Device Evaluations - Interventional Cardiology (2014) Volume 6, Issue 4
The Lotus transcather aortic valve: a next-generation repositionable, resheathable and recapturable prosthesis
- Corresponding Author:
- Robert Gooley
Monash Cardiovascular Research Centre, Monash University, Clayton, Australia
Tel:+ 61 39594 6666
Fax:61 39594 6239
E-mail:robertgooley@hotmail.com
Abstract
Symptomatic severe aortic stenosis occurs in 2–4% of people aged over 65 years, with calcific degeneration being the predominant etiology in the developed world. Surgical valve replacement has been and remains the gold-standard treatment modality, yet a significant number of high-risk individuals are denied or refuse this potentially life-saving treatment. Transcatheter aortic valve replacement has proven efficacy in this high-risk cohort. Current-generation transcatheter aortic valve replacement devices, however, have significant limitations. The Lotus transcatheter device represents an evolution of this technology that allows full resheathing, repositioning and retrieval, which together with features designed to minimize para-prosthetic regurgitation address a number of the limitations of first-generation devices.
Keywords
Aortic Stenosissst, Boston Scientific Lotus Valve, Transcatheter Aortic valvec Replacement, Transcatheter Aortic valve implantation, Valvular heart disease
Background
Aortic stenosis due to calcific degeneration occurs with a prevalence of 2–4% among people over 65 years. While surgical aortic valve replacement remains the gold–standard treatment, a significant number of patients decline or are denied surgery due to actual or perceived risks [1]. Transcatheter aortic valve replacement (TAVR) is an accepted treatment alternative in appropriately selected high–risk patients [2] and nonsurgical candidates [3].
Second–generation TAVR devices have been developed with design features aimed to overcome some of the limitations observed with first–generation devices. The Lotus prosthesis utilizes a unique method of deployment, which together with other device features aims to improve procedural safety and efficacy. The device demonstrates early function following implantation, and is fully repositionable, resheathable and retrievable. The clinical efficacy and safety of the Lotus device has been assessed in the REPRISE clinical trial program.
Current devices & limitations
An increasing number of TAVR devices are entering trial and clinical practice. The majority of worldwide experience, however, remains with the Sapien (Edwards Lifesciences, CA, USA) and CoreValve (Medtronic, MN, USA) devices. Both devices have proven efficacy and safety in high– and extreme–risk patients but a number of limitations remain [4].
Second–generation devices have been designed to overcome some of these limitations. The Portico (St Jude Medical, MN, USA) and Direct Flow (Direct Flow Medical Inc., CA, USA) have received approval (CE mark) for clinical use in Europe while a number of others remain in trial phase.
Positioning
Achieving an optimal deployment position is imperative, as it has been shown to correlate with outcomes such as degree of para–prosthetic aortic regurgitation (PAR) and pacing requirement. First–generation devices, however, are not truly repositionable or recapturable.
Slight repositioning of the CoreValve is possible via indirect tension on the delivery catheter, although this increases the risk of inadvertent device migration or embolization. Once the balloon expandable, Sapien valve is deployed it cannot be further maneuvered percutaneously. Accurate positioning is, therefore, reliant on initial placement and the use of rapid ventricular pacing during deployment to maximize device stability.
The Portico device is retrievable to the point of device functionality although it remains incompletely expanded in the outflow portion. The Direct Flow prosthesis can be fully expanded into its final position prior to exchange of the pressurized saline for the permanent polymer. This affords the ability to assess the functioning device and reposition or remove the prosthesis if required.
Para–PAR
PAR following TAVR with current–generation devices correlates with morbidity and mortality [2]. PAR results from a number of factors. First, placement of circular TAVR devices within noncircular native annuli often results in residual paravalve interstices [5,6]. Further to this, the presence of eccentric or protuberant calcification at the level of the annulus or sub–annular level can prohibit complete sealing.
Efforts to reduce PAR with first–generation devices focused on oversizing the prosthesis in order to create better apposition between valve frame and annulus circumferentially. This practice, however, may increase the risk of annular injury/rupture, the need for pacing, sinus obliteration and coronary occlusion [7,8]. Secondgeneration devices utilize features such as sealing skirts or more precise placement to overcome PAR.
