
The common thread connecting these multiple variables is the relative inability of the high-risk patient to withstand the hemodynamic sequelae of arrhythmias and even transient periods of ischemia-reperfusion, for instance, during balloon inflation and stent deployment or from the distal embolization of atherogenic material (i.e., the no-reflow phenomenon). These individuals have significantly attenuated hemodynamic reserve and may develop postischemic stunning, leading to a deleterious cascade of decreased diastolic compliance, depressed systolic function leading to a fall in cardiac output and worsening ischemia culminating in cardiogenic shock (CS) or ventricular arrhythmias. Furthermore, high-risk patients with subnormal left ventricular function tend to be older and have more pre-existing comorbidities, both of which are independently associated with poorer outcome post-PCI [6].
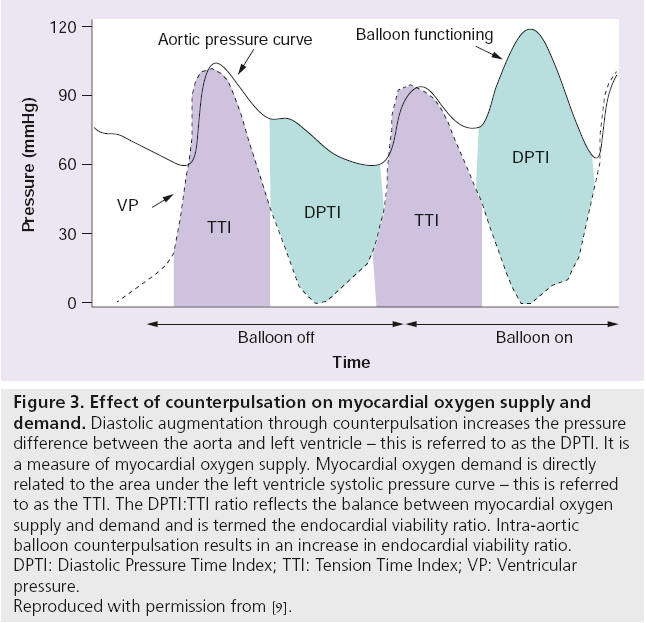
As there is a heightened propensity to suffer catastrophic hemodynamic collapse, either as a direct consequence of the procedure or as a result of the pre-existing proischemic milieu, it seems intuitively attractive to use an adjunctive device that can augment the coronary circulation and reduce the workload of the endangered myocardium during high-risk PCI. One such device is the intra-aortic balloon pump (IABP; MAQUET Cardiovascular, NJ, USA) and the physiological benefits of mechanical counterpulsation it provides (Box 2, Figure 3) [7–14].
Figure 3: Effect of counterpulsation on myocardial oxygen supply and
demand. Diastolic augmentation through counterpulsation increases the pressure
difference between the aorta and left ventricle – this is referred to as the DPTI. It is
a measure of myocardial oxygen supply. Myocardial oxygen demand is directly
related to the area under the left ventricle systolic pressure curve – this is referred
to as the TTI. The DPTI:TTI ratio reflects the balance between myocardial oxygen
supply and demand and is termed the endocardial viability ratio. Intra-aortic
balloon counterpulsation results in an increase in endocardial viability ratio.
DPTI: Diastolic Pressure Time Index; TTI: Tension Time Index; VP: Ventricular
pressure.
Reproduced with permission from [9].
The diastolic augmentation of coronary perfusion
“In short, it seems likely that the returned arterialized blood should be returned during diastole when the aortic valve is closed. This would probably offer less resistance to the ailing heart’s systolic effort and therefore presumably less cause for myocardial work which of course is our objective … For medical auxiliary circulation it will probably be better to have this micro-switch mechanism replaced by a synchronizing oscillator so we can have slave correlation to the QRS complex of the electrocardiogram.”
– Dwight E Harken
Dwight Emary Harken, a pioneering US army cardiac surgeon, speaking at a meeting on extracorporeal circulation in 1957, had clearly grasped the concept of diastolic augmentation [15]. Four years previously, the Kantrowitz brothers had published a paper in which they described a 22–53% increase in flow through a canine circumflex artery by retardation of the arterial pressure pulse so that peak arterial pressure was made to occur during diastole [16]. The term ‘counterpulsation’ was coined from another canine study conducted by Harken’s group in which an external ‘mechanical ventricle’ was used [17]. Here, an electronically controlled arterial counterpulsator allowed the injection of blood, withdrawn in ventricular systole, into the abdominal aorta during diastole. There were problems with the procedure: most pertinently the need for bilateral femoral arteriotomies and technical difficulties with the extracorporeal pump. The authors did, however, postulate this new device and its underlying physiological principles could be utilized in acute heart failure.
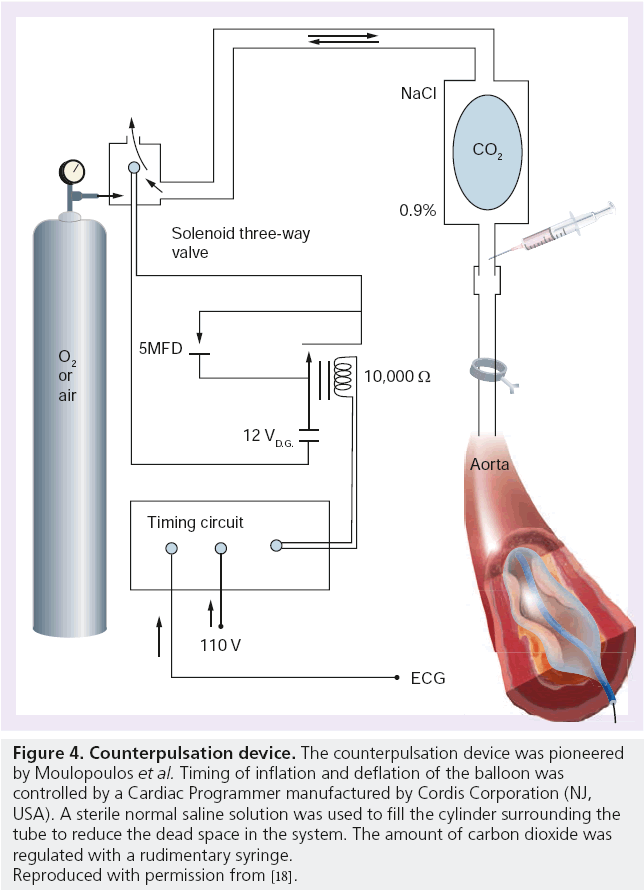
Moulopoulos et al. at The Cleveland Clinic (OH, USA) went on to develop a method of counterpulsation that avoided removing blood out of the body. They constructed a precursor to the modern-day IABP by using latex tubing tied around the end of a polyethylene catheter with multiple side holes (Figure 4) [18]. A 20 cm length of the latex tubing was inserted into the descending thoracic aorta via the left carotid or subclavian artery of live anesthetized dogs. By occluding the distal end of the catheter, the latex tubing could be rhythmically inflated and deflated with carbon dioxide, the volume of which could be controlled by a syringe. This apparatus was connected to a circuit timed according to the ECG of the animal. Stroke length and delay after the R wave were preset to allow diastolic augmentation to occur. They were able to demonstrate an increase in diastolic blood flow through the arterial system and an associated lowering of the end diastolic arterial pressure. And so was born the IABP.
Figure 4: Counterpulsation device. The counterpulsation device was pioneered
by Moulopoulos et al. Timing of inflation and deflation of the balloon was
controlled by a Cardiac Programmer manufactured by Cordis Corporation (NJ,
USA). A sterile normal saline solution was used to fill the cylinder surrounding the
tube to reduce the dead space in the system. The amount of carbon dioxide was
regulated with a rudimentary syringe.
Reproduced with permission from [18].
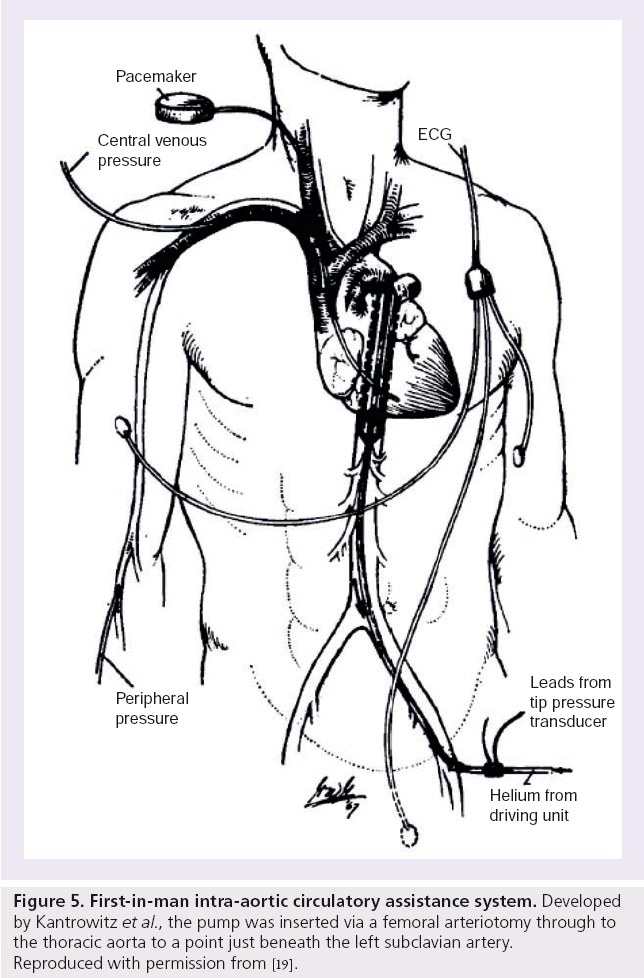
The first-in-man experience of IABC was performed by Kantrowitz et al. [19]. By this time, helium had replaced carbon dioxide as a means of expanding the balloon, since its lower density assured rapid passage through the catheter. A femoral arteriotomy was fashioned to insert the pump to a point just beneath the subclavian artery in two patients presenting with CS (Figure 5). One patient survived maintaining adequate blood pressure and urine output after several hours of intermittent IABC. The other patient suffered intractable circulatory collapse leading to florid pulmonary edema, ventricular fibrillation and ultimately death, 1 h and 29 min after IABC, which had to be stopped for balloon repositioning; the latter case reflecting a complete dependence of the patient’s circulation on the balloon pump. The investigators had the foresight to list criteria that a temporary cardiac assist device should fulfill to be of value in these particular clinical scenarios:
Figure 5: First-in-man intra-aortic circulatory assistance system. Developed by Kantrowitz et al., the pump was inserted via a femoral arteriotomy through to the thoracic aorta to a point just beneath the left subclavian artery. Reproduced with permission from [19].
