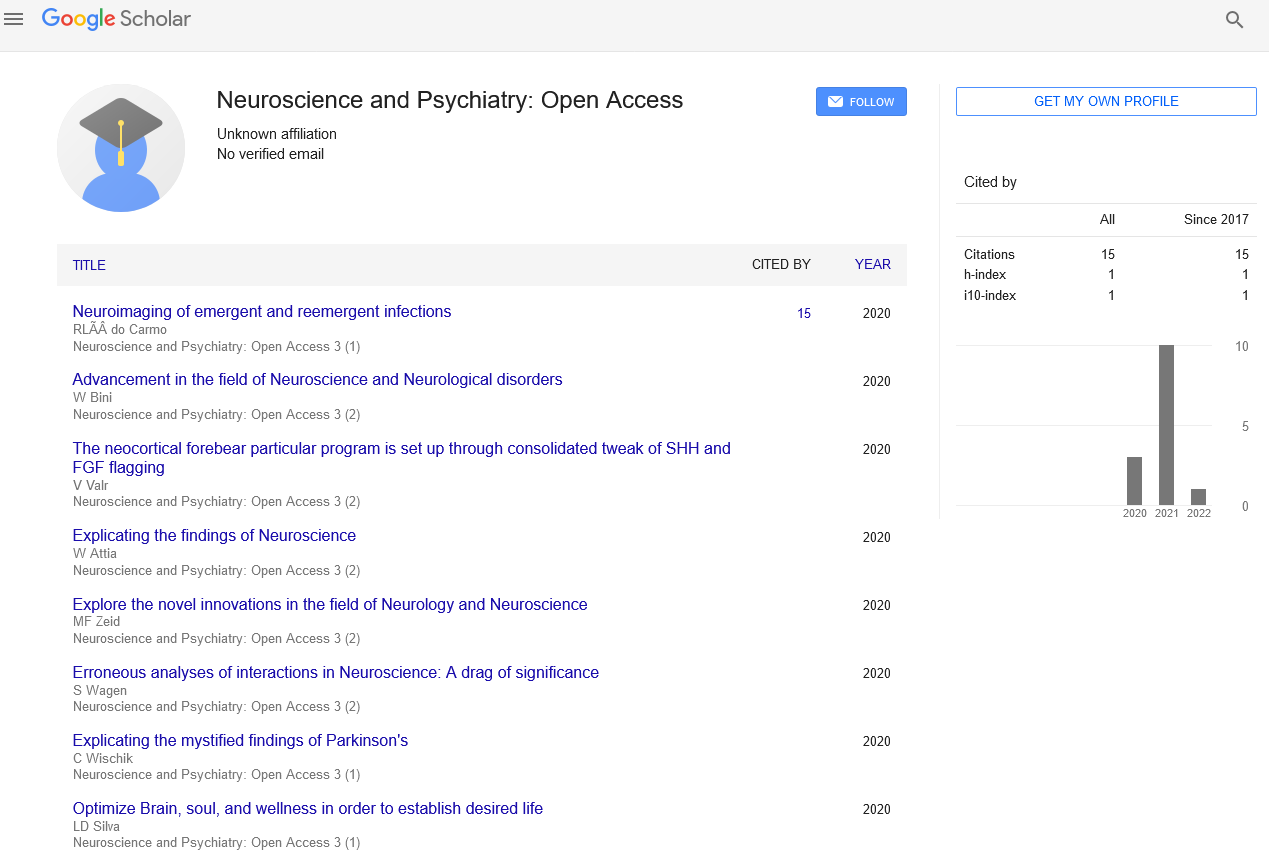
Short Article - Neuroscience and Psychiatry: Open Access (2020) Volume 3, Issue 2
The neocortical forebear particular program is set up through consolidated tweak of SHH and FGF flagging
Vanitha Valr
Aberdeen University, UK
Abstract
Neuronal forebears in the creating forebrain experience dynamic capability states to guarantee opportune age of explicit excitatory and inhibitory neuronal subtypes from particular neurogenic specialties of the dorsal and ventral forebrain, separately. Here we show proof of forebear pliancy when Sonic hedgehog (SHH) flagging is left unmodulated in the early stage neocortex of the mammalian dorsal forebrain. We found that at beginning phases of corticogenesis, loss of Suppressor of Fused (Sufu), a powerful inhibitor of SHH motioning, in neocortical ancestors, changed the transcriptomic scene of male mouse incipient organisms. Ectopic initiation of SHH flagging happened, through debasement of Gli3R, bringing about noteworthy upregulation of Fibroblast Growth Factor 15 (FGF15) quality articulation in all E12.5 Sufu-cKO neocortex regardless of sex. Subsequently, enactment of FGF flagging, and its downstream effector the MAPK flagging, encouraged articulation of qualities normal for ventral forebrain forebears. Our investigations recognize the significance of regulating extraneous specialty signals, for example, SHH and FGF15 to keep up the competency and detail program of neocortical ancestors all through corticogenesis.