Conduction disturbance
Reported rates of requirement for pacing following TAVR vary between studies and between device types. Contemporary pacing rates are generally declining although rates following CoreValve implantation are still reported at 10–25% [9–11] and following Sapien at 4–8% [11–13]. The reduction in pacing requirement has been driven by improved device positioning, avoidance of excessive oversizing and higher operator thresholds for pacemaker implantation [9,10,14].
Stroke & transient ischemic attacks
The reported rates of new neurological events have declined [2,15]. This has been variably attributed to improved operator technique, selective avoidance of procedural steps (e.g., predilatation and postdilation) and lower profile devices [16].
Despite this, contemporary MRI studies have demonstrated very high rates of subclinical cerebral lesions [17,18]. Studies investigating the efficacy of embolic protection devices have likewise shown high rates of MRI detected lesions, although with a suggestion that the volume of such lesions may be reduced [19]. This has not yet translated to a proven reduction in clinical neurological events.
With the advent of repositionable and resheathable devices concerns have/were raised that increased manipulation of the device in the annulus may result in higher rates of stroke. To date, this has not been demonstrated in reported clinical trials. It is possible that nontraumatic resheathing mechanisms may actually result in less debris embolization than gross traction, which is often applied to first–generation devices in an attempt to repositioning.
Lotus transcatheter valve design
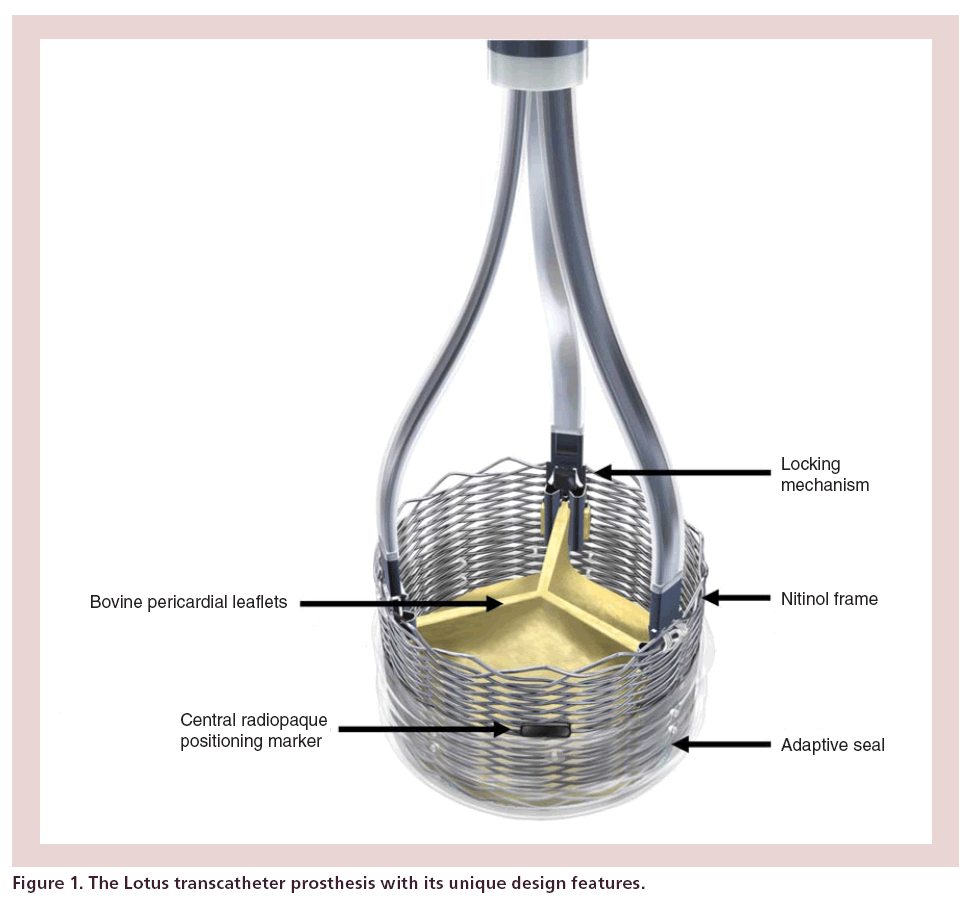
The Lotus transcatheter prosthesis is a novel device with a number of features designed to improve ease of use, efficacy and safety (Figure 1).
