Case Report - Clinical Investigation (2017) Volume 7, Issue 1
The management of contralateral axillary sentinel lymph nodes detected by lymphoscintigraphy in a patient of breast cancer that had never undergone breast related medical treatment
Hisako Ono1,4*, Masahiko Tanabe1,5, Kiyomi Kimura1,6, Yumi Miyagi1, Rie Horii2,3, Futoshi Akiyama2,3, Takuji Iwase1 and Shinji Ohno1
1Breast Oncology Center, the Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo, Japan
2Division of Pathology, the Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo, Japan
3Division of Pathology, the Cancer Institute of the Japanese Foundation for Cancer Research, Tokyo, Japan
4Department of Endocrine and Breast Surgery, Graduate School of Medical Science, Kyoto Prefectural University of Medicine
5Department of Breast Surgical Oncology, Kyoundo Hospital, Japan
6Breast Center, International University of Health and Welfare Mita Hospital, Japan
- Corresponding Author:
- Hisako Ono
Breast Oncology Center
the Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo, Japan
E-mail: hisako-o@koto.kpu-m.ac.jp
Submitted: 25 April 2017; Accepted: 08 May 2017; Published online: 15 May 2017
Abstract
In breast cancer, lymphoscintigraphy-based sentinel lymph node biopsy examinations usually show lymphatic drainage to the ipsilateral axilla, and sometimes to the extra-axillary regions. Drainage to the contralateral axilla is rarely observed in patients that have never undergone any medical treatment involving their breasts. We experienced a case of breast cancer in which bilateral sentinel lymph nodes were detected by lymphoscintigraphy in a patient that had never undergone any medical treatment involving her breasts. Here, we report a rare case and review the clinical and pathological features of several reported cases. In addition, we discuss the necessity of subjecting contralateral sentinel lymph nodes to a biopsy examination.
Keywords
Breast cancer, Contralateral sentinel lymph node, Lymphoscintigraphy
Introduction