▪ Effective insertion with minimal surgical application;
▪ Capability for aiding the coronary and peripheral circulation intermittently or continuously for hours or days;
▪ Significant support for the ischemic myocardium by reducing its work;
▪ Simplicity of initiation and maintenance for widespread use by minimally trained professional personnel [19].
These basic principles continue to underlie the clinical effectiveness and safety of modern-day devices. By 1979 Bregman and Casarella had published their experience with an IABP that could be inserted percutaneously through a 12 F sheath via the Seldinger technique [20].
Intra-aortic counterpulsation: a physiologist’s perspective
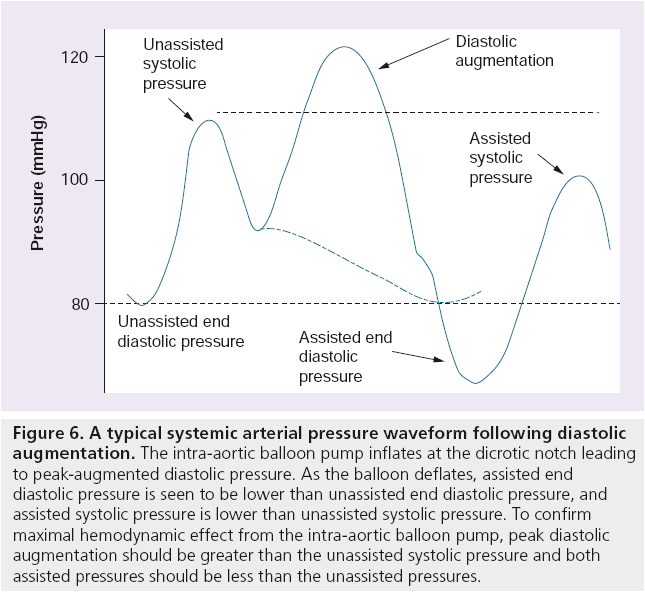
IABP systems are typically inserted within the multidisciplinary environment that is the cardiac catheterization laboratory. The specific role of the physiologist during IABP insertion is to ensure correct system set-up and maintenance during what is likely to be either a complex or acute PCI case. This includes providing and selecting appropriate ‘trigger’ sources for the system, setting up a pressurized fluid-filled line for accurate pressure monitoring, waveform analysis to ensure correct inflation and deflation timings (see Figure 6) and selecting the most appropriate settings for each specific clinical situation.
Figure 6: A typical systemic arterial pressure waveform following diastolic augmentation. The intra-aortic balloon pump inflates at the dicrotic notch leading to peak-augmented diastolic pressure. As the balloon deflates, assisted end diastolic pressure is seen to be lower than unassisted end diastolic pressure, and assisted systolic pressure is lower than unassisted systolic pressure. To confirm maximal hemodynamic effect from the intra-aortic balloon pump, peak diastolic augmentation should be greater than the unassisted systolic pressure and both assisted pressures should be less than the unassisted pressures.
Depending on the patient’s hemodynamic status, the balloon can be programmed to assist with every heartbeat in a 1:1 fashion, or less frequently in a 1:2 or 1:3 ratio, the latter sequence often used to wean down support prior to removal. Successful weaning from the IABP requires the patient to have no signs of CS with a satisfactory blood pressure on minimal or no inotropic support (target mean arterial pressure of ≥65 mmHg and if available, a cardiac index of 2 l/min/m2). The device should never be left switched off in situ as this would increase the risk of thrombus formation.
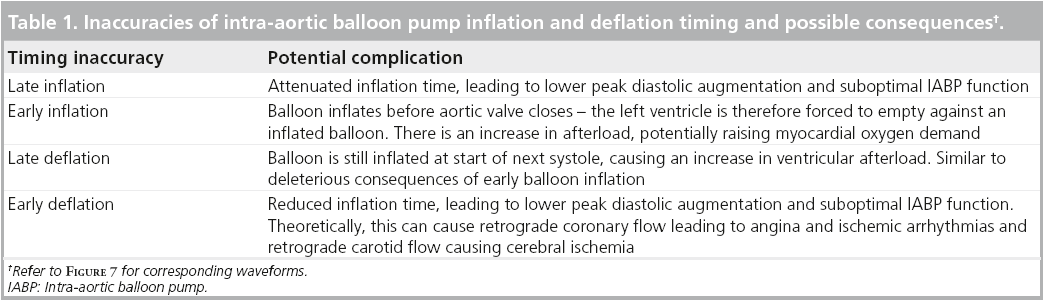
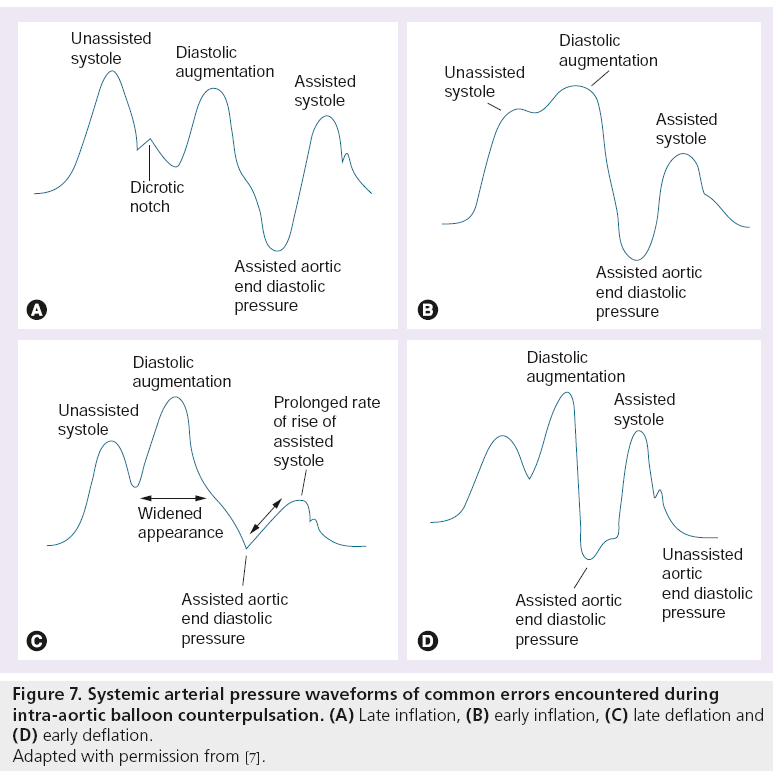
Recognizing correct inflation and deflation balloon catheter timings is an important physiologist skill. Modern IABP systems have built-in features that enable fairly accurate baseline inflation and deflation timings to be selected – however, these timings may not always be optimum. It is important to assess inflation and deflation timings at balloon insertion and on a regular basis whilst the balloon is in situ to avoid any related complications (see Table 1, Figure 7).
In many circumstances, the IABP system will need to remain with the patient after their catheter laboratory procedure until they are hemodynamically stable or have further intervention (e.g., CS patients or patients with left main/triple vessel disease requiring Coronary artery bypass graft [CABG]).
There are many situations in the ward environment that risk compromising the function of the IABP. Patient movement can cause the balloon catheter to displace either upwards towards the head and neck vessels or downwards causing possible occlusion of the renal arteries. In these situations, the waveform may show little change to reflect the new position, so other clinical signs should be regularly observed – such as the radial pulse and the urine output, as these may give more accurate clues to balloon displacement.
It is also a common complication for the pressure line to become compromised. This can be due to a number of causes; for instance if the catheter becomes twisted or kinked, the pressure signal through the fluid-filled line will be lost. The screen will display a flat line sitting at the top of the pressure range. If the kink is not visible on the portion of the line outside of the body, the catheter itself may be kinked and the patient may have to be taken back to the catheter laboratory for this to be resolved. Clots may also form in the pressure line. This could be due to insufficient pressure in the line causing retrograde blood flow back towards the transducer, insufficient anticoagulation therapy to prevent clotting within the lumen of the catheter or as a consequence of using the pressure line to collect blood or administer medication. If a clot does develop within the lumen of the pressure line, the waveform seen on the IABP screen will become damped. All prominent waveform features will become lost and the pulse pressure of the signal will decrease. Damping can be seen at varying levels depending on clot size from a subtle smoothing out of the waveform, to a complete loss of all recognizable waveform features. If this occurs, the line should be aspirated to remove the clot and then thoroughly flushed through again to ensure the heparinized-saline solution reaches the very tip of the IAB catheter (see Box 3).
As with most medical devices, advancement and improvements are being made in counterpulsation systems regularly. The predominant systems currently in use are the CS100 and the CS300 systems, manufactured by MAQUET Cardiovascular. Both of these systems have many intuitive features that can reduce pump set-up time, virtually obviate the need for clinician adjustment and optimize pump function.
Both systems are built to recognize the optimum trigger source and can set the inflation and deflation times fairly accurately to optimize patient care. An algorithm known as R-Trac© is available in these systems, which means that the regularity of the patients rhythm is monitored and, if the patient changes from a regular rhythm to an irregular rhythm, the system will adjust the deflation timings on a beat-to-beat basis.