Importance Statement: Low degrees of FGF15 control forebear expansion and separation during neocortical improvement however little is known on how FGF15 articulation is kept up. Our investigations distinguished SHH motioning as a basic activator of FGF15 articulation during corticogenesis. We found that Sufu, through Gli3R, guaranteed low degrees of FGF15 was communicated to forestall anomalous detail of neocortical begetters. These investigations advance our insight on the subatomic systems managing the age of explicit neocortical neuronal genealogies, their suggestions in neurodevelopmental sicknesses, and may direct future examinations on how ancestor cells might be used for cerebrum fix. Determination abandons are obvious in discrete districts of the neocortex of E12.5 early stage mice lacking Sufu: The job of SHH motioning in neocortical neuron determination is basic preceding E13.5, a timepoint at which shallow projection neurons are simply starting to separate.
Examination of mice in which Sufu is restrictively erased at E10.5 in neocortical forebears utilizing the Emx1-Cre driver (Emx1-cre/+;Sufu-fl/fl or Sufu-cKO), uncovered that balancing SHH flagging is basic to appropriately determine unmistakable shallow and profound layer projection neurons, after dorsoventral designing of the forebrain (Yabut et al., 2015). While particular imperfections were clear at E14.5 in SufucKO cortex, any atomic changes before this timepoint were not profoundly inspected in the past investigation. Since changes in Gli2 and Gli3R levels were evident at E12.5, we proposed that basic sub-atomic adjustments more likely than not happened at this timepoint. We in this manner started our investigations via cautious assessment of Pax6 articulation, which is profoundly communicated in neocortical RG begetters (Ypsilanti and Rubenstein, 2016). True to form, we found that Pax6 solely communicated in dorsal forebrain districts of the E12.5 control and Sufu-cKO cerebrums, and not in the ganglionic distinction (GE) . Be that as it may, Pax6 articulation was discernibly irregular in front locales of the E12.5 Sufu-. Examination of comparing districts indicated that the E14.5 Sufu-cKO neocortex comparatively showed columnar dispersion of Pax6+ and Pax6-areas in front locales (sharpened stones, Figure 1B), yet this circulation was not common in back locales (Figure 1B). These imperfections were absent at E10.5, in which the dissemination of Pax6+ cells were to a great extent indistinct among controls and Sufu-cKO undeveloped organisms.