Sentinel lymph node (SN) biopsy examinations are frequently performed in cases of breast cancer that do not involve clinical lymph node metastasis [1-3]. Lymphoscintigraphy is generally performed to identify SN before surgery. SN are usually located in the ipsilateral axilla, but some are found outside of the ipsilateral axilla. According to previous reports, extra-axillary SN is detected in as many as 19- 25% of breast cancer patients. They are often located in the region around internal mammary chain, but are rarely found in the contralateral axilla [4-6]. There have been a few reports about contralateral axillary SN in patients that had never undergone surgery or radiotherapy for breast cancer or cosmetic mammoplasty [7-12]. We report the case of a breast cancer patient with bilateral axillary SN who had never undergone breast surgery or radiotherapy. This is the first such case we have experienced at our institution.
Case Report
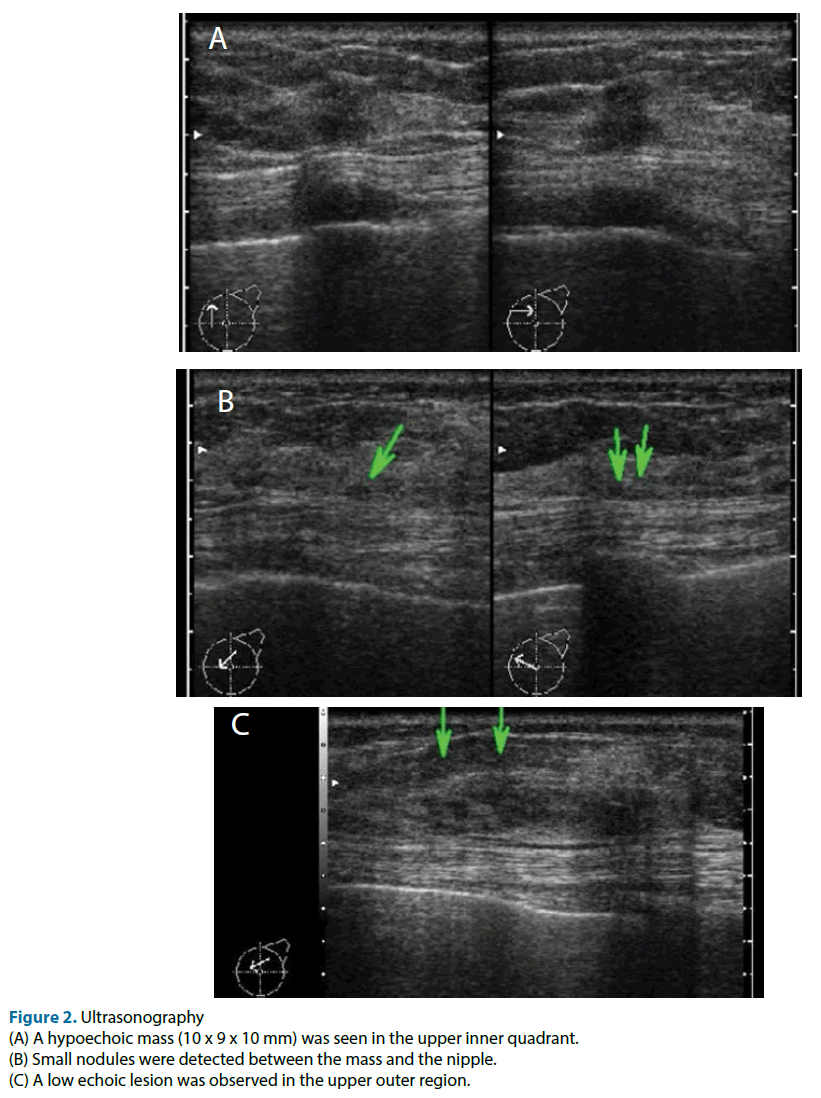
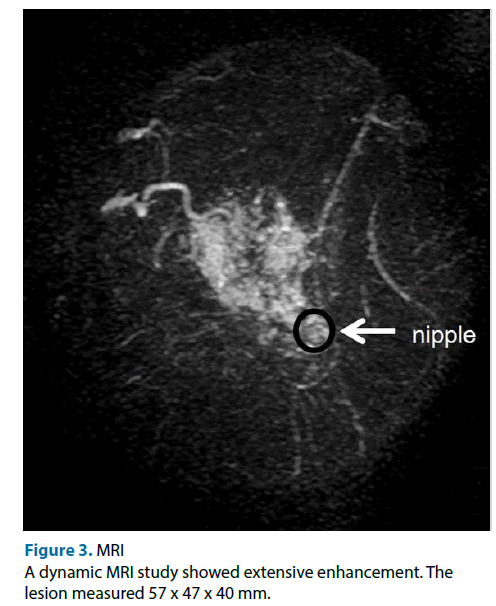
This case was a 51-year-old female. Neither the patient nor her family had any relevant medical history. She was referred to our hospital because of the detection of an abnormality during screening mammography, and a further examination at our institution revealed cancer in her left breast. The main lesion was located in the upper inner part of the patient’s left breast, and extensive ductal spread was seen in the upper region. Any tumor was not detected during palpation of the patient’s breasts. Mammography showed focal asymmetrical density and areas of distortion together with segmentally distributed amorphous calcifications in her left breast (Figure 1). Ultrasonography showed a hypoechoic tumor (diameter: 10 mm) with irregular margins in the upper inner quadrant of her left breast. We suspected that it was an invasive ductal carcinoma. The ultrasonography also showed hypoechoic lesions, which were indicative of ductal spread in the upper outer region (Figure 2). Dynamic magnetic resonance imaging (MRI) demonstrated that small nodes had spread segmentally and directly towards the left nipple from the upper inner region. The lesion measured 57 × 47 × 40 mm on MRI (Figure 3). The pathological diagnosis of ductal carcinoma in situ was confirmed by a stereo-guided Mammotome® biopsy targeting the calcifications. There were no clinical signs of metastasis to the axillary lymph nodes. Considering wide spread of breast cancer, we planned left mastectomy for curative resection of the disease. In addition, SN biopsy guided by blue dye and radioisotope (RI) injections were planned because the possibility of invasiveness of the cancer was not ruled out at that time.