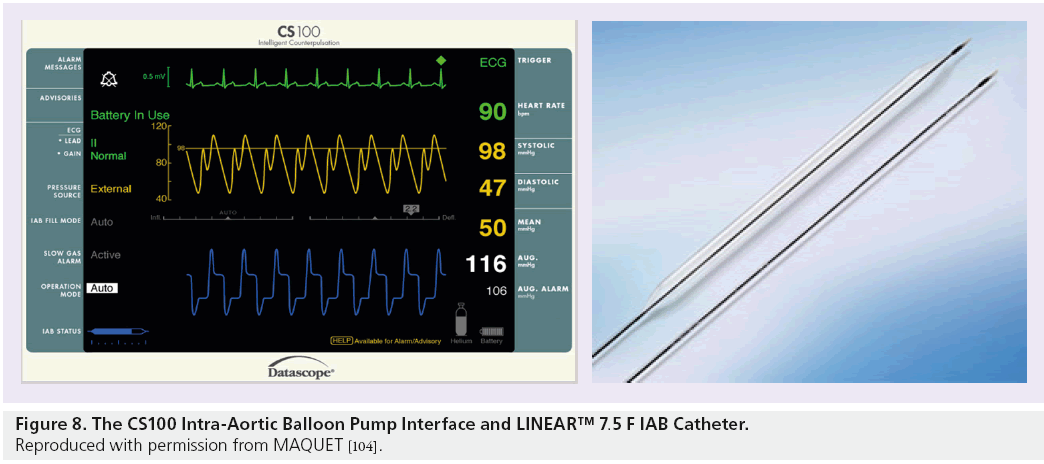
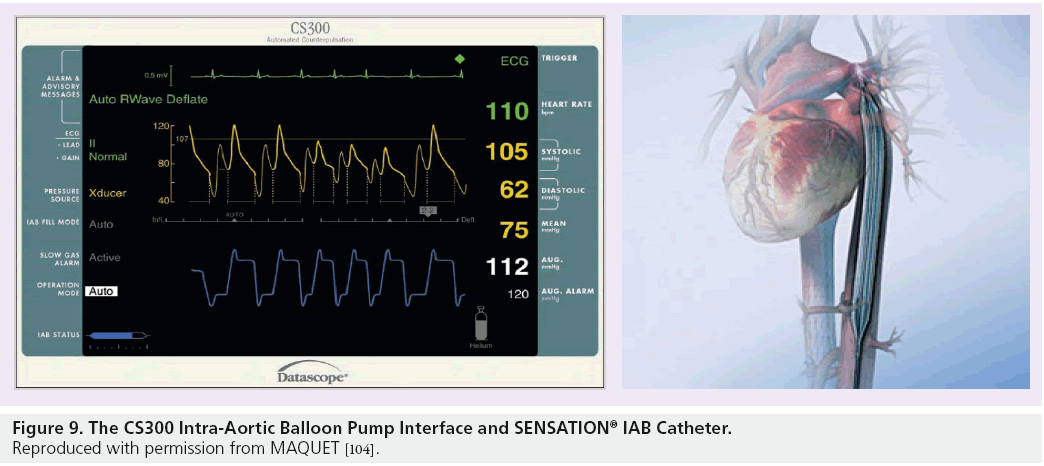
The CS100 is compatible with fluid-filled IAB catheters such as the 7.5 F LINEAR™ catheter, which comes in a variety of sizes to correspond with patient height. These fluid-filled catheters are, however, subject to the complications described above. One of the main advantages of the CS300 system is that a more advanced fiber-optic catheter called the SENSATION® 7 F can be used. The SENSATION catheter has many advantages over the LINEAR catheter; the main advantage being the use of fiber-optic technology to measure the IABP waveform. The SENSATION catheter removes the need for a fluid-filled pressure line completely – and therefore, all of the complications associated with it. The pump set-up time when using the SENSATION catheter is also reduced as a transducer and pressurized fluid-filled line are no longer required which, in an acute situation, is a great advantage (see Figures 8 and 9).
Figure 8: The CS100 Intra-Aortic Balloon Pump Interface and LINEAR™ 7.5 F IAB Catheter. Reproduced with permission from MAQUET [104].
Figure 9: The CS300 Intra-Aortic Balloon Pump Interface and SENSATION® IAB Catheter. Reproduced with permission from Maquet [104].
With further improvements to the current counterpulsation systems on the horizon, it seems possible that IABP therapy will soon become almost fully automated after balloon insertion, allowing more time for staff to concentrate directly on the patients themselves. It is still very important that the necessary skills needed to recognize correct inflation/deflation times and to troubleshoot any pump problems, are still taught to all staff involved in the use of IABP systems as there will always be times, despite technological advancements, that human experience and intuition will be required.
A low-risk therapeutic option in a high-risk patient cohort
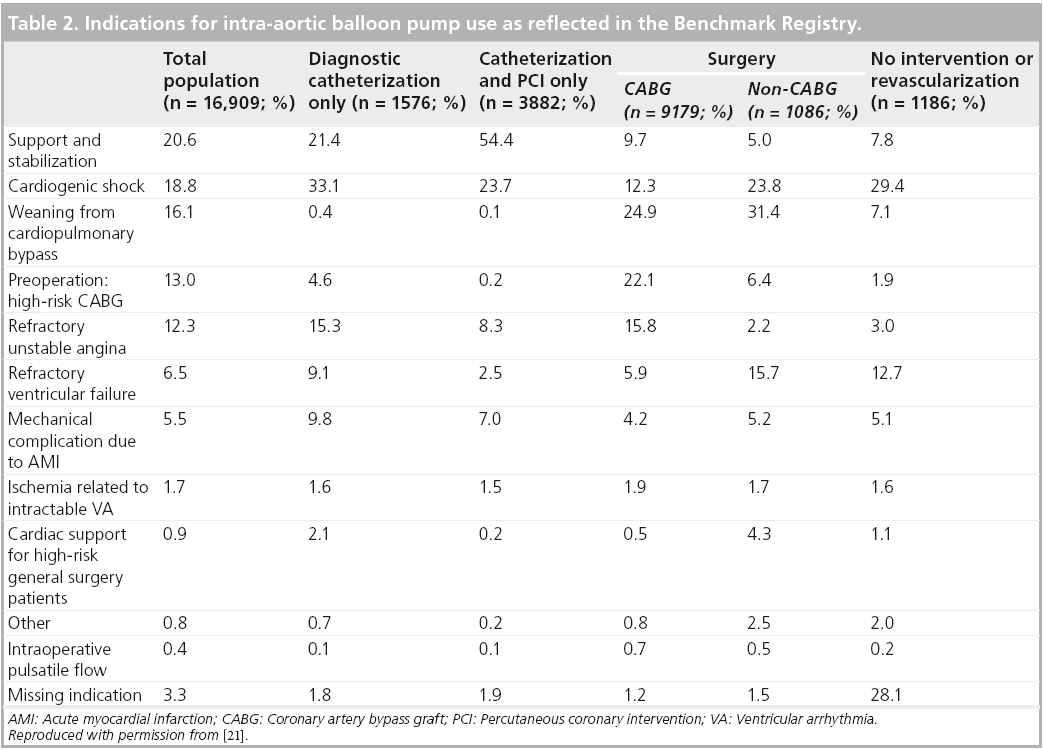
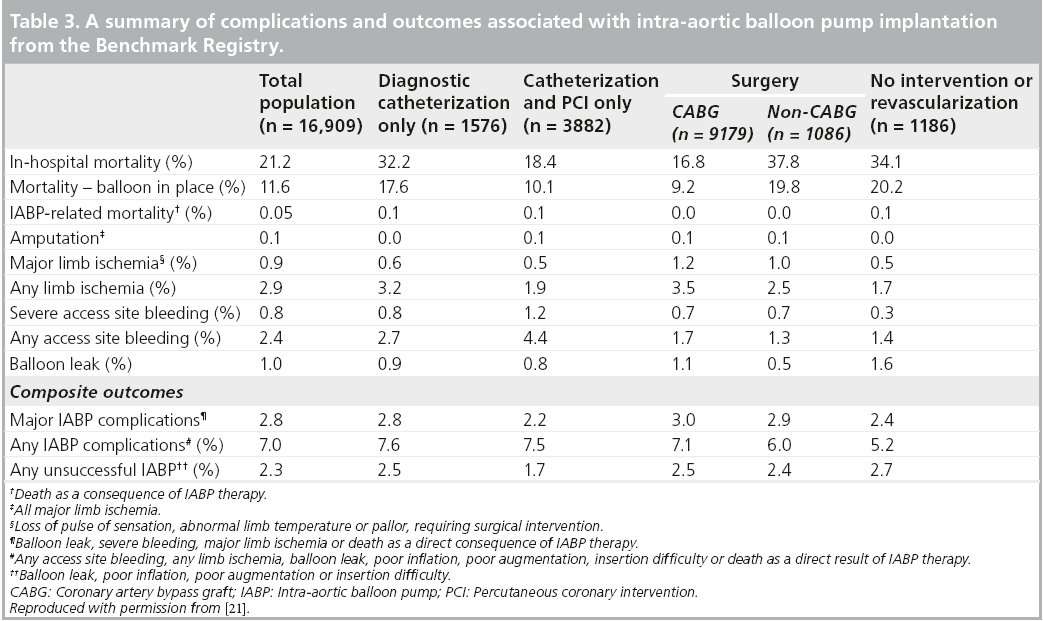
The Benchmark Counterpulsation Outcomes Registry is perhaps the most robust barometer of real-world contemporary IABP practice we have at our disposal. The first report, published in 2001, documented, most pertinently, the indications, clinical outcomes and complications associated with IABP implantation across 243 institutions in 18 countries [21]. Data collection was initiated in June 1996 and by August 2000, there were 17,540 IABP records from 16,909 patients available for analysis from this prospective computerized database.