Frame
The frame of the prosthesis is braided from a single nitinol wire, the ends of which are joined by a radioopaque, tantalum marker. The tantalum marker is positioned at the mid-frame height and acts as a fluoroscopic aid for accurate valve positioning. The orientation of the tantalum marker also assists the operator in understanding delivery catheter orientation and thus allowing the operator to maneuver the catheter if necessary when dealing with tortuous anatomy.
Leaflets
Three bovine pericardial leaflets are hand–sewn onto the valve frame. The leaflets are positioned within the inflow portion of the frame to achieve a true annular position once deployed.
Adaptive seal
A blended polymer membrane surrounds the lower half of the Lotus device. As the device shortens and radially expands this membrane concertinas and occupies any small interstices that may remain between the annulus and the frame that could result in PAR.
Delivery catheter & premounted valve
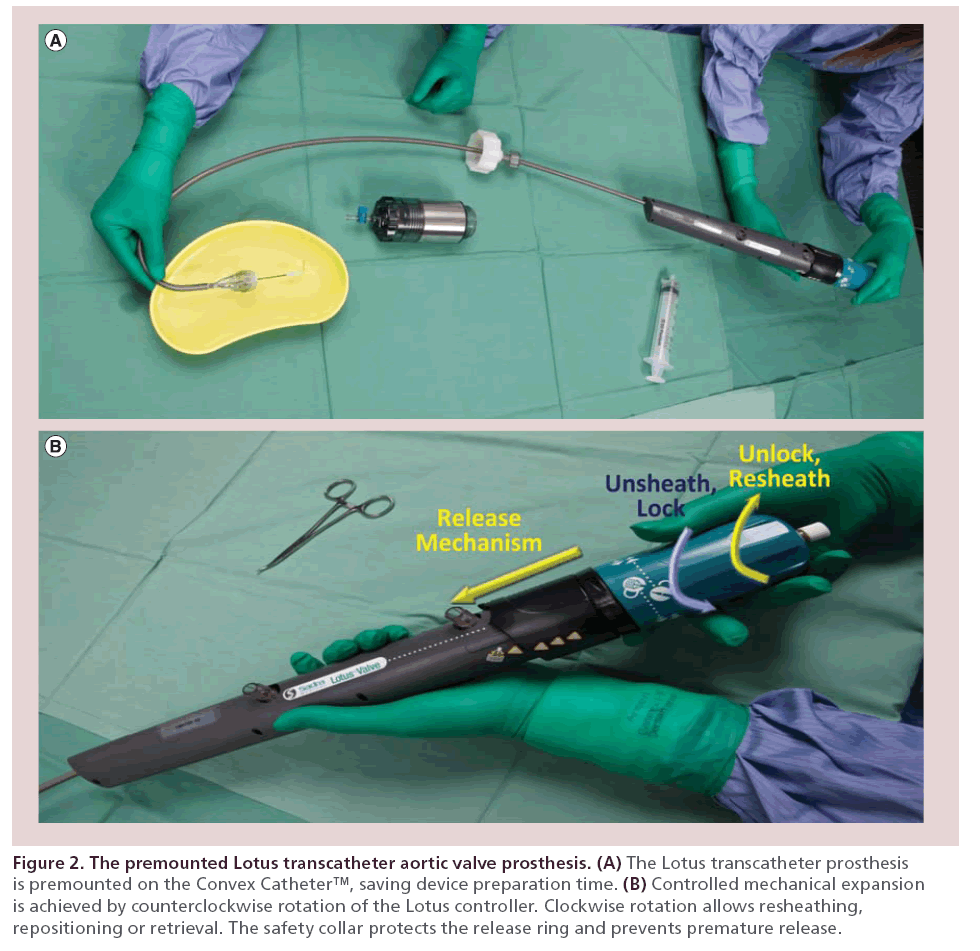
The Lotus prosthesis is pre–mounted on the Convex Catheter™, which reduces device preparation time in the catheterization laboratory (Figure 2). The catheter is preshaped and has a lubricious coating, which together allow steerable passage through peripheral tortuosity and across the aortic arch. The preshaped catheter also aids coaxial positioning of the device in the aortic flow plane. On unsheathing, the valve functions immediately, thus reducing the likelihood of hemodynamic instability. Rapid pacing is not required.