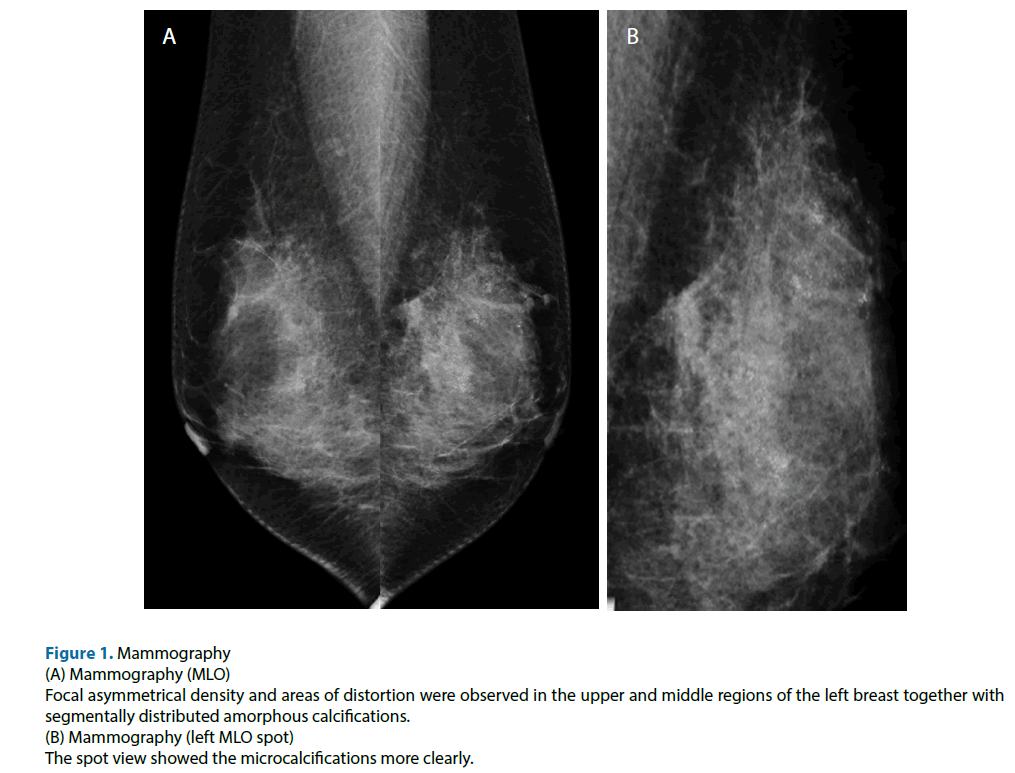
Figure 1: Mammography
(A) Mammography (MLO)
Focal asymmetrical density and areas of distortion were observed in the upper and middle regions of the left breast together with
segmentally distributed amorphous calcifications.
(B) Mammography (left MLO spot)
The spot view showed the microcalcifications more clearly.