As expected, the most frequent IABC indication was for hemodynamic support during or after cardiac catheterization (20.6% of patients). It is unclear what proportion of these constituted high-risk PCI procedures although, given that 15.4% of patients presented with left main coronary artery (LMCA) disease, 28.5% triple vessel disease and 23.0% of the overall cohort underwent PCI, we can potentially assume a significant proportion of the hemodynamic support provided for cardiac catheterization was indeed for a high-risk PCI subset. Other common indications included CS (18.8%) and refractory unstable angina (12.3%) (see Table 2).
One of the major findings of this study was the encouragingly low incidence of major complications associated with IABP implantation (see Table 3). The profiles of balloons and catheters have evolved and diameters narrowed along with marked improvements in anticoagulation regimes and increasing clinical experience and familiarity with the device. This is reflected in the deaths attributable to IABP failure or insertions being as low as five in 10,000. The incidence of major complications (2.8%) and any unsuccessful IABP implantations (2.3%) as composite outcomes were relatively low. This led the investigators to conclude that IABC represented a “low-risk therapeutic option in a high-risk patient cohort” [21]. They also went on to identify independent predictors of major complications of IABC using multivariate logistic regression analysis. Of the 15 variables screened, the following significantly increased the risk of a major complication:
▪ Female gender;
▪ Peripheral vascular disease;
▪ Small body surface area (<1.65 m2);
▪ Age ≥75 years.
Clearly, as with the utilization of any medical device, appropriate patient selection is paramount (see Box 4).
The question remains, however, as to whether real-world utilization of a device matches up with national and international guidelines? In the latest European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS) guidelines on myocardial revascularization, the use of IABC is given a class I, level of evidence C, recommendation for those patients presenting with hemodynamic instability in the setting of acute myocardial infarction (MI) with particular emphasis paid to those in CS or those suffering mechanical complications (e.g., acute mitral regurgitation secondary to papillary muscle rupture, ventricular septal defect, free wall rupture or cardiac tamponade) [22]. The IABP should be inserted prior to any attempt at revascularization in the context of acute ST-elevation MI (STEMI) complicated by hemodynamic compromise. The ESC does not recommend, however, the prophylactic use of IABC when there is no hemodynamic impairment. Interestingly there is no reference to, or guidelines on, a stand alone ‘high-risk’ PCI subset in this publication although most observers would agree that revascularization for STEMI complicated by acute heart failure is indeed a high-risk clinical scenario.
The American College of Cardiology Foundat ion (ACCF)/American Hea r t Association (AHA) Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions (SCAI) are more explicit on the role of periprocedural IABC as an adjunct to PCI in the most recent iteration of their PCI practice guidelines published in 2011 [23]. Like the Europeans they have assigned a class I level of evidence B recommendation on the use of IABC in STEMI patients presenting with CS refractory to pharmacological therapy. They have, however, gone one step further than the ESC/EACTS guidelines by delineating what constitutes high-risk PCI:
▪ PCI to an unprotected LMCA or last remaining conduit;
▪ PCI to a vessel supplying a significant area of myocardium in a patient with severely depressed LV function;
▪ PCI in those patients in CS.
Under these circumstances the ACCF/AHA/ SCAI have assigned a class IIb, level of evidence C, recommendation for the elective insertion of an appropriate hemodynamic support device as an adjunct to PCI, the caveat being judicious patient selection and the following factors taken in to careful consideration:
▪ Risk of vascular injury;
▪ Ease of application/removal of the device;
▪ Related complications;
▪ Operator/catheterization laboratory expertise.
It is evident from the Benchmark Registry that the IABP is commonly used in specific high risk scenarios. It is familiar to the majority of catheterization laboratories throughout the world; has a relatively low acquisition cost; is easy to implant and, if used diligently and in the correct patient subtype, is associated with a significant but reassuringly low incidence of directly attributable complications. Furthermore, contemporary IABP systems require minimal technical support and automated algorithm advancements now allow for seamless adjustment to changing patient and environmental factors. In addition, intra-aortic counterpulsation in high-risk revascularization procedures is currently supported by European and US class I recommendations, albeit with levels of evidence that are not entirely robust. There are many reasons, therefore to use the IABP, but what of the data?
The evidence for intra-aortic counterpulsation in high-risk PCI
▪ Registry data
The applicability and validity of registry data remains open to debate. When studying a patient cohort that is notoriously difficult to enroll in randomized trials, registry data can certainly add value by giving an all-comers, real-world, viewpoint on management trends and, since registries can be easily maintained, subsequent longer-term outcomes. Clearly there is the scepter of selection bias overhanging any registry, but with adjustment for baseline differences and the utilization of propensity score matching, the data accrued can be robust and can be used to evaluate the safety and efficacy of a particular intervention in everyday practice. Furthermore the larger registries allow investigators to study relatively rare outcomes and generate statistically significant inferences from them. This has certainly been the case for studying trends pertaining to IABP usage in high-risk clinical scenarios.
Brodie et al. looked at a registry of 1490 consecutive patients admitted to a single center with acute MI treated with primary PCI (the majority with balloon angioplasty but also coronary stenting in the last 3 years) without prior thrombolytic therapy from 1984 to 1997 [24]. An IABP was implanted in 213 (14.2%) of these patients, 133 had CS, 80 were hemodynamically stable but high-risk, 108 received counterpulsation before intervention and 105 after intervention. In 119 of the CS patients, the prophylactic use of IABC before intervention resulted in significantly lower rates of catheterization laboratory events (defined as ventricular fibrillation/tachycardia, cardiopulmonary arrest and prolonged hypotension) compared with no IABC or IABC after intervention. In 119 high-risk patients (defined as congestive heart failure or left ventricular ejection fraction (LVEF) ≤30%) preintervention IABC was also associated with a nonsignificant reduction in laboratory events. IABC led to an increased propensity for major bleeding although the investigators did recognize the potential adverse effect of larger sheaths, high dose heparin anticoagulation and longer indwelling of the sheath, could have had on this outcome.
The SHOCK Trial Registry concurrently enrolled 1190 patients with suspected CS from 36 participating centers during the SHOCK Trial recruitment period [25]. Of these, 856 patients with CS secondary to acute MI were available for analysis. Those patients supported with an IABP were more likely to proceed to coronary angiography (p < 0.001) and as such, IABC use was associated with a lower mortality rate compared with no IABC (50 vs 72%; p < 0.0001). Patients proceeding to revascularization (PCI or CABG) following thrombolysis and IABP insertion had a significantly lower mortality than those who remained on a conservative strategy (39 vs 78%; p < 0.0001) [26]. A subgroup analysis of patients undergoing PCI was also undertaken to examine the effect of diastolic augmentation. Interestingly, there was no difference in in-hospital mortality for patients with an IABP in situ prior to PCI (n = 98, 47% mortality) versus those receiving counterpulsation after PCI (n = 95, 47% mortality) versus patients undergoing PCI without IABP support altogether (n = 56, 46% morality) [26], although this result could be attributed to differences in patient selection, a well recognized flaw of all registries.
In the National Registry of Myocardial Infarction-2 (NRMI-2), investigators studied the outcomes of 23,180 participants presenting with CS or in whom CS developed during their hospital admission [27]. IABC was used in 7268 (31%) patients. In those that received thrombolytic therapy, supplemental IABC conferred a significant mortality advantage (thrombolysis + IABP 49% vs thrombolysis alone 67%), perhaps reflecting the synergistic effect of both therapies working in tandem to establish patency of the infarct-related artery. High-risk primary PCI in this setting produced the lowest mortality rate, although insertion of an IABP did not lead to any further advantage (primary PCI 42% vs primary PCI + IABP 47%) and, in fact, was associated with higher hospital mortality rates. Unlike thrombolysis, PCI does not rely on coronary perfusion pressure to establish patency. It should also be borne in mind that the specific timing of IABP insertion was not available for this analysis.
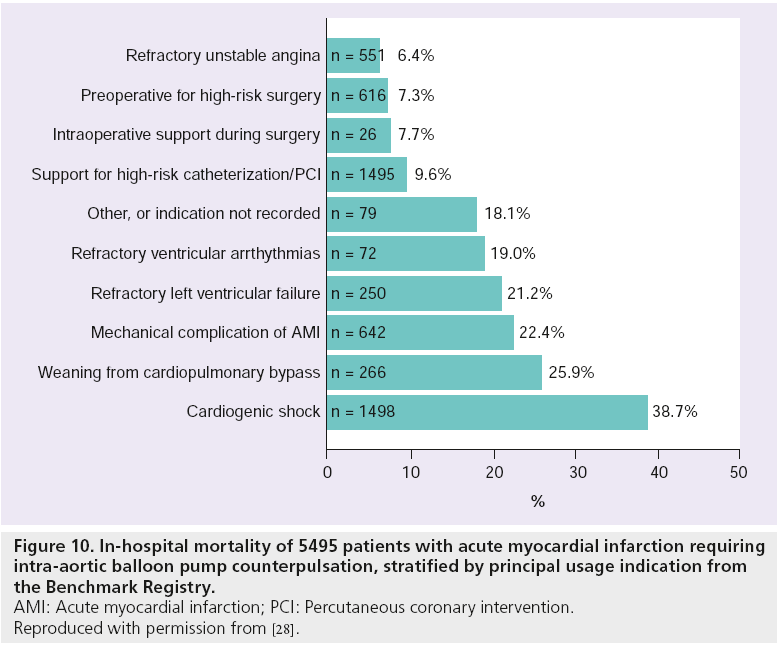
Further iterations of the Benchmark Registry also provide a noteworthy insight in to IABP usage patterns in acute MI [28]. Of the 22,663 patients that received an IABP at 250 centers between June 1996 and August 2001, 5495 (24%) were documented as having an acute MI. CS (n = 1498, 27.3%) and high-risk catheterization and angioplasty (n = 1495, 27.2%) were the principal indications for IABC emanating from the analysis. Unfortunately the investigators do not specifically state what constitutes ‘high risk’ in the paper, but with 59% of those undergoing cardiac catheterization having triple vessel disease and 16% LMCA involvement in addition to a mean ejection fraction of 36.5 ± 14.3% within the cohort, we can confidently surmise the type of characteristics that may have been used. As with the first Benchmark Registry analysis [21], rates of major IABP-related complications remained low and were similar between patients undergoing PCI (2.8%), surgery (2.6%) or conservative management with or without angiography (2.7%) [28]. Indeed only three (0.05%) of the 5495 patients died as a direct result of IABP placement. Furthermore, the process of IABP insertion was itself shown to be safe, with only 2.2% of patients suffering a failed procedure as a consequence of balloon leak, poor inflation, poor augmentation or insertion difficulty [28]. And finally, in-hospital mortality of those acute MI patients requiring IABC support for highrisk catheterization/PCI was proportionately low (9.6%) despite it being the primary indication for hemodynamic assistance in over a quarter of the entire cohort (see Figure 10).