The 23–mm Lotus system is compatible with an 18–Fr proprietary femoral sheath while the 27–mm Lotus system requires a 20–Fr proprietary femoral sheath. At present, due to the pre–shaped Convex Catheter, the device is only deployed from the femoral route.
Figure 2: The premounted Lotus transcatheter aortic valve prosthesis. (A) The Lotus transcatheter prosthesis is premounted on the Convex Catheter™, saving device preparation time. (B) Controlled mechanical expansion is achieved by counterclockwise rotation of the Lotus controller. Clockwise rotation allows resheathing, repositioning or retrieval. The safety collar protects the release ring and prevents premature release.
Locking mechanism
The Lotus prosthesis is neither balloon nor selfexpanding but utilizes a unique controlled mechanical expansion mechanism. Counterclockwise rotation of the Lotus controller results in shortening of the prosthesis along three mandrels, which are spaced evenly around the frame. This brings the ventricular and aortic portions of the locking mechanism together. As the frame shortens from its constrained form to the final height of 19 mm, it radially expands to its final diameter of 23 or 27 mm (Figure 3).
The device is fully repositionable, resheathable and retrievable even from the fully expanded and locked position. This allows complete assessment of the expanded device by fluoroscopy, angiography and/or echocardiography prior to final release.
REPRISE trials
The clinical efficacy and safety of the Lotus device is currently being investigated in the REPRISE clinical trial program [20].
REPRISE I
REPRISE I, a first–in–man feasibility trial, enrolled 11 patients at three Australian centers. The primary end point was clinical procedural success at discharge or 7 days, defined as successful device implantation without in–hospital major cardiovascular or cerebrovascular events using Valve Academic Research Consortium definitions [21].
The results of REPRISE I through 1–year postprocedure were recently published [20]. All patients were female and received a 23–mm device. The mean Society of Thoracic Surgeons score was 4.9 ± 2.5 and mean logistic EuroSCORE 9.5 ± 4.4. All patients were deemed to be high risk by institutional Heart Teams.
The primary end point was met in nine of 11 patients (81.8%) at discharge. One patient suffered a major stroke and one patient had a mean trans–prosthesis gradient of 22 mmHg, which while functioning normally with a valve area of 1.6 cm2 was above the specified Valve Academic Research Consortium threshold of 20 mmHg for device success.
There were very low rates of PAR with only two cases deemed mild, one trivial and the remainder none. Four patients (36.4%) required implantation of a permanent pacemaker. There was a reduction in mean aortic gradient from 53.9 ± 20.9 to 13.7 ± 3.7 mmHg at discharge and an increase in aortic valve area from 0.68 ± 0.19 to 1.53 ± 0.18 cm2.
All patients were alive at 12 months with no additional strokes. The observed changes in mean aortic gradient and aortic valve area at discharge were sustained.
REPRISE II & REPRISE II Extension
The REPRISE II CE mark trial enrolled 120 patients at Australian and European centers. It was a singlearm study with similar inclusion criteria to REPRISE I but with the addition of a 27–mm device. The primary performance end point was mean aortic pressure gradient at 30 days and the primary safety end point was all–cause mortality at 30 days.
The primary end point results were presented at Transcatheter Cardiovascular Therapeutics 2013. More than half (56.7%) of participants were female. The mean Society of Thoracic Surgeons score was 7.1 ± 4.6 and mean EuroSCORE II 6.9 ± 5.8. These risk scores are in keeping with those in the recently published CoreValve IDE trial [22] and many contemporary registries. To ensure that subjects were at high surgical risk, however, a number of frailty indices including gait speed, handgrip strength, Charlson Index and Katz Index were collected.
The performance end point was met with a reduction in mean gradient from 46.4 ± 15.0 to 11.5 ± 5.2 mmHg (p < 0.001 compared with a performance goal of 18.0 mmHg) and an increase in aortic valve area from 0.7 ± 0.2 cm2 to 1.7 ± 0.4 cm2. There were five (4.2%) deaths at 30 days and two (1.7%) disabling strokes.