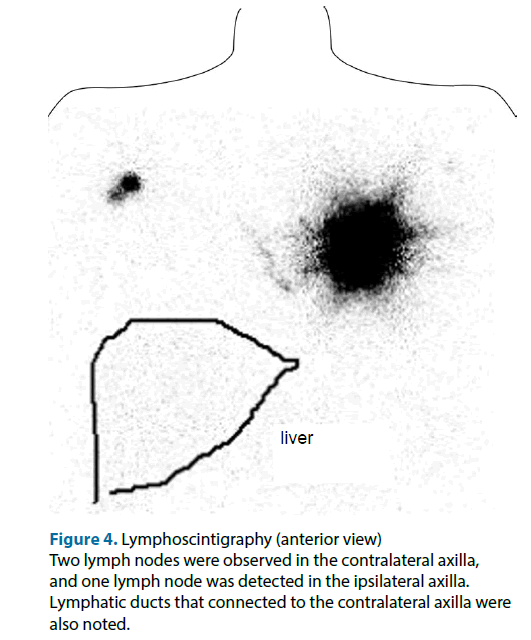
On the day before the surgery, intradermal (just above the main tumor)-plus-deep (peritumoral) injections of 1.5 mCi (total: 1.0 ml) 99mTc-labeled phytate colloid were performed. About 60 minutes after the injection procedure, lymphoscintigraphy of the anterior, oblique, and lateral views were performed. Unexpectedly, it revealed the presence of SN in the bilateral axillae, even though the patient had never undergone any medical treatment involving her breasts. The anterior view showed an ipsilateral axillary lymph node as well as two contralateral axillary lymph nodes with lymph ducts that connected to the contralateral axilla (Figure 4). The contralateral axillary lymph nodes seemed to exhibit stronger RI accumulation than the ipsilateral lymph node. Based on the results of the lymphoscintigraphy, we decided to perform left mastectomy and bilateral axillary SN biopsies.
During the operation, we injected indigo carmine into an intradermal site associated with the main tumor and a subareolar site in the left breast. Although none of the SN were dyed blue, they were all labeled by the radioactive tracer. As shown on lymphoscintigraphy, we found three radioactive SN during the operation. One was located in the ipsilateral axilla, and the other two were located in the contralateral axilla. The radioactive counts of the lymph nodes were 17 for the lymph node in the ipsilateral axilla, and 71 and 247, respectively, for those in the contralateral axilla. The contralateral SN exhibited the highest radioactive counts. All of the frozen sections of the SN were free from metastases, so we did not perform complete axillary lymph node dissection. The pathological diagnosis of the surgical specimen was invasive ductal carcinoma with predominant intraductal components. The invasive component extended 1.5 mm in diameter, and extensive ductal spread and slight fat infiltration were also observed. The precise pathological examination of paraffin-embedded lymph node sections did not reveal metastases in any of the lymph nodes. The immunohistochemical examination showed that the tumor was found to be positive for the estrogen and progesterone receptors and negative for Her2/neu. The patient did not suffer any postoperative complications and was treated with tamoxifen for five years and injections of a luteinizing hormone-releasing hormone agonist for two years as an adjuvant treatment. She is currently free from recurrence at 7 years after the operation.
Discussion
Drainage to extra-axillary (sites other than the ipsilateral axilla) sites was discovered in as many as 25% of cases of breast cancer in which SN mapping was performed. The majority of extra-axillary sites were internal mammary lymph nodes, and a few were intramammary or supraclavicular lymph nodes [4-6]. Previous studies have reported that the frequency of SN detection depended on the radiocolloid particle size and injection site [13-19]. The analysis in our institution showed that SN biopsy procedures performed with the intradermal-plus-deep injection method were found to be significantly superior to those involving intradermal injection alone in terms of their ability to detect axillary SN (No. of axillary SN detected [mean ± SD]: 1.82 ± 0.94 vs. 1.63 ± 0.80, respectively) and to facilitate the visualization of internal mammary lymph nodes on lymphoscintigraphy (51/329 vs. 5/325) [13]. Based on these results, the injection method employed at our institution was changed from intradermal injection alone to the intradermal-plus-deep method in April 2004.
This is the first case in which we have detected contralateral axillary SN in a patient who had never had undergone medical treatment involving her breasts, even though we have conducted several thousand SN mapping procedures involving such patients. During a search for previously published reports, we found 6 cases of breast cancer involving the detection of contralateral axillary SN [7-12]. Here, we summarize the clinicopathological features of our case and the other 6 reported cases in Table 1. Table 1 shows information about the age of the patients, the tumor site, tumor size, the extent of any invasion, the type of 99mTc radiocolloid, the injection method, the number of SN visualized on lymphoscintigraphy, the number of SN identified and removed during the operation, the number of metastatic lymph nodes, and the final procedure. Two cases of breast cancer involving contralateral axillary SN and metastasis have been reported (Table 1) [11,12]. We speculate that performing contralateral axillary SN biopsies based on lymphoscintigraphy would help to prevent the development of local disease; i.e., contralateral axillary lesions.