Figure 10: In-hospital mortality of 5495 patients with acute myocardial infarction requiring intra-aortic balloon pump counterpulsation, stratified by principal usage indication from the Benchmark Registry. AMI: Acute myocardial infarction; PCI: Percutaneous coronary intervention. Reproduced with permission from [28].
Results from an analysis of the National Cardiovascular Data Registry CathPCI Registry, in which the use and effectiveness of IABC for high-risk PCI was examined, give the most contemporaneous and provocative look at recent trends [29]. Importantly, PCI was designated as high-risk if one of the following factors were present:
▪ Unprotected LMCA as the target vessel;
▪ CS;
▪ Severely depressed LV function (<30%);
▪ STEMI.
A total of 181,599 high-risk patients undergoing PCI at 681 hospitals between 1 January 2005 and 31 December 2007 were included in the analysis. The primary outcome measure was in-hospital mortality. Of the high-risk PCIs, 144,190 (79.4%) presented with STEMI, 21,259 (11.7%) had CS, 3592 (2.0%) had unprotected LMCA PCI and 37,394 (20.6%) had significant LV systolic dysfunction. An IABP was implanted in 44.4% of CS patients, 10.3% of STEMI patients, 28.1% receiving LMCA PCI and 13.9% of those with depressed LV function. Overall the perceived hemodynamic benefits of IABP were only deemed necessary for 10.5% of all high-risk cases, a relatively low figure given the widespread availability of the device. The investigators went on to categorize hospitals according to their proportional IABP use and grouped them in to corresponding quartiles. They found significant variation in IABP use across all participating centers, with a median odds ratio of 1.93 for a hospital effect, indicating a substantial influence held by actual location on whether a patient would receive IABC or not. Most poignantly, and after adjustment for multiple variables, in-hospital mortality or complication rates did not vary across hospital quartiles, despite differences in the rate of IABP implantation. Furthermore, a meticulous analysis of subgroups found no particular subset of patients benefitted from an increased frequency of IABP use. A fundamental limitation of this analysis was a lack of information on the timing of IABP insertion, one that has been recognized by the investigators. Nevertheless, they conclude there is little evidence to support the increased use of IABP at hospitals which were more selective on implementing this adjunct to high-risk PCI. Based on these results, it appears the use of IABP in a high-risk setting is not only low for such a commonly available device but also appears discretionary and is influenced more by local expertise, familiarity and protocols as opposed to definitive evidence from clinical trials and direction from guidelines.
▪ Trial data
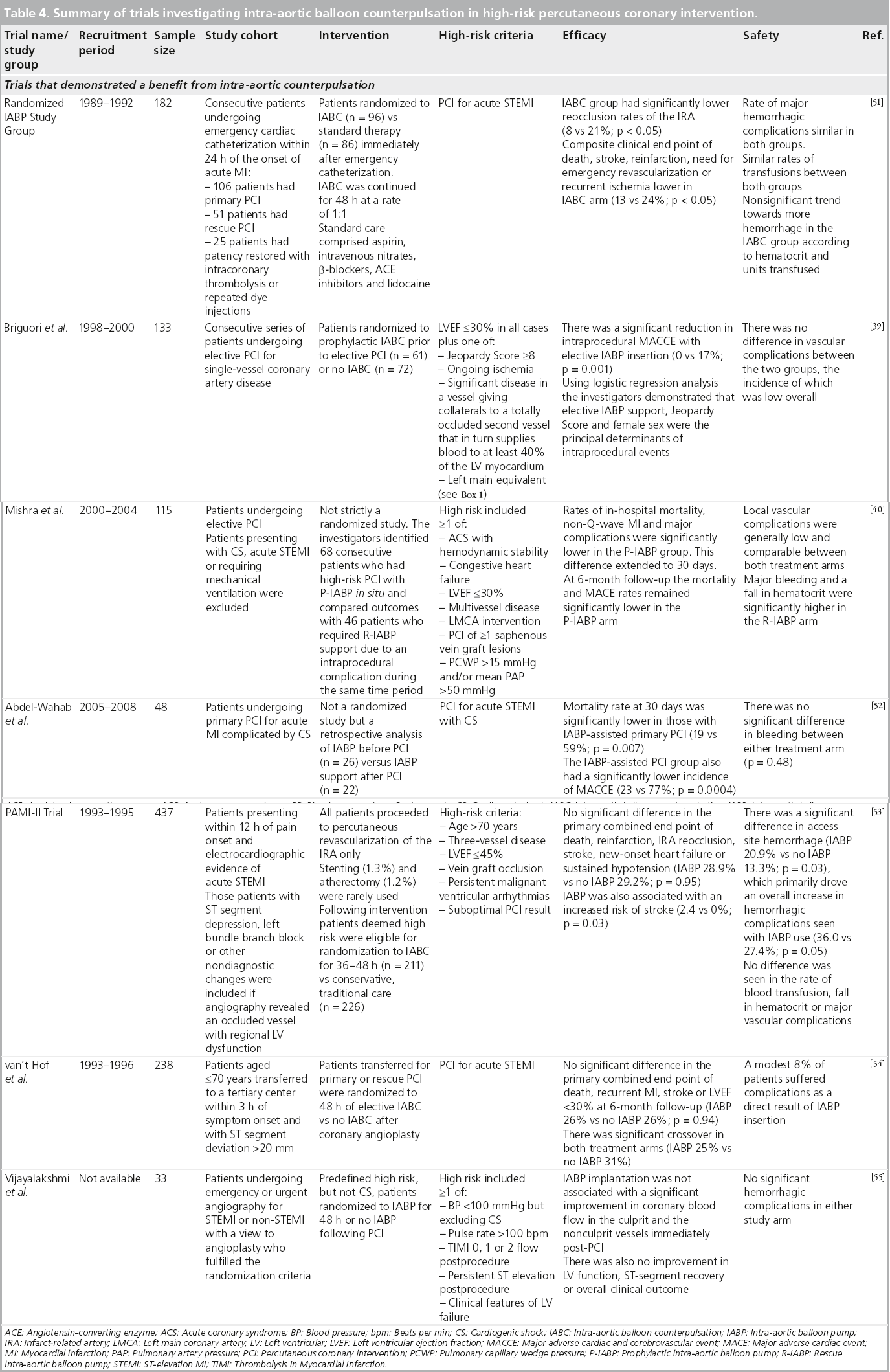
There have been several randomized trials on the use of IABC in high-risk PCI, results of which, much like the registry data above, have produced conflicting results that serve to convolute the matter, rather than provide a uniform statement on the clinical effectiveness of IABP. These are summarized in Table 4. Three landmark trials are, however, worthy of closer scrutiny.
The SHOCK Trial randomized, in a 1:1 fashion, 302 patients presenting with acute MI complicated by CS precipitated by LV failure to emergency revascularization versus initial medical stabilization [30]. Although an IABP was inserted in 86% of the patients in both groups, no clear benefit of IABC could be delineated for either treatment strategy. Indeed the high uptake of IABC in the conservative treatment arm, along with the frequent use of thrombolytic therapy, could well have led to a convergence in overall 30-day mortality, which was not significantly reduced by early revascularization (46.7% revascularization vs 56.0% medical therapy, p = 0.11). By 6 months, however, overall mortality was significantly lower in the revascularization arm (50.3 vs 63.1%; p = 0.027), a trend that persisted to improved 1-year survival rates in favor of revascularization (46.7 vs 33.6%; p < 0.03) [31]. Although not a direct investigation of IABC, this seminal study highlighted the frequent use of IABC in the context of revascularization for CS secondary to acute MI. By virtue of the improved 1-year survival rate, but by no means a statistically valid inference, we might surmise that the use of IABC during PCI in this high-risk cohort of patients appeared to be beneficial. The results of this landmark study have been used by both the ESC/EACTS and the ACCF/AHA/SCAI to justify their class I recommendation for IABC in acute MI complicated by CS [22,23]. The survival benefit of early revascularization persisted to 6-year follow-up (32.8 vs 19.6%), irrespective of the emergency mode of revascularization used (i.e., PCI or CABG) [32].
The BCIS-1 study was the first prospective, open, multicenter, randomized controlled trial to determine whether elective IABP insertion prior to high-risk single-vessel or multivessel PCI was able to reduce MACCE (i.e., death, acute MI, cerebrovascular event or the need for further revascularization by either PCI or CABG at hospital discharge capped at 28 days) [33]. High-risk in this instance was characterized by the following:
▪ Depressed LV function (ejection fraction ≤30%);
▪ A BCIS-1 Jeopardy Score of ≥8 (a modification of the Duke Jeopardy Score);
▪ LMCA PCI;
A target vessel that provided collateral supply to an occluded second vessel that in turn supplied more than 40% of myocardium.
Those patients with pre-existing class I or II indications for IABP insertion (i.e., CS, acute MI within the last 48 h, and complications of acute MI) were excluded. Elective IABP insertion took place before coronary intervention. Bailout IABC was permitted in the no planned IABP group if clinical circumstances warranted it. Overall 301 patients with multivessel disease and LV systolic dysfunction were randomized in a 1:1 fashion between December 2005 and January 2009. There were similar rates of the primary end point of MACCE between both arms of the trial (15.2% elective IABP vs 16.0% no planned IABP; p = 0.85). There was no significant difference in the secondary end points of 6-month mortality (it should be noted that the trial was not sufficiently powered to detect a difference in mortality at 6 months) or overall rates of bleeding although, when broken down, there were significantly more minor bleeds in the elective IABP arm (15.9% elective IABP vs 7.3% no planned IABP; p = 0.02). This was perhaps tempered by more periprocedural complications occurring in the no planned IABP arm, predominantly due to procedural hypotension (1.3 vs 10.7% in favor of elective IABP; p < 0.001), which might explain the need for rescue/bailout IABC in 18 (12%) patients overall.