Permanent pacemaker implantation was required in 34 patients (28.6%) with approximately half of these cases occurring in the setting of significant oversizing [23]. Of 103 echocardiograms performed at 30 days, 96 were evaluable for PAR. None of the patients had severe PAR, 1% had moderate, 16% had mild, 5% had trivial and 78% had no PAR as adjudicated by an independent core laboratory.
The REPRISE II trial included an evaluation of the device by the implanting investigators. The investigators considered the major advantages of the Lotus prosthesis, as demonstrated in the REPRISE trials, to be early valve function during the implantation process and very low PAR rates. The investigators attributed the latter to the ability to accurately position and reposition the device as well as the influence of the adaptive seal.
The REPRISE II Extension study is ongoing with plans to extend the REPRISE II cohort by 130 patients.
REPRISE III
REPRISE III is a planned global randomized pivotal study that will compare the Lotus device to a contemporary competitor device. Enrollment is planned to commence in 2014, and will also include a 25–mm diameter valve.
Respond
The Lotus device obtained CE mark in October 2013 and the first commercial device was implanted in November 2013. Real–world safety and efficacy data will be collected in the post–market RESPOND study, which will also include a 25–mm diameter valve and is expected to commence in 2014.
Procedural details
Preprocedural anatomical assessment is critical to efficacious and safe valve deployment. Patients undergo echocardiographic, angiographic and multidetector computed tomographic assessment to determine anatomical suitability while institutional Heart Teams and an independent clinical review committee adjudicate clinical suitability.
A description of the Lotus transcatheter valve system has previously been published [24]. The Lotus transcatheter device is deployed via the femoral access route. The 23–mm device is delivered via an 18–Fr proprietary Lotus introducer while the 27–mm device requires a 20–Fr Lotus introducer.
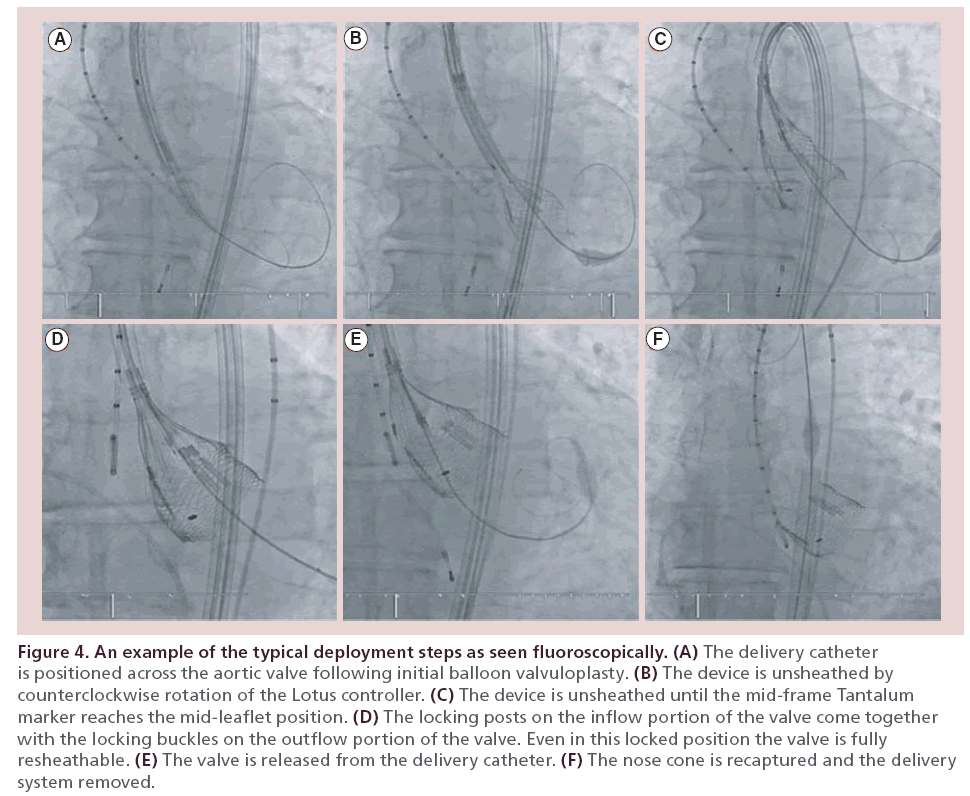
In the REPRISE I and REPRISE II trials, an initial balloon valvuloplasty was performed, although this is not mandated in commercial use. The preshaped Convex Catheter delivery system is used to steer through any peripheral tortuosity and across the aortic arch. At this point the catheter shape allows coaxial positioning of the device in the aortic flow plane. The prosthesis is gradually deployed by counterclockwise rotation of the Lotus controller. This results in shortening and radial expansion of the prosthesis. The mid–frame height tantalum marker is used to guide positioning during this deployment phase (Figure 4).