| report [reference] | age (yr) | location of lesion | tumor size (invasion(mm)) | colloid type | injection method | visualized Ax* SN | removed Ax* SN | Ax* metastasis | Ax* procedure | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| contra | ipsi | contra | ipsi | contra | ipsi | contra | ipsi | ||||||
| Our case | 51 | upper inner | 57 (1.5) | phytate | intradermal + deep | 2 nodes | 1 node | 2 nodes | 1 node | none | none | only biopsy | only biopsy |
| Vicente J. [7] | 48 | retroareolar | 20 (unknown) | nanocollid | peritumoral | unknown** | unknown** | unknown** | unknown** | none | none | only biopsy | only biopsy |
| Luján B. [8] | 66 | inner | unknown (2.7) | albumin | intratumoral | 1 node | 2 nodes | 1 node | 2 nodes | none | none | only biopsy | only biopsy |
| Carmon M. [9] | 55 | medial | 18 (unknown) | sulfur | peritumoral | unknown** | unknown** | 2 nodes | 1 node | none | none | only biopsy | only biopsy |
| Chakura A. [10] | 62 | lower medial | unknown | nanocollid | peritumoral | 2 nodes | 2 nodes | not explored | 4 nodes | unknown | none | not done | only biopsy |
| Allweis T. [11] | 48 | lower inner | 18 (unknown) | nanocollid | peritumoral | 1 node | 1 node | 1 node | 1 node | 1 node | 1 node | sampling | dissection |
| Krause A. [12] | unknown | medial | unknown | nanocollid | peritumoral | 1 node | unknown** | 1 node | unknown** | 1 node | none | only biopsy | sampling? |
Contra: contralateral axillary ipsi: ipsilateral axillary
** No details were provided, but there were one or more SN.
Table 1: Clinicopathological features of breast cancer patients with bilateral axillary SN who had never undergone any breast-related medical treatment
There are two main discussion points about cases of breast cancer involving contralateral axillary SN. The first point is the reason why lymph drainage rarely flows to the contralateral axilla. The lymphatic drainage route to the contralateral breast passes through the subdermal plexus [20]. In patients that undergo breast surgery or radiotherapy targeting the breast, it is assumed that the main lymphatic drainage route to the ipsilateral axilla will have been dissected or disrupted, so it is understandable if lymph starts flowing towards the contralateral axilla in such cases, as has been reported previously [21]. However, such changes in the direction of lymphatic flow are rare in patients who have never received any medical treatment involving their breasts, as was seen in this case. Therefore, in the present case the lymph drainage to the contralateral axilla was considered to have occurred via an aberrant route directly from the primary tumor.
The other point that needs discussing is whether it is necessary to carry out biopsy examinations of contralateral axillary SN. Few studies have examined this point; therefore, the diagnostic and therapeutic impact of the detection of contralateral axillary SN remains unclear. In cases of breast cancer in which it is considered that a contralateral axillary SN might be the primary lymph node, it is not possible to precisely determine the clinical stage of the patient’s disease without performing an SN biopsy of the contralateral axilla. In some cases, metastatic SN have been detected in the contralateral axilla [11,12]; therefore, contralateral axillary SN biopsy examinations might be worth performing because the results of such examinations can affect the subsequent treatment.