Overall the study did not support the use of elective/prophylactic IABP insertion prior to high-risk PCI in terms of reducing the incidence of MACCE. Despite this, evidence from the study helped to justify the current class IIb recommendations assigned by the new ACCF/AHA/SCAI PCI guidelines on the elective use of hemodynamic support devices before performing high-risk PCI [23]. Given the fact that 12% of the no planned IABP arm required rescue/bailout IABC during their procedure, does suggest, however, that an initial strategy of standby IABC for PCI in those patients with compromised LV functional reserve and extensive CAD could attenuate the delay in gaining arterial access and therefore prevent patients from entering a cascade of irreversible hemodynamic collapse. Interestingly, those patients requiring rescue IABC had a significantly higher BCIS-1 Jeopardy Score than those not requiring salvage in the no planned IABP group (Jeopardy Score: 11.2 vs 10.2; p = 0.02) further emphasizing the potential need for provisional hemodynamic support in those at the extreme end of the risk spectrum.
The CRISP AMI trial was a prospective, multicenter, open, randomized controlled trial undertaken to determine whether prophylactic IABP insertion within 6 h of pain onset and planned primary PCI for acute anterior STEMI (without CS) was able to reduce infarct size, as measured by cardiac MRI between 3 and 5 days postintervention, when compared with standard care [34]. As with BCIS‑1, the insertion of an IABP in the primary PCI alone group was at the operator’s discretion for indications such as persistent hypotension or overt CS, malignant arrhythmias, or acute MI complications such as mitral regurgitation or ventricular septal defect. Of note, 15 (8.5%) patients initially receiving standard care crossed over to receive IABC.
By subtracting average times from symptom onset to the insertion of first device, it took less than 10 min more to insert the IABP when both groups were compared and so was unlikely to hamper any gains made by IABP insertion. The overall primary efficacy measure of mean infarct size in all patients was not significantly different between the two groups. Secondary cardiac magnetic resonance measures such as mean LVEF and LV systolic volume were also similar between the two groups. There were no significant differences in terms of major bleeding/ transfusion or major vascular complications in the two groups. By 6 months, mortality rates along with the composite end point of death, recurrent MI, or new or worsening heart failure had not diverged. The investigators postulate that mean ischemic times (time between symptom onset to first device application) of just over 3 h may have been outside a therapeutic window in which significant myocardial salvage could occur [34]. This is in contrast to the results of a randomized trial conducted by the BRAVE‑2 investigators, in which left ventricular infarct size (as measured by single-photon emission computed tomography) was significantly reduced by an early invasive strategy of coronary stenting and use of abciximab in patients with acute STEMI, despite presenting 12–48 h after symptom onset [35]. Much like BCIS‑1, CRISP AMI was one of the largest randomized trials of elective IABP use conducted to date. The intervention, albeit in a high-risk cohort outside of the recognized class I indication of CS, did not however translate into improved outcomes. The 8.5% crossover of patients to IABC does, however, signal the propensity of these patients to succumb to hemodynamic collapse and therefore provides further credence to the need for standby mechanical circulatory assistance.
Meta-analyses
The meta-analysis of IABP therapy for STEMI with or without CS conducted by Sjauw et al. has been used by both the ESC/EACTS [22] and ACCF/AHA/SCAI [23] to support their most recent class I recommendation for IABC in this high-risk clinical scenario [36]. Since there is a distinct paucity of randomized trial data directly examining IABP use in STEMI with CS, the investigators performed two meta-analyses, the first covering a total of seven randomized trials (n = 1009) and the second including nine cohort studies (n = 10,529). In the former, IABP support in STEMI was not associated with a reduction in 30‑day mortality or improvement in LVEF at follow-up. To its detriment, IABP was also associated with an increased stroke rate of 2% and bleeding rate of 6% with no difference in overall results when analyzed according to revascularization strategy (i.e., thrombolysis vs primary PCI) adopted. In the latter analysis, there was a divergence in outcomes according to type of reperfusion. IABP support in the context of thrombolysis was shown to stimulate an absolute decrease in 30‑day mortality of 18% (p < 0.0001), echoing the results of the NRMI-2 registry described above, whereas primary PCI and IABP caused a significant increase in 30‑day mortality of 6% (p = 0.0008). The perceived benefit of IABP use in thrombolyzed patients should be taken with a note of caution as patients in this group tended to be younger, male and were more likely to undergo subsequent revascularization [36]. We should also be aware that all observational data are prone to varying degrees of bias and confounding and that, ultimately, a meta-analysis can only be as good as the sum of its parts.
A more recent meta-analysis performed by Bahekar et al. also confirms much of what we already know [37]. The authors identified 16 (13 prospective and three retrospective) studies (n = 11,778) that compared IABC with no IABC in the management of acute MI, with or without CS. They found no difference in in-hospital mortality in the management of STEMI with or without CS between those who received IABP support and those who did not (p = 0.67). When the analysis was limited to those with STEMI complicated by CS, a significantly improved outcome was noted in those receiving IABP support (p < 0.0004) although much of the benefit gained was in the thrombolysis era. In terms of safety parameters, IABP insertion was also associated with an increased incidence of moderate (p = 0.04) and major (p < 0.0001)bleeding, although the investigators make a point of highlighting the heterogeneity of bleeding definitions between studies.
Future perspective
To answer the question ‘is counterpulsation counterproductive in high-risk PCI?’ we must first bear in mind what high risk actually means. The term high risk in the context of coronary revascularization clearly represents a spectrum of factors: anatomical, hemodynamic and clinical, that can interact to cause periprocedural, short-, medium- and long-term MACCE. For instance, a patient about to undergo elective unprotected LMCA PCI, who is comfortable at rest with no evidence of ongoing ischemia is still deemed high risk and still vulnerable to the same set of adverse sequelae as a patient with an acute STEMI secondary to total occlusion of the proximal left anterior descending artery presenting in CS. Although not listed in Box 1, we must also pay due care and diligence to a patient’s renal function, comorbidities (especially diabetes), degree of ‘frailty’, BMI, surface area and gender when formulating an intervention strategy for their CAD. The ACCF/AHA/SCAI guideline writing committee should be commended on documenting what they regard as high-risk features for PCI [23]. They have attached certain caveats on the use of hemodynamic support devices in such scenarios, and although we must remember this statement is effectively the opinion of a group of experts, it is a fundamental first step in establishing a degree of uniformity between future trials of high-risk PCI, so that researchers, policy makers and clinicians alike can compare outcomes of a specific intervention with a certain degree of scientific and statistical reassurance.
It seems reasonable that IABC be implemented in the context of pre-existing hemodynamic compromise. Both the European and US guidelines attach class I recommendations for the use of IABC in STEMI patients with CS and we see no reason why this should be contested, although the available evidence is not entirely incontrovertible. The SHOCK trial [30] and its Registry [26] were not resoundingly proscriptive on the use of IABC in CS, and indeed were not a direct investigation of counterpulsation anyway, but they do provide robust evidence on the need for an early revascularization strategy in this patient subset. The landmark meta- analysis by Sjauw et al. did not show a clear survival advantage or improvement in LVEF for IABC in CS, but was prevented from making completely ironclad statements on the subject matter by the type and quality of data available [36]. And herein lies the problem. Symptomatic of all trials of CS, enrollment is extremely difficult and much of the data we already have is either conflicting or predates significant advancements in primary PCI, such as aspiration thrombectomy and the constantly evolving pharmacotherapeutic armamentarium. It does, however, seem perfectly intuitive to use a PCAD that has been in evolution for the past four decades, is safe and easy to implant and has reliable hemodynamic data to support its use in this particular high-risk scenario. As with any mechanical intervention, familiarity and accrued clinical experience is of paramount importance. Chen et al. highlighted this in their analysis of the NRMI-2 data [38]. Of those patients presenting with acute MI and CS, IABP placement in high-IABP volume centers was associated with a significantly lower rate of mortality when compared with low-IABP volume centers (odds ratio: 0.71, 95% CI: 0.56–0.90). However, this contradicts the most recent CathPCI registry analysis conducted by a group from Yale University School of Medicine, which found no association between in-hospital mortality and frequency of IABP use [29]. The IABP-SHOCK II (ClinicalTrials.gov Identifier: NCT00491036) trial is currently looking to resolve this issue by recruiting 600 patients with acute MI and CS and randomizing them to PCI plus IABP versus PCI alone [101]. The results are eagerly awaited.
The case of elective IABC in hemodynamically stable high-risk PCI is perhaps more clear-cut. There is currently no evidence to advocate prophylactic IABP insertion, although this statement is based on evidence gleaned from the sum total of 886 patients, a significant minority of whom formed part of a retrospective series [33,34,39,40]. Although both the BCIS‑1 and CRISP AMI investigators deserve huge credit for well-constructed trials, both were woefully underpowered to detect differences in mortality, and a mortality advantage is often the only commodity that forces clinicians to stand up and take note. This is especially the case in the counterpulsation arena, in which uptake of the device for even well established indications such as CS and acute MI complications remains at 10–30% despite class I recommendations. The significant proportion of patients crossing over to rescue IABC, seen in both BCIS‑1 and CRISP AMI, does indicate a subset of patients at the extreme end of the risk spectrum that could benefit from elective IABC. Future trials should aim to identify such patients and would be well advised to use the SYNTAX Score [41], a better-validated marker of myocardium at risk, in place of the Duke or BCIS‑1 Jeopardy Scores. Should we go as far as to say counterpulsation is counterproductive in this context? No we should not. Furthermore, we suggest that provisional or standby IABP is a preferred option to rescue or bailout IABP in patients with a strong susceptibility to rapid hemodynamic decline. To have the contralateral groin prepped prior to intervention and the IABP equipment readied for use takes no time at all and could save valuable minutes during a periprocedural emergency.