Figure 4: An example of the typical deployment steps as seen fluoroscopically. (A) The delivery catheter is positioned across the aortic valve following initial balloon valvuloplasty. (B) The device is unsheathed by counterclockwise rotation of the Lotus controller. (C) The device is unsheathed until the mid–frame Tantalum marker reaches the mid–leaflet position. (D) The locking posts on the inflow portion of the valve come together with the locking buckles on the outflow portion of the valve. Even in this locked position the valve is fully resheathable. (E) The valve is released from the delivery catheter. (F) The nose cone is recaptured and the delivery system removed.
The unique locking mechanism of the Lotus device facilitates partial or complete resheathing and hence repositioning or retrieval. The prosthesis can, therefore, be assessed for anatomical and functional integrity while in the fully expanded position and any fine adjustments made prior to final release. If the position needs to be altered, simple clockwise rotation of the Lotus controller results in partial or full resheathing, at which point the position can be optimized or the device removed.
Alternative devices
The Lotus device enters the commercial market in Europe at a time when other second generation devices have also become available along with the existing first generation CoreValve and Sapien prostheses (Table 1).
| Approval | Frame | Leaflets | Sheath size (Fr) | Prosthesis size (mm) | Annulus size (mm) | Mechanism of deployment | Access site | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Medtronic CoreValve |
US FDA CE mark |
Nitinol | Porcine Pericardium | 18 | 23 26 29 31 |
18–20 20–23 23–27 26–29 |
Self–expanding | Femoral Subclavian Direct aortic | [25] |
| Edwards Sapien XT |
FDA CE mark |
CoCr | Bovine Pericardium |
16† 18† 20† |
23 26 29 |
18–22 21–25 24–27 |
Balloon expandable | Femoral Apical Direct aortic |
[26] |
| Direct Flow Medical Inc. TAV System |
CE mark | Dacron Polymer |
Bovine Pericardium |
18 | 25 27 29 |
21–24 24–26 26–28 |
Pressurized inflation of inflow and outflow rings | Femoral | [27] |
| St Jude Medical Portico |
CE mark | Nitinol | Bovine Pericardium |
18 | 23 25 |
19–21 21–23 |
Self–expanding | Femoral | [28] |
| Boston Scientific LOTUS |
CE mark | Nitinol | Bovine Pericardium |
18 20 |
23 25‡ 27 |
19–23 23–27 |
Mechanically expanded | Femoral | [20] |
25-mm Lotus prosthesis will be available in the second quarter of 2014.
CoCr: Cobalt chromium; TAVR: Trancatheter aortic valve replacement.
Comparison of Lotus with US FDA/CE mark transcatheter aortic valve replacement devices.
How the Lotus device fits in contemporary practice
The Lotus 23– and 27–mm devices are currently CE mark–approved for use in Europe. The Lotus device is not approved for clinical use in the USA although the planned REPRISE III IDE trial will enroll at centers in the USA with the aim of obtaining US FDA approval.
Conclusion
The Lotus prosthesis is a second–generation fully resheathable, repositionable and retrievable TAVR device. Through the REPRISE suite of trials it has proven to have very high rates of device and procedural success with an excellent safety and efficacy profile. A modest pacing rate may be reduced with the advent of further device sizes negating the degree of oversizing. The near absence of significant PAR, in the reported trials to date, suggests that this previous Achilles heel of TAVR may have been overcome.
Future perspective
TAVR is currently limited to treatment of high and extreme surgical risk populations. The extrapolation of current generation TAVR devices to lower risk cohorts has been limited by modest yet significant complication rates. The Lotus valve represents a next–generation device that may overcome some of these obstacles through its ability to be fully repositioned and retrieved in order to achieve ideal positioning and to allow function interrogation prior to release. This together with design features such as the adaptive seal, which has proven in the REPRISE suit of trials to contribute to very low rates of significant PAR, may result in improved outcomes and the extrapolation of TAVR into a broader population.