Regarding the treatment of metastasis to the internal mammary lymph nodes, the use of surgery in such cases remains controversial. It has been suggested that surgery can improve patient prognosis in such cases, but there is insufficient evidence to support this assertion at present [22-26]. At our institution, when an internal mammary node is suspected to be metastatic based on ultrasonography, we subject it to fine needle aspiration. When metastasis is cytologically proven, we administer radiotherapy rather than resecting the metastatic lymph nodes. In cases in which lymphoscintigraphy shows an internal lymph node in a patient with clinical stage N0 disease, we try to safely remove the SN to confirm its metastatic status. In cases in which metastasis is detected in an internal mammary SN, the disease is pathologically defined as pN1 according to the National Comprehensive Cancer Network guidelines [27], which should be taken into consideration during decisions regarding the use of adjuvant therapy, including chemotherapy. When an incision made during breast-conserving surgery is located far from the internal mammary region, no operation for SN involving the internal mammary region is considered, providing that the patient and the surgeon in charge agree.
If a contralateral axillary node is considered to be anatomically similar to an internal mammary node, an SN biopsy examination of the contralateral axilla should be considered. Metastasis to a contralateral axillary lymph node is considered to be a form of distant metastasis (M1) according to conventional guidelines [27,28], but when the metastasis does not produce clinical symptoms and is found during an SN biopsy, the contralateral axillary node can be treated as the primary node. We can also consider that such disease should be pathologically staged as pN1, as would be the case for metastases to the internal mammary lymph nodes. Cases of stage pN1 disease can be treated with chemotherapy with the aim of achieving a complete cure. However, the significance of biopsy examinations of the contralateral axillary SN and internal mammary lymph nodes remains unclear. Clinical studies evaluating the significance of SN biopsy examinations of the contralateral axilla in breast cancer are difficult to conduct because such cases are very rare. After considering both clinical case reports and the merits of the procedure for our patient, we decided to conduct SN biopsy examinations of the contralateral and ipsilateral axillae in the present case. We consider that our report of this rare case will aid the treatment of breast cancer patients in whom SN are incidentally detected in the contralateral axilla.
Conclusion
We experienced a rare case of contralateral axillary SN in a breast cancer patient who had never received any medical treatment involving her breasts. Although this report only describes one case, we consider that this case deserves attention because few similar cases have been reported. It is important to accumulate more cases to be able to draw definitive conclusions about the management of contralateral axillary SN in breast cancer.
Funding
This study did not receive any financial support.
Conflicts of Interest Statement
The authors declare that they have no conflicts of interest.
Authorship Statement
Clinical studies: Hisako Ono, Kiyomi Kimura, and Yumi Miyagi
Data acquisition and analysis: Hisako Ono, Kiyomi Kimura, and Yumi Miyagi
Literature research: Hisako Ono and Kiyomi Kimura
Pathological analysis: Rie Horii and Futoshi Akiyama
Statistical analysis: None
Manuscript preparation: Hisako Ono and Masahiko Tanabe
Manuscript editing: Masahiko Tanabe, Rie Horii, and Takuji Iwase
Manuscript review: Shinji Ohno, Takuji Iwase, Futoshi Akiyama, Rie Horii, Miyagi Yumi, Kiyomi Kimura, Masahiko Tanabe, and Hisako Ono
Acknowledgements
We thank Medical English Service (www.med-english.com) for English language editing.
References
- Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med, 349: 546-553 (2003).
- Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet, 349: 1864-1867 (1997).
- Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer: a multicenter validation study. N Engl J Med,. 339: 941-946 (1998).
- Jansen L, Doting MH, Rutgers EJ, et al. Clinical relevance of sentinel lymph nodes outside the axilla in patients with breast cancer. Br J Surg, 87: 920-925 (2000)
- Nieweg OE. Lymphatics of the breast and the rationale for different injection techniques. Ann Surg Onco,8: 71-73 (2001).
- Tanis PJ, Nieweg OE, Valdés Olmos RA, et al. Impact of non-axillary sentinel node biopsy on staging and treatment of breast cancer patients. Br J Cancer, 87: 705-710 (2002).
- Vicente JS, Grande ML, Barquero CD, et al. Bilateral axillary and internal mammary drainage in breast cancer without prior surgery during sentinel node mapping. Indian J Nucl Med, 26: 205-207 (2011).