The future of IABC cannot, however, be taken in isolation. It must be analyzed in the context of other PCADs available for in-human use, namely the Impella Recover LP and the TandemHeart devices. Although a thorough review of these devices is beyond the scope of this article, their performance in comparison with IABC should be noted.
The use of the Impella device in high-risk PCI has been shown to be both safe and feasible with demonstrable improvements in cardiac index and overall LVEF [42–45]. It is able to augment cardiac output by up to 2.5–5 l/min (depending on the size of device), does not require a stable cardiac rhythm or output to function (unlike the IABP) and is relatively easy to insert. The ISAR-SHOCK study randomized 25 patients with CS secondary to acute MI to IABP (n = 13) or Impella LP 2.5 (n = 12) implanted after revascularization therapy [46]. The Impella achieved a significantly greater increase in cardiac index but this did not translate in to improved 30‑day mortality. There were nonsignificant trends towards greater requirements for packed red blood cells and fresh frozen plasma for the Impella group, in which the degree of hemolysis was also significantly greater in the first 24 h. Lest we forget the Impella is a high profile device with 13 F access required for the LP 2.5 and 21 F access required for the LP 5.
Furthermore the PROTECT II Trial (ClinicalTrials.gov Identifier NCT00562016), a large prospective, multicenter randomized controlled trial of IABP versus Impella in nonemergent high-risk PCI has been terminated for futility in reaching the primary end point of 30‑day adverse events having recruited 305 patients between October 2007 and December 2010 [102]. Indeed a retrospective study of prophylactic IABC in elective high-risk PCI undertaken by Syed et al. had preempted the study termination by concluding that the PROTECT II trial would have had to recruit 908 patients to demonstrate superiority for the Impella device [47].
The TandemHeart device can augment cardiac output by up to 5 l/min. It is, however, yet another high-profile system that requires a transeptal puncture and placement of a 17 F left atrial cannula via a 21 F femoral venous sheath. Blood is returned to the body via a 15–17 F arterial perfusion catheter via the femoral artery. As a consequence, critical limb ischemia, bleeding and vascular complications are a major concern. This was borne out in a randomized comparison of IABC (n = 20) versus the TandemHeart device (n = 21) in patients revascularized for acute MI complicated by CS [48]. Although the TandemHeart device improved hemodynamic and metabolic variables more effectively, this did not translate into a better 30‑day mortality. Furthermore, severe bleeding and limb ischemia occurred more frequently with the ventricular assist device.
A meta-analysis by Cheng et al. [49] encompassing the trials by Thiele et al. [48], ISAR-SHOCK [46] and a trial by Burkhoff et al. [50] compared IABC with both the Impella and TandemHeart devices. As expected the ventricular assist devices produced a higher cardiac index, mean arterial pressure and lower pulmonary capillary wedge pressure when compared with IABC. However, there were similar 30‑day mortality rates for all three. There were also similar rates of limb ischemia but bleeding was found to occur significantly more often with the TandemHeart device.
It is clear, therefore, that adequately powered comparative trials between all three devices in the context of both acute MI complicated by CS and in nonemergent high-risk PCI are required in the future. In the meantime, advancements in IABP development have seen the release of lower profile devices, fiber optic technology and larger 50 cc balloons that offer greater diastolic augmentation and improved unloading. We therefore predict, with some degree of certainty, that the IABP will be around for the foreseeable future.
Executive summary
How do we define high-risk percutaneous coronary intervention?
▪ There is no universally accepted definition of what constitutes high-risk percutaneous coronary intervention (PCI). All patients falling into this category, however, share an inability to withstand the hemodynamic sequelae of arrhythmias and even transient periods of ischemia-reperfusion.
▪ High-risk PCI represents a spectrum of factors: anatomical, hemodynamic and clinical, that can interact to cause periprocedural, short-, medium- and long-term major adverse cardiac and cerebrovascular events.
The diastolic augmentation of coronary perfusion
▪ The intra-aortic balloon pump (IABP) constitutes a mechanical adjunct that conveys an attractive physiological means of diastolic augmentation, reducing end diastolic pressure and improving coronary perfusion. The introduction of larger counterpulsation balloons will also help to stimulate greater blood volume displacement resulting in more diastolic augmentation and systolic unloading.
Intra-aortic counterpulsation: a physiologist’s perspective
▪ Intra-aortic balloon counterpulsation (IABC) is familiar to the majority of catheterization laboratories throughout the world; has a relatively low acquisition cost; is easy to implant and, if used diligently and in the correct patient subtype, is associated with a significant but reassuringly low incidence of directly attributable complications.
▪ Furthermore, contemporary IABP systems require minimal technical support, and automated algorithm advancements now allow for seamless adjustment to changing patient and environmental factors. The introduction of fiber-optic technology in current IAB catheters will also help to facilitate much faster set-up times.
A low-risk therapeutic option in a high-risk patient cohort
▪ Significant independent predictors of major complications directly attributable to IABP insertion are: female gender, peripheral vascular disease, small body surface area (<1.65 m2) and age ≥75 years.
▪ The ESC/EACTS 2010 revascularization guidelines recommend the use of IABC at a class I, level of evidence C, in patients with hemodynamic instability following acute myocardial infarction.
▪ The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions (SCAI; 2011) have also assigned a class I level of evidence B recommendation on the use of IABC in ST-elevation myocardial infarction patients presenting with cardiogenic shock refractory to pharmacological therapy.
▪ The ACCF/AHA/SCAI have gone a step further and defined high-risk coronary intervention as: PCI to an unprotected left main coronary artery or last remaining conduit; PCI to a vessel supplying a significant area of myocardium in a patient with severely depressed left ventricular function; and/or PCI in those patients in cardiogenic shock. Under such circumstances the elective use of IABC carries a class IIb, level of evidence C recommendation.
The evidence for IABC in high-risk PCI
▪ Despite these guidelines, registry data indicate significant under-utilization of IABC, even in class I-recommended high-risk PCI scenarios, leading many to label the use of IABC as ‘discretionary’ and prone to widespread variation.
▪ The elective/prophylactic use of IABC precoronary intervention can only be recommended for those acute MI patients presenting with hemodynamic instability.
▪ There is no evidence to recommend the elective/prophylactic use of IABC in high-risk PCI not complicated by hemodynamic instability. It seems intuitive, however, to recommend standby/provisional IABC in this setting.
Future perspective
▪ Both the Impella® and TandemHeart® devices have been shown to promote significantly greater degrees of circulatory augmentation when compared with the IABP, but this has so far not translated in to improved clinical outcomes.
▪ Both the Impella and TandemHeart devices are high profile and are therefore prone to more bleeding and vascular complications when compared with the IABP.
▪ We thoroughly recommend the availability of IABC in every catheterization laboratory performing emergent or planned high-risk PCI and predict that the IABP will be ever present for the foreseeable future.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Cutlip DE, Windecker S, Mehran R et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115, 2344–2351 (2007).
- Mehran R, Rao SV, Bhatt DL et al. Standardized bleeding definitions for cardiovascular clinical trials. A consensus report from the Bleeding Academic Research Consortium. Circulation 123, 2736–2747 (2011).
- Hartzler GO, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV. ‘High-risk’ percutaneous transluminal coronary angioplasty. Am. J. Cardiol. 61, 33G–37G (1988).
- Califf RM, Phillips HR III, Hindman MC et al. Prognostic value of a coronary artery Jeopardy Score. J. Am. Coll. Cardiol. 5, 1055–1063 (1985).
- APPROACH Investigators (Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease; Graham MM, Faris PD, Ghali WA et al. Validation of three myocardial Jeopardy Scores in a population-based cardiac catheterization cohort. Am. Heart J. 142(2), 254–261 (2001).
- Keelan P, Johnston J, Koru-Sengul T et al. Comparison of in-hospital and one-year outcomes in patients with left ventricular ejection fractions <or=40%, 41% to 49%, and >or=50% having percutaneous coronary revascularization. Am. J. Cardiol. 91(10), 1168–1172 (2003).
- Trost JC, HIllis DL. Intra-aortic balloon counterpulsation. Am. J. Cardiol. 97, 1391–1398 (2006).
- Santa-Cruz RA, Cohen MG, Ohman EM. Aortic counterpulsation: a review of the hemodynamic effects and indications for use. Catheter Cardiovasc. Interv. 67, 68–77 (2006).
- Parissis H. Hemodynamic effects of the use of the intraaortic balloon pump. Hellenic J. Cardiol. 48, 346–351 (2007).
- Kimura A, Toyota E, Lu S et al. Effects of intraaortic balloon pumping on septal arterial blood flow velocity waveform during severe left main coronary artery stenosis. J. Am.Coll. Cardiol. 27(4), 810–816 (1996).
- Kern MJ, Aguirre F, Bach R et al. Augmentation of coronary blood flow by intra-aortic balloon pumping in patients after coronary angioplasty. Circulation 87, 500–511 (1993).
- Kern MJ, Aguirre FV, Tatineni S et al. Enhanced coronary blood flow velocity during intraaortic balloon counterpulsation in critically ill patients. J. Am. Coll. Cardiol. 21(20), 359–368 (1993).
- Takeuchi M, Nohtomi Y, Yoshitani H et al. Enhanced coronary flow velocity during intra-aortic balloon pumping assessed by transthoracic Doppler echocardiography. J. Am. Coll. Cardiol. 43, 368–376 (2004).
- Williams DO, Korr KS, Gewirtz H, Most AS. The effect of intraaortic balloon counterpulsation on regional myocardial blood flow and oxygen consumption in the presence of coronary artery stenosis in patients with unstable angina. Circulation 66,593–597 (1982).