In this manner, in spite of having appropriately framed dorsal forebrain spaces, a subpopulation of neocortical RG forebears showed deviant conduct in the E12.5 Sufu-cKO neocortex. Forebrain Organotypic Slice Culture: Entire minds from E12.5 wildtype CD-1 mice were painstakingly dismembered and set in super cold Hanks Balanced Salt Solution (HBSS; Invitrogen). Cerebrums were inserted in 4% Low Melting Point Agarose (Nueve)/HBSS blend and permitted to harden on ice. Implanted cerebrums were cut utilizing a VT1000S vibratome (Leica) into 400 μm thick cuts and set in Recovery Media (MEM (Invitrogen) with Glutamax (Invitrogen) and Pennicillin/Streptomycin (Invitrogen)). Cuts were moved into uncoated Millicell-CM film embeds (EMD-Millipore) in 6-well plates (BD Biosciences) and refined in Neurobasal (Invitrogen) enhanced with Glutamax (Invitrogen), Pennicillin/Streptomycin (Invitrogen), B-27 (Invitrogen) and N2 (Invitrogen) at 37°C, 5% CO2, and 100% moistness.
Following 2 days in vitro (DIV), cell culture media were suctioned, and cuts were washed in 1X PBS, fixed in cold 4% PFA for 30 minutes, cryoprotected in 30% sucrose, and implanted in OCT. Cuts were cryosectioned into 20 μm thick coronal areas and put away at −80°C until utilized for immunofluorescence investigation as portrayed previously. Treatment (as depicted in text) of organotypic cuts were directed 2-3 hours after beginning plating and hatching of cuts with the accompanying fixations: 100 ng/ml recombinant FGF15 (Prospec Bio, #CYT-027), 200 ng/ml recombinant SHH (GenScript, #Z03050-50), and 5 uMcyclopamine (Toronto Research Chemicals, #C988400).
Following medicines, cut societies were brooded for 2 days and handled as depicted previously. Picture Analysis and Acquisition: Pictures were procured utilizing a Nikon E600 magnifying instrument outfitted with a QCapture Pro camera (QImaging), Zeiss Axioscan Z.1 (Zeiss, Thornwood, NY, USA) utilizing the Zen 2 blue release programming (Zeiss, Thornwood, NY, USA), or the Nikon Ti transformed magnifying instrument with CSU-W1 huge field of view confocal and AndorZyla 4.2 sCMOS camera. All pictures were imported in spat or jpeg design. Splendor, difference, and foundation were balanced similarly for the whole picture among controls and freak utilizing the "Brilliance/Contrast" and "Levels" work from "Picture/Adjustment" choices in Adobe Photoshop or NIH ImageJ with no further change. NIH Image J was utilized to edge foundation levels among controls and freak tissues to measure fluorescence naming. For Phospho-Erk1/2 evaluation, the absolute territory with positive Phospho-Erk1/2 marking were estimated, which started in the pallial-subpallial limit in the controls and broadened dorsally in Sufu-cKO neocortex, for every half of the globe over the front to back pivot. One forebrain segment in every delegate front to back district were estimated from the two sides of the equator were arrived at the midpoint of. All investigations were led in at any rate 2-3 20 μm thick segments that were histologically coordinated at the rostralcaudal level between genotypes.
Acknowledgement: Al-Ayadhi LY. 2012. Connection Between Sonic Hedgehog Protein, Brain-Derived Neurotrophic Factor and Oxidative Stress in Autism Spectrum Disorders. Neurochem Res 37:394–400. doi:10.1007/s11064-011-0624- xCrossRefPubMedGoogle Scholar Beattie R, Hippenmeyer S. 2017. Systems of outspread glia begetter cell heredity movement. FEBS Lett 591:3993– 4008. doi:10.1002/1873- 3468.12906CrossRefGoogle Scholar Borello U, Cobos I, Long JE, Murre C, Rubenstein JL. 2008. FGF15 advances neurogenesis and restricts FGF8 work during neocortical turn of events. Neural Dev 3:17. doi:10.1186/1749-8104-3- 17CrossRefPubMedGoogle Scholar Dave RK, Ellis T, Toumpas MC, Robson JP, Julian E, Adolphe C, Bartlett PF, Cooper HM, Reynolds BA, Wainwright BJ. 2011. Sonic hedgehog and score flagging can coordinate to manage neurogenic divisions of neocortical forebears.