Financial & competing interests disclosure
i meridith and P Antonis report receiving consulting fees from Boston Scientific. In addition to tha peer–review process, with the author(s) consent, the manufacturer of the product(s) discussed in this article was given the opportunity to review the manuscipt for factual accuracy. Changes were made at the discretion of the author(s) and based on scientific or editori merit only. The authors have no other relevant affiliations or financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
• Transcatheter aortic valve replacement (TAVR) is an acceptable treatment modality in high– and extreme–risk patients.
• The Lotus TAVR prosthesis is a new–generation device that has CE mark in Europe.
Current devices & limitations
• First–generation devices have modest yet significant complication rates.
• Para–prosthetic regurgitation is associated with increased morbidity and mortality.
• Pacing requirement varies between 3 and approximately 25% in contemporary studies.
• Stroke rates are declining and to date have not been shown to be significantly higher with repositionable devices.
Lotus transcatheter valve design
• The first fully resheathable, repositionable and retrievable TAVR prosthesis.
• Utilizes a unique mechanical expansion and locking mechanism.
• Nitinol frame and bovine pericardial leaflets.
• Adaptive seal surrounds the inflow portion of the valve frame and is designed to reduce para–prosthetic aortic regurgitation.
Clinical efficacy
• The REPRISE suite of trials have shown high procedural and device success..
• Near absence of significant para–prosthetic aortic regurgitation.
Procedural details
• The 23–mm device is delivered through an 18–Fr sheath and the 27–mm device through a 20–Fr sheath.
• A 25–mm device is planned for release in the second quarter of 2014.
• The device is currently only indicated for transfemoral delivery.
Alternative devices
• The Medtronic CoreValve Revalving System, Edwards Sapien, Direct Flow Medical and St Jude Medical Portico TAVR devices have obtained CE Mark approval in Europe.
• The Edwards Sapien and Medtronic CoreValve devices have US FDA approval.
Conclusion
• The Lotus prosthesis is a fully resheathable, repositionable and retrievable TAVR device.
• The Lotus has demonstrated excellent efficacy and safety in the REPRISE suite of trials.
• Modest pacing rates may be reduced as more device sizes become available, reducing the degree of oversizing.
References
- Iung B, Cachier A, Baron G et al. Decision–making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur. Heart J. 26, 2714–2720 (2005).
- Kodali SK, Williams MR, Smith CR et al. Two–year outcomes after transcatheter or surgical aortic–valve replacement. N. Engl. J. Med. 366, 1686 –1695 (2012).
- Makkar RR, Fontana GP, Jilaihawi H et al. Transcatheter aortic–valve replacement for inoperable severe aortic stenosis. N. Engl. J. Med. 366, 1696 –1704 (2012).
- Khatri PJ, Webb JG, Rodes–Cabau J et al. Adverse effects associated with transcatheter aortic valve implantation: a meta–analysis of contemporary studies. Ann. Int. Med. 158, 35– 46 (2013).
- Sherif MA, Abdel–Wahab M, Stocker B et al. Anatomic and procedural predictors of paravalvular aortic regurgitation after implantation of the Medtronic CoreValve bioprosthesis. J. Am. Coll. Cardiol. 56, 1623–1629 (2010).
- Wong DT, Bertaso AG, Liew GY et al. Relationship of aortic annular eccentricity and paravalvular regurgitation post transcatheter aortic valve implantation with CoreValve. J. Invasive Cardiol. 25, 190 –195 (2013).
- Barbanti M, Yang TH, Rodes Cabau J et al. Anatomical and procedural features associated with aortic root rupture during balloon–expandable transcatheter aortic valve replacement. Circulation 128, 244–253 (2013).
- Eker A, Sozzi FB, Civaia F, Bourlon F. Aortic annulus rupture during transcatheter aortic valve implantation: safe aortic root replacement. Eur. J. Cardiothorac. Surg. 41, 1205 (2012).
- Khawaja MZ, Rajani R, Cook A et al. Permanent pacemaker insertion after CoreValve transcatheter aortic valve implantation: incidence and contributing factors (the UK CoreValve Collaborative). Circulation 123, 951–960 (2011).
- Munoz–Garcia AJ, Hernandez–Garcia JM, Jimenez–Navarro MF et al. Factors predicting and having an impact on the need for a permanent pacemaker after CoreValve prosthesis implantation using the new Accutrak delivery catheter system. JACC Cardiovasc. Interv. 5, 533–539 (2012).
- Chieffo A, Buchanan GL, Van Mieghem NM et al. Transcatheter aortic valve implantation with the Edwards SAPIEN versus the Medtronic CoreValve Revalving system devices: a multicenter collaborative study: the PRAGMATIC Plus Initiative (Pooled–RotterdAm–Milano–Toulouse In Collaboration). J. Am. Coll. Cardiol. 61, 830–836 (2013).
- Laynez A, Ben–Dor I, Barbash IM et al. Frequency of conduction disturbances after Edwards SAPIEN percutaneous valve implantation. Am. J. Cardiol. 110, 1164–1168 (2012).
- Webb JG, Altwegg L, Boone RH et al. Transcatheter aortic valve implantation: impact on clinical and valve–related outcomes. Circulation 119, 3009–3016 (2009).
- Bleiziffer S, Ruge H, Horer J et al. Predictors for new–onset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 3, 524–530 (2010).
- Cao C, Ang SC, Indraratna P et al. Systematic review and meta–analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann. Cardiothorac. Surg. 2, 10–23 (2013).
- Eggebrecht H, Schmermund A, Voigtlander T, Kahlert P, Erbel R, Mehta RH. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta–analysis of 10,037 published patients. EuroIntervention 8, 129–138 (2012).
- Kahlert P, Knipp SC, Schlamann M et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion–weighted magnetic resonance imaging study. Circulation 121, 870–878 (2010).
- Ghanem A, Muller A, Nahle CP et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion–weighted magnetic resonance imaging. J. Am. Coll. Cardiol. 55, 1427–1432 (2010).
- Rodes Cabau J. PROTAVI–C Pilot Study. Prospective randomised outcome study in patients undergoing TAVI to examine cerebral ischaemia or bleeding complications. Presented at: EuroPCR 2013. Paris, France, 21–24 May 2013.
- Meredith IT, Worthley SG, Whitbourn RJ et al. Transfemoral aortic valve replacement with the repositionable Lotus Valve System in high surgical risk patients: the REPRISE I study. EuroIntervention 9, 1264–1270 (2014).
- Kappetein AP, Head SJ, Genereux P et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium–2 consensus document. J. Thorac. Cardiovasc. Surg. 145, 6–23 (2013).
- Adams DH, Popma JJ, Reardon MJ et al. Transcatheter aortic–valve replacement with a self–expanding prosthesis. N. Engl. J. Med. 370, 1790–1798 (2014).
- Dumonteil N. Need for permanent pacemaker following implantation of the repositionable second–generation LOTUS device for transcatheter aortic valve replacement: results from the pivotal REPRISE II trial. J. Am. Coll. Cardiol. 62, B39 (2013).
- Gooley R, Lockwood S, Antonis P, Meredith IT. The SADRA Lotus Valve System: a fully repositionable, retrievable prosthesis. Minerva Cardioangiol. 61, 45–52 (2013).
- Linke A, Wenaweser P, Gerckens U et al. et al. Treatment of aortic stenosis with a self–expanding transcatheter valve: the international multi–centre ADVANCE study. Eur. Heart J. doi:10.1093/eurheartj/ehu162 (2014) (Epub aheadof print).
- Freeman M, Webb JG. Edwards Sapien and Edwards Sapien XT transcatheter heart valves for the treament of severe aortic stenosis. Expert Rev. Med. Devices 9(6), 563–569 (2012).
- Schofer J, Colombo A, Klugmann S et al. Prospective multicenter evaluation of the direct flow medical transcatheter aortic valve. J. Am. Coll. Cardiol. 63(8), 763–768 (2014).
- Manoharan G, Spence MS, Rodes–Cabau J et al. St Jude Medical Portico valve. EuroIntervention 8(Suppl. Q), Q97–Q101 (2012).