- Shumacker HB Jr. Further development of heart-lung machines. In: The Evolution of Cardiac Surgery. Shumacker HB Jr (Ed.). Indiana University Press, IN, USA, 266–279 (1992).
- Kantrowitz A, Kantrowitz A. Experimental augmentation of coronary flow by retardation of the arterial pressure pulse. Surgery 34(4), 678–687 (1953).
- Clauss RH, Birtwell WC, Albertal G et al. Assisted circulation. I. The arterial counterpulsator. J. Thorac. Cardiovasc. Surg. 41, 447–458 (1961).
- Moulopoulos SD, Topaz S, Kolff WJ. Diastolic balloon pumping (with carbon dioxide) in the aorta – a mechanical assistance for the failing circulation. Am. Heart J. 63, 669–675 (1962).
- Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA 203, 113–118 (1968).
- Bregman D, Casarella WJ. Percutaneous intraaortic balloon pumping: initial clinical experience. Ann. Thorac. Surg. 29(2), 153–155 (1980).
- Ferguson JJ III, Cohen M, Freedman JJ Jr et al. The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. J. Am. Coll. Cardiol. 38, 1456–1462 (2001).
- The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); Wijns W, Kolh P, Danchin N et al. Guidelines on myocardial revascularization. Eur. Heart J. 31, 2501–2555 (2010).
- Writing Committee Members; Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 124(23),2574–2609 (2011).
- Brodie BR, Stuckey TD, Hansen C, Muncy D. Intra-aortic balloon counterpulsation before primary percutaneous transluminal coronary angioplasty reduces catheterization laboratory events in high-risk patients with acute myocardial infarction. Am. J. Cardiol. 84, 18–23 (1999).
- SHOCK Investigators; Hochman JS, Buller CE, Sleeper LA et al. Cardiogenic shock complicating acute myocardial infarction – etiologies, management and outcome: a report from the SHOCK Trial Registry. J. Am. Coll. Cardiol. 36, 1063–1070(2000).
- SHOCK Investigators; Sanborn TA, Sleeper LA, Bates ER et al. Impact of thrombolysis, intra-aortic balloon pump counterpulsation, and their combination in cardiogenic shock complicating acute myocardial infarction: a report from the SHOCK Trial Registry. J. Am. Coll. Cardiol. 36, 1123–1129 (2000).
- Barron HV, Every NR, Parsons LS et al. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: data from the National Registry of Myocardial Infarrction-2. Am. Heart J. 141, 933–939 (2001).
- Stone GW, Ohman EM, Miller MF et al. Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocardial infarction. The benchmark registry. J. Am. Coll. Cardiol. 41, 1940–1945 (2003).
- Curtis JP, Rathore SS, Wang Y et al. Use and effectiveness of intra-aortic balloon pumps among patients undergoing high-risk percutaneous coronary intervention: Insights from the National Cardiovascular Data Registry. Circ. Cardiovasc. Qual. Outcomes 5(1), 21–30 (2011).
- SHOCK Investigators; Hochman JS, Sleeper LA, Webb JG et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N. Engl. J. Med. 341, 625–634 (1999).
- SHOCK Investigators; Hochman JS, Sleeper LA, White HD et al. One-year survival following early revascularization for cardiogenic shock. JAMA 285, 190–192 (2001).
- Hochman JS, Sleeper LA, Webb JG et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA 295, 2511–2515 (2006).
- Perera D, Stables R, Thomas M et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention; a randomized controlled trial. JAMA 304(8), 867–874 (2010).
- Patel MR, Smalling RW, Thiele H et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock. The CRISP AMI randomized trial. JAMA 306(12), 1329–1337 (2011).
- Beyond 12 hours Reperfusion AlternatiVe (BRAVE-2) Trial Investigators; Schomig A, Mehilli J, Antoniucci D et al. Mechanical reperfusion in patients with acute myocardial infarction presenting more than 12 hours form symptom onset. JAMA 293(23), 2865–2872 (2005).
- Sjauw KD, Engstrom AE, Vis MM et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines. Eur. Heart J. 30, 459–468 (2009).
- Bahekar A, Singh M, Singh S et al. Cardiovascular outcomes using intra-aortic balloon pump in high-risk acute myocardial infarction with or without cardiogenic shock: a meta-analysis. J. Cardiovasc. Pharmacol. Ther. 17(1), 44–56 (2011).
- Chen EW, Canto JG, Parsons LS et al. Relation between hospital intra-aortic balloon counterpulsation volume and mortality in acute myocardial infarction complicated by cardiogenic shock. Circulation 108, 951–957(2003).
- Briguori C, Sarais C, Pagnotta P et al. Elective versus provisional intra-aortic balloon pumping in high-risk percutaneous transluminal coronary angioplasty. Am. Heart J. 145, 700–707 (2003).
- Mishra S, Chu WW, Torguson R et al. Role of prophylactic intra-aortic balloon pump in high-risk patients undergoing percutaneous coronary intervention. Am. J. Cardiol. 98, 608–612 (2006).
- Sianos G, Morel M-A, Kappetein AP et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 1, 219–227 (2005).
- Dixon SR, Henriques JP, Mauri L et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial U.S. experience. JACC Cardiovasc. Interv. 2, 91–96 (2009).
- Sjauw KD, Knorza T, Erbel R et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J. Am. Coll. Cardiol. 54, 2430–2434 (2009).
- Henriques JP, Remmelink M, Baan J et al. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2.5. Am. J. Cardiol.97, 990–992 (2006).
- Alasnag MA, Gardi DO, Elder M et al. Use of the Impella 2.5 for prophylactic circulatory support during elective high-risk percutaneous coronary intervention. Cardiovasc. Revasc. Med. 12(5), 299–303 (2011).
- Seyfarth M, Sibbing D, Bauer I et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J. Am. Coll. Cardiol. 52, 1584–1588 (2008).
- Syed AI, Karkar A, Torguson R et al. Prophylactic use of intra-aortic balloon pump for high-risk percutaneous coronary intervention: will the Impella LP 2.5 device show superiority in a clinical randomized study? Cardiovasc. Revasc. Med. 11, 91–97 (2010).
- Thiele H, Sick P, Boudriot E et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 26, 1276–1283 (2005).
- Cheng JM, den Uil CA, Hoeks SE et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a metaanalysis of controlled trials. Eur. Heart J. 30, 2102–2108 (2009).
- Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am. Heart J. 152, e461–e468 (2006).
- Ohman EM, George BS, White CJ et al. Use of aortic counterpulsation to improve sustained coronary artery patency during acute myocardial infarction. Results of a randomized trial. The Randomized IABP Study Group. Circulation 90, 792–799 (1994).
- Abdel-Wahab M, Saad M, Kynast J et al. Comparison of hospital mortality with intra-aortic balloon counterpulsation before versus after primary percutaneous coronary intervention for cardiogenic shock complicating acute myocardial infarction. Am. J. Cardiol. 105, 967–971 (2010).
- Stone GW, Marsalese D, Brodie BR et al. A prospective, randomized evaluation of prophylactic intraaortic balloon counterpulsation in high-risk patients with acute myocardial infarction treated with primary angioplasty. Second Primary Angioplasty in Myocardial Infarction (PAMI-II) Trial Investigators. J. Am. Coll. Cardiol. 29, 1459–1467 (1997).
- van’t Hof AWJ, Liem AL, de Boer MJ et al. A randomized comparison of intra-aortic balloon pump after primary coronary angioplasty in high-risk patients with acute myocardial infarction. Eur. Heart J. 20, 659–665 (1999).
- Vaijayalakshmi K, Kunadian B, Whittaker VJ et al. Intra-aortic counterpulsation does not improve coronary flow early after PCI in a high-risk group of patients: observations from a randomized trial to explore its mode of action. J. Invasive Cardiol. 19(8), 339–346 (2007).
▪ Excellent, albeit slightly dated, overview of intra-aortic balloon counterpulsation.
▪ Excellent, albeit again slightly dated, overview of the hemodynamic effects of counterpulsation.
▪▪ Seminal paper on the post-stentotic diastolic augmentation of coronary blood flow by counterpulsation.
▪▪ Seminal work looking at coronary flow velocities during intra-aortic counterpulsation measured using a Doppler-tipped catheter.
▪▪ Seminal paper describing a method of counterpulsation, tested in live anesthetized dogs, that avoided an extracorporeal circulation.
▪▪ The first-in-man experience of intra-aortic counterpulsation.
▪▪ First large-scale registry of intra-aortic counterpulsation examining the indications, outcomes and complications associated with this device.
▪▪ The writing panel committee has been bold and listed, for the first time in international guidelines, features that they feel are consistent with high-risk percutaneous coronary intervention (PCI).
▪ Messages from the SHOCK Trial Registry with regard to counterpulsation and acute myocardial infarction complicated by cardiogenic shock.
▪▪ A recent registry analysis by a group from Yale University School of Medicine outlining significant variation in the uptake of intra-aortic counterpulsation for high-risk PCI.
▪▪ Landmark trial of acute myocardial infarction complicated by cardiogenic shock and the value of early revascularization in this high-risk patient cohort.
▪▪ Landmark study, and the largest randomized controlled trial to date, studying the impact of prophylactic intra-aortic counterpulsation in an elective high-risk PCI cohort.
▪▪ Landmark study looking at intra-aortic counterpulsation in the context of primary PCI for ST-elevation myocardial infarction not complicated by cardiogenic shock.
▪▪ Landmark meta-analysis of intra-aortic counterpulsation in the context of STelevation myocardial infarction, which has formed the basis of the recommendations on intra-aortic counterpulsation emanating from the most current US and European PCI guidelines.
▪ Websites
- Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II). http://clinicaltrials.gov/ct2/show/NCT00491036
- Protect II, A Prospective, Multicenter Randomized Controlled Trial (PROTECT II). http://clinicaltrials.gov/ct2/show/NCT00562016?term=NCT00562016&rank=1
- British Cardiovacular Intervention Society.
- MAQUET. www.maquet.com