- Luján B, Carrera D, Picas J, et al. Detection of contralateral axillary sentinel lymph node by lymphoscintigraphy in breast cancer: Prognostic implications. Rev Esp Med Nucl, 29: 135-137 (2010).
- Carmon M, Mintz A, Hain D, et al. Clinical implications of contralateral axillary sentinel lymph nodes. The Breast, 15: 266-268 (2006).
- Chakera AH, Law I, Friis E, et al. Bilateral axillary sentinel nodes from breast cancer. Clin Nucl Med, 29: 840 (2004).
- Allweis TM, Parson B, Klein M, et al. Breast cancer draining to bilateral sentinel lymph nodes. Surgery, 134: 506-508 (2003).
- Krause A, Dunkelmann S, Makovitzky J, et al. Detection of atypical site of sentinel lymph drainage scintigraphy in patients with breast carcinoma. Zentralbl Gynakol, 122: 514-518 (2000).
- Koizumi M, Koyama M, Yamashita T, et al. Experience with intradermal injection and intradermal-plus-deep injection in the radioguided sentinel node biopsy of early breast cancer patients.Eur J Surg Oncol, 32: 738-742 (2006).
- Koizumi M, Nomura E, Yamada Y, et al. Radio-guided sentinel node detection in breast cancer patients: comparison between Tc-99m phytate and Tc-99m rhenium colloid. Nucl Med Commun, 25: 1031-1037 (2004).
- Shimazu K, Tamaki Y, Taguchi T, et al. Lymphoscintigraphic visualization of internal mammary nodes with subtumoral injection of radiocolloid in patients with breast cancer. Ann Surg, 237: 390-398 (2003).
- Roumen RM, Geuskens LM, Valkenburg JG. In search of the true sentinel node by different injection techniques in breast cancer patients. Eur J Surg Oncol, 25: 347-351 (1999).
- Kimberg VS, Rubio IT, Henry R, et al. Subareolar versus peritumoral injection for location of the sentinel lymph node. Ann Surg, 229: 860-864 (1999).
- Maza S, Valencia R, Geworski L, et al. Peritumoral versus subareolar administration of technetium-99m nanocolloid for sentinel lymph node detection in breast cancer: preliminary results of a prospective intra-individual comparative study. Eur J Nucl Med Mol Imaging, 30: 651-656 (2003).
- Nieweg OE, Estourgie SH, van Rijk MC, et al. Rationale for superficial injection techniques in lymphatic mapping in breast cancer patients. J Surg Oncol, 87: 153-156 (2004).
- Osborne MP. Breast anatomy and development. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the breast. 2nd ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2000. p.1-15
- Intra M, Viale G, Vila J, et al. Second Axillary Sentinel Lymph Node Biopsy for Breast Tumor Recurrence: Experience of the European Institute of Oncology. Ann Surg Oncol, 22: 2372-2377 (2015)
- Leidenius MH, Krogerus LA, Toivonen TS, et al. The clinical value of parasternal sentinel node biopsy in breast cancer. Ann Surg Oncol, 13: 321-326 (2006).
- Madsen E, Gobardhan P, Bongers V, et al. The impact on post-surgical treatment of sentinel lymph node biopsy of internal mammary lymph nodes in patients with breast cancer. Ann Surg Oncol,14: 1486-1492 (2007).
- Heuts EM, van der Ent FW, von Meyenfeldt MF, et al. Internal mammary lymph drainage and sentinel node biopsy in breast cancer-A study on 1008 patients. Eur J Surg Oncol, 35:252-257 (2009)
- Gnerlich JL, Barreto-Andrade JC, Czechura T, et al. Accurate staging with internal mammary chain sentinel node biopsy for breast cancer. Ann Surg Oncol, 21:368-374 (2014)
- Koo MY, Lee SK, Bae SY, et al. Long-term outcome of internal mammary lymph node detected by lymphoscintigraphy in early breast cancer. J Breast Cancer, 15:98-104 (2012).
- NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Ver.1.2016. National Comprehensive Cancer Network.
- The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual.