Short Article - Interventional Cardiology (2011) Volume 3, Issue 2
The NovoStent̨̉ SAMBĄ̉ stent: a novel alternating helix self-expanding nitinol stent design
- Corresponding Author:
- Thomas Zeller
Department of Angiology
Herz- Zentrum Bad Krozingen
Südring 15, D-79189 Bad Krozingen, Germany
Tel: +49 763 340 22431
Fax: +49 763 340 22439
E-mail: thomas.zeller@herzzentrum.de
Abstract
Keywords
nitinol, popliteal artery, stent, stent fracture, superficial femoral artery
The need for effective treatments for peripheral arterial disease (PAD) is clear. It is estimated that up to 27 million people in Europe and North America alone suffer from some form of PAD [1,2]. Current treatment modalities range from noninvasive lifestyle modification to bypass surgery and, at the extreme, amputation of an affected limb. All these treatments have varying levels of effectiveness that can be influenced by their design, the skill level of the practitioner and, perhaps most importantly, the behavior of the patient. The focus of this article will be to describe a radically new stent type that has been designed specifically for the treatment of PAD in the superficial femoral (SFA) and popliteal arteries.
Current stent technology used to treat SFA and popliteal disease is based on slotted-tube designs and is made from nitinol, a shape-memory alloy of nickel and titanium that is especially well suited to the mechanical stresses endured by the SFA and popliteal arteries. These selfexpanding, slotted-tube stents have been used to treat PAD for over 10 years [3–10]. During this time there has been some advancement in stent design. This advancement has come mostly in the form of modifications to the axial bridges used to connect the individual slotted-tube rings that create the stent in an effort to increase flexibility and reduce fracture rates. Slotted-tube stents offer several advantages. In general, the coaxial catheter delivery systems are simple to use and often incorporate some mechanical advantage (e.g., a wheel) for the user to overcome high pull-back forces. The integration of delivery catheters and stents has produced high technical success rates in straightforward lesions. Finally, the nickel–titanium material of the implant is well tolerated by the body.
Despite these advantages, stents are not without their limitations. They lack the ability to treat a wide variety of disease and inherent in their design are tradeoffs and in order to improve the flexibility necessary to bend with the artery, designers have sacrificed the strength necessary to hold arteries open in the face of significant, and sometimes only moderate, calcium burdens. Stent struts remain quite thick in order to maintain the strength that they are able to achieve. This can create blood turbulence and lead to conditions that can promote restenosis. Current stents are also limited in the amount of vessel coverage they can provide (~20%) without becoming unworkable from the standpoint of the delivery profile. Fracture rates of some of the newest stent designs have decreased to low single digit levels [5,7–10]. However, not all stents are created equal in this regard and the fracture rates of some designs are still too high and must be reduced [4,10–14]. Most importantly, failure rates of stents remain too high [4,13–15]. Stents provide a proven advantage over balloon angioplasty in all but the shortest lesions [3–10]. However, with restenosis rates of 20–40% at 12 months, there is still ample room for improvement.
SAMBA® technology
▪ Femoro–popliteal biomechanics
The primary objective of a vascular stent is to maintain luminal clearance so that adequate blood flow can pass to downstream tissues. Mechanically, this means the stent needs to provide radial support. For stents that are implanted into very mobile anatomies, such as in the lower extremity vessels, structural rigidity in any orientation other than the radial direction may be detrimental to long-term performance. For example, the femoro–popliteal artery (FPA) experiences significant axial, twist and bending deformations due to musculoskeletal movement [16–18], and even with normal hip and knee flexion, the femoro–popliteal segment undergoes visually striking deformations, as presented by Cheng et al. [17]. Stents implanted into the FPA exhibit a fracture rate of up to 37% and for severe fractures there is a correlation with clinical sequelae [13,18]. These stent fractures are caused by metal fatigue, which is intimately linked to high material strains resulting from anatomic deformations and current stent designs. As such, there is increasing interest from the engineering and clinical community in improving stent flexibility, which may have other commensurate mechanobiologic benefits in addition to decreasing stent fracture rate.
SAMBA design
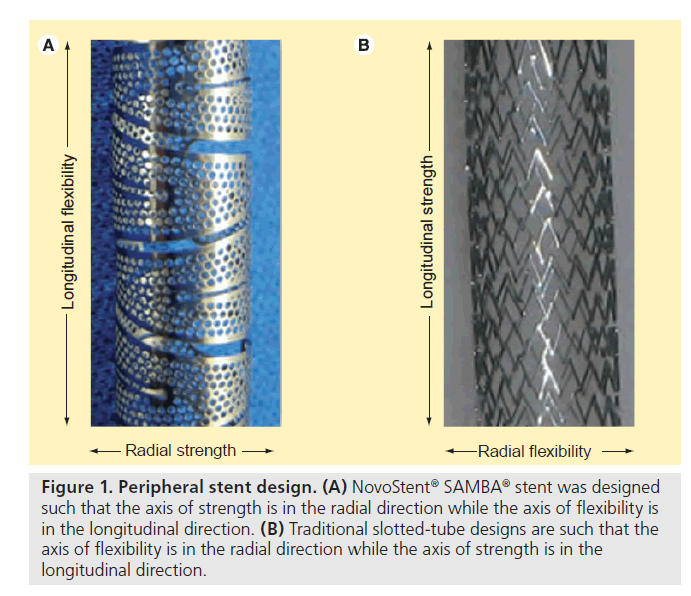
In traditional slotted-tube stent designs, increased axial, twist and bending flexibility are generally accompanied by decreased radial strength. The NovoStent® design incorporates a 60-micron thin, alternating helical ribbon comprised of small holes (~0.4 mm diameter) that enables simultaneous higher flexibility and radial strength compared with slotted-tube designs. This benefit is derived from orienting the axis of strength of the stent macrostructure in the radial direction, as opposed to in the longitudinal direction as in traditional slotted-tube designs (Figure 1).
Figure 1. Peripheral stent design. (A) NovoStent® SAMBA® stent was designed such that the axis of strength is in the radial direction while the axis of flexibility is in the longitudinal direction. (B) Traditional slotted-tube designs are such that the axis of flexibility is in the radial direction while the axis of strength is in the longitudinal direction.
The following sections report measurements of axial, twist and bending flexibility, as well as stent conformability and the metrics of radial strength for the NovoStent SAMBA, Cordis® SMART®, and Bard LifeStent® peripheral stent designs. The SMART and LifeStent designs were chosen for comparison because they are the most commonly used stent in the femoral artery and the only utilized bare-metal stent US FDA approved for the femoral artery, respectively. In addition, we report benchtop fatigue testing for these three stent designs, including twist, bending, and buckle and twist modes. Many of the tests were designed to approximate the tests of prior studies such that comparisons to larger bodies of data could be made [19–21].
SAMBA flexibility/conformability
▪ Devices & testing equipment
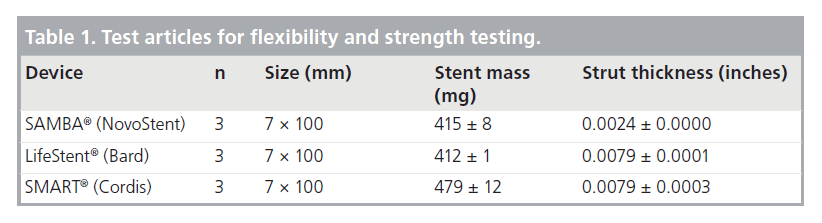
All tests were performed on three samples of each stent design (Table 1). Forces were measured using an Instron® Universal Materials Testing Machine, Model 3342. All testing was performed inside a temperature-controlled chamber at 37°C. Prior to testing, mass and thickness were recorded for each stent; average ± standard deviation for each stent design is presented in Table 1. Note that while the stent masses of the SAMBA and slotted-tube designs are similar, the SAMBA stent has one third the strut thickness and approximately three-times the area coverage of the slotted-tube stents. In order to demonstrate the extreme flexibility of the SAMBA stent, several of the tests were repeated with a sample of two completely overlapped SAMBA stents, representing the worst-case clinical scenario for flexibility.
Table 1. Test articles for flexibility and strength testing.
▪ Flexibility
For axial elongation, two 7.25-mm mandrels were inserted into each end of the stent samples such that 90 mm of stent remained free between the mandrels; the stent was then affixed to the mandrels with shaft collars (Figure 2A). This fixture was then placed into the jaws of the Instron tester and the force to stretch the samples by 15% of the original 90-mm test length (13.5 mm) was measured. For axial compression, stent samples were placed into a compression fixture consisting of a flat base with a 6-mm diameter mandrel and a top with a hole that allows the mandrel to pass through while axially compressing the stent sample without kinking (Figure 2B). The force to compress the sample by 15% of the original 100 mm test length (15 mm) was measured. For axial twist, stent samples were affixed to a torque fixture using shaft collars at each end with 90 mm of stent between the attachment points (Figure 2C). Linear force was translated to the torque through a 12.7‑mm diameter shaft and the force required to twist the stent sample 3°/cm (27° total) was measured. The torque required was found to be independent of the direction (clockwise or counter-clockwise) of the applied torque. For three-point bend flexibility testing, stent samples were placed into a fixture with three pins. The two outer pins were 50 mm apart and contacted one side of the stent while the third contacted the other side and pulled the center of the test segment by 5 mm (Figure 2D). The force to move the center pin by 5 mm was measured. Due to the asymmetric macrostructure of the SAMBA stent, and potential orientation effects, the SAMBA stent samples were tested in four different orientations (90° apart); orientation differences were found to be small.
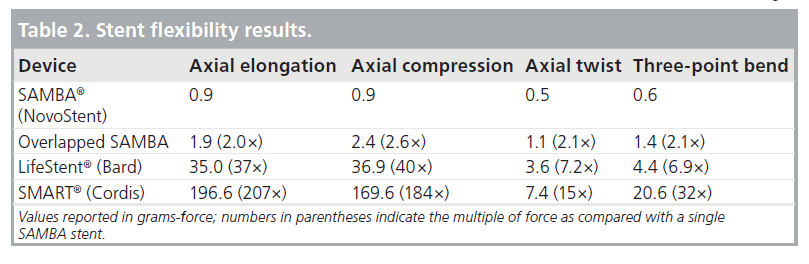
The flexibility data shown in Table 2 illustrates the superior flexibility of the SAMBA stent as compared with the LifeStent and SMART designs in axial elongation, axial compression, axial twist and three-point bend. Note that the LifeStent and SMART stent designs are even less flexible than two SAMBA stents that are completely overlapped together for all four testing methods.
▪ Conformability
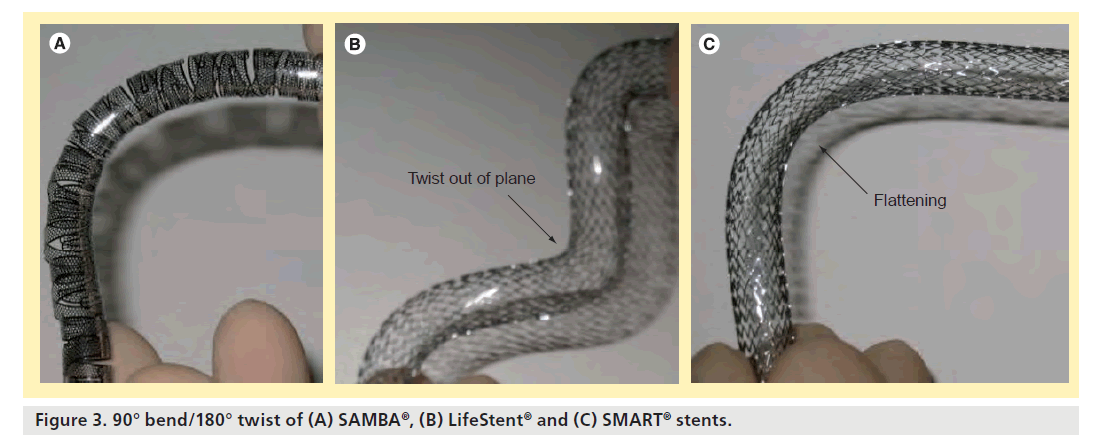
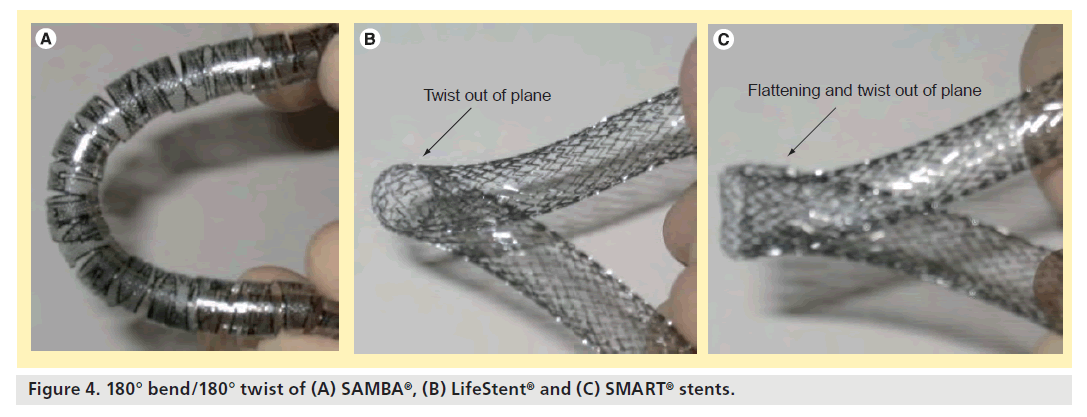
A conformability test was performed to show a visual, qualitative comparison between the stent designs. Each stent design was twisted 180° over 9 cm of stent length and then bent into 90 and 180° curves. The SAMBA stent remains planar and circular in cross-section for 90° bend/180° twist and 180° bend/180° twist, while the SMART and LifeStent designs take on out-of-plane curves and/or noncircular cross-sections (Figures 3 & 4).
SAMBA flexibility/conformity discussion
The axial, twisting, and bending flexibility/conformability described in the previous sections are critical for stent performance. While much attention has been paid to how the motions of a vessel affect stent fatigue and fracture, possibly the more important issue is how the stent affects the vessel. The ability and ease of a stent to follow the complex and substantial dynamic motions of a vessel determines how much loading a stent imparts on the vessel. This loading, arising from any of the deformations described above, may be highly influential on biological reaction and stent performance [22,23]. Of note, owing to the relationship between stress and strain, stents that exhibit high flexibility (how the stent affects the vessel) perform well in fatigue (how the vessel affects the stent). In addition, stents that do not fatigue and fracture also avoid broken, sharp features that may be biologically traumatic [13,18].
Stent flexibility, with regards to tortuous vascular anatomy, is important when considering the potentially trauma-inducing stiff, sharp features on traditional slotted-tube designs. When a stent is implanted into a vessel that deforms dramatically enough to kink, as depicted by Smouse et al., adverse mechanobiologic responses would be minimized with a flexible stent with rounded ends, as with the SAMBA stent [21]. Based on mechanical performance data provided in a prior study, the SAMBA stent is more flexible in the longitudinal, torsional and bending directions than the ev3 Protégé, Guidant Absolute™, Gore Viabahn® and Bard Fluency® devices [19].
SAMBA strength
▪ Diametric crush & radial resistive force
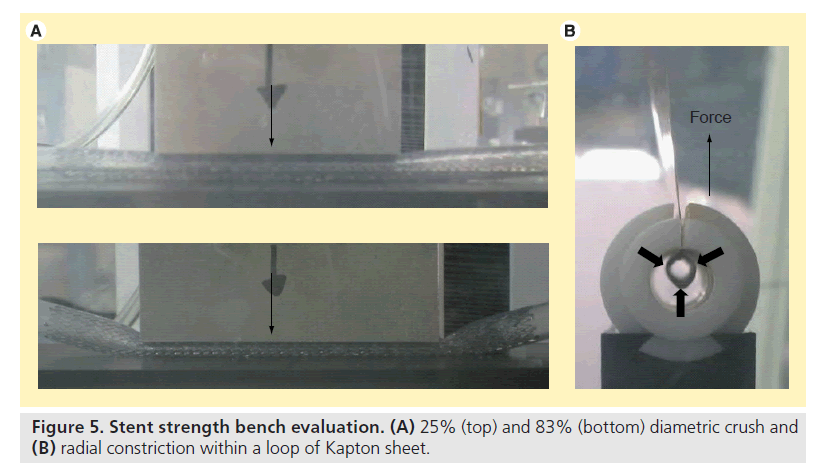
Two different diametric crush tests were performed on each stent sample (Figure 5A). In both tests, samples were placed inside 6-mm inner diameter silicone tubing (the maximum treatment diameter for all designs). The first test placed each sample between 51 mm long plates. The force to diametrically crush the sample by 25% (1.5 mm) was measured. The second test placed each sample between 64 mm long plates, and then the force to diametrically crush the sample by 83% (5 mm) was measured. For radial resistive force, each stent sample was placed into a loop of DuPont Kapton® (DuPont, NC, USA) sheet and then inserted into a cylinder. The constraining sheet ends pass through a slit in the cylinder and into the jaws of the Instron tester. As the constraining sheet is pulled through the slit, the perimeter of the sheet reduces, thus radially compressing the stent (Figure 5B). The forces required to radially reduce the stent sample to the maximum (6 mm) and minimum (5 mm) treatment diameters were measured.
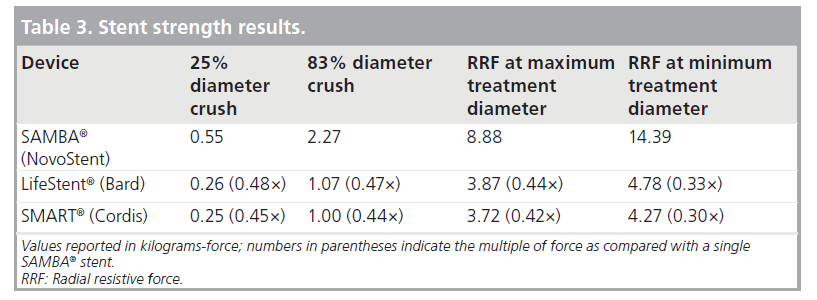
As shown in Table 3, the SAMBA stent resists diametric crush and radial constriction substantially more than the LifeStent and SMART designs.
▪ Strength: discussion
The SAMBA stent provides more than twice the diametric crush and radial constriction resistance than that of SMART and LifeStent stents. Radial strength is important for lumen diameter maintenance, especially in the presence of diseased vessels that tend to recoil after balloon dilatation. In addition, while the slottedtube designs increase their radial resistive forces by approximately 20% when constraining from maximum to minimum treatment diameter, the SAMBA stent increases its radial resistive force by 60%. This means that not only does the SAMBA stent provide better resistance to radial constriction; but it does so with increasing tenacity at smaller vessel diameters, when lumen maintenance is more critical. Based on mechanical performance data provided in a prior study, the SAMBA stent exhibits greater radial strength than the ev3 Protégé, Guidant Absolute and Gore Viabahn devices [19].
SAMBA fatigue resistance
▪ Devices & testing equipment
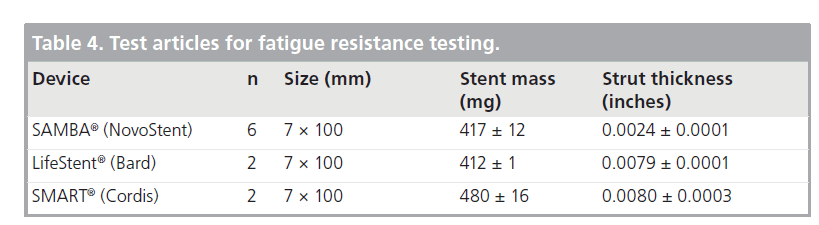
Tests were performed on three stent designs (Table 4). All testing was performed inside temperature- controlled chambers at 37°C. Prior to testing, mass and thickness was recorded for each stent; average ± standard deviation for each stent design is presented in Table 4.
Table 4. Test articles for fatigue resistance testing.
▪ Twist, bending & buckle twist fatigue
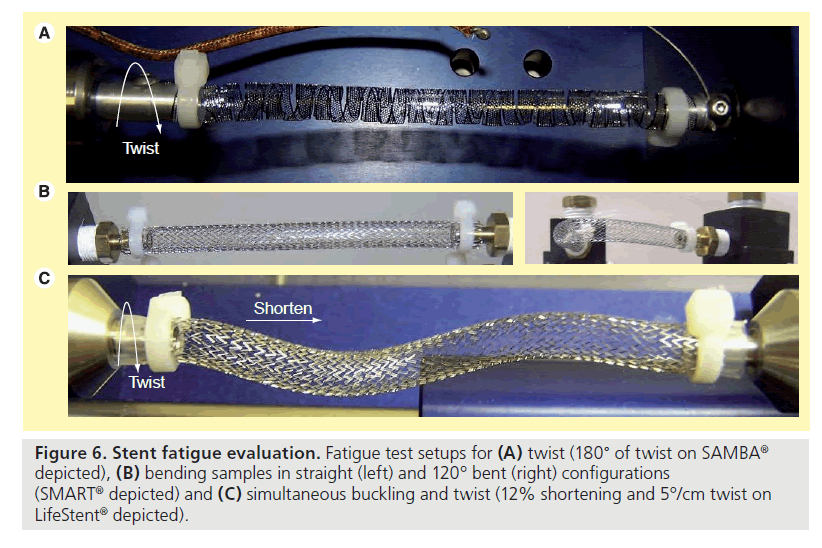
Two samples of SAMBA stent were twisted from neutral to 180° and back (Figure 6A) at 4.4 Hz for 10 million cycles and was similar to that reported by others [20]. Two SAMBA stents, one LifeStent and one SMART stent were cycled between straight to a bend of 120° and back (Figure 6B) at 1.4 Hz for 10 million cycles and was similar to that performed by others [20]. A multimodal buckling and twist test was also performed on two SAMBA stents, one LifeStent and one SMART stent where the stent ends were moved towards one another so as to shorten the sample’s length by 12%, and also simultaneously twisted about its axis by 5°/cm of length (Figure 6C) at 2.0 Hz for 10 million cycles. These parameters represent a worst case and are calculated from the average plus two standard deviations of deformation measured in a population of older adults, aged 50–70 years [17].
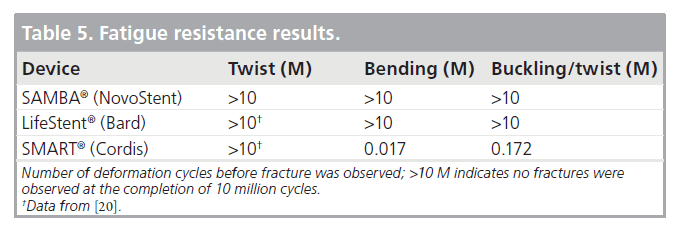
Table 5 shows the fatigue performance of the SAMBA, LifeStent, and SMART stent designs for 10 million cycles of twist, bending and buckle/twist.
For the twist testing, both samples of SAMBA stent endured 10 million cycles with no fractures. In a previous study, similar torsion testing was endured by the IDev SUPERA™, the Bard LifeStent, the Cordis SMART Control and the Zilver® 635, while the ev3 Protégé GPS fractured under these conditions at approximately 300,000 cycles (equivalent to 4 months) [20]. With the exception of the SUPERA stent, which is a braided design, all the other stents mentioned have similar slotted-tube designs.
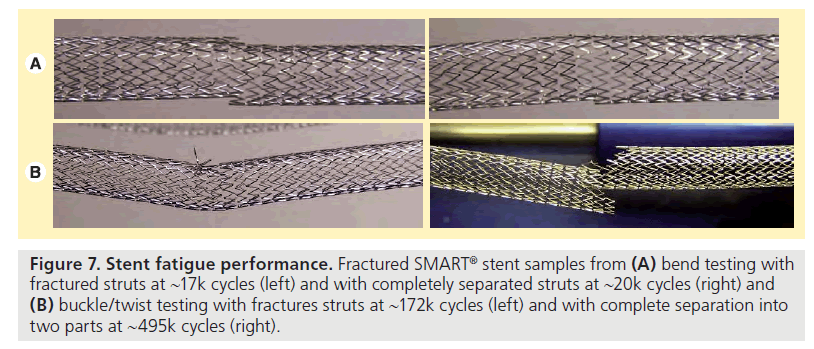
For the bend testing, the SAMBA stent and LifeStent samples successfully endured 10 million cycles (equivalent to 10 years) with no fractures. The SMART sample displayed fractures at 17,045 cycles (equivalent to 6 days) with small sections of struts completely free from the sample at 20,622 cycles (equivalent to 7 days) (Figure 7A). Complete separation of the sample into two parts was observed at 46,958 cycles (equivalent to 17 days). In a previous study, similar bending testing was endured by the IDev SUPERA, while the Bard LifeStent fractured at 21,087 cycles (equivalent to 8 days) and 92,415 cycles (equivalent to 1 month), the Cordis SMART Control fractured at 21,087 cycles (equivalent to 8 days), the Zilver 635 fractured at 1738 cycles (equivalent to 1 day), and the ev3 Protégé Everflex® fractured at 21,087 cycles (equivalent to 8 days) under similar conditions [20].
Figure 7. Stent fatigue performance. Fractured SMART® stent samples from (A) bend testing with fractured struts at ~17k cycles (left) and with completely separated struts at ~20k cycles (right) and (B) buckle/twist testing with fractures struts at ~172k cycles (left) and with complete separation into two parts at ~495k cycles (right).
For the buckle and twist testing, the SAMBA stent and LifeStent samples endured 10 million cycles (equivalent to 10 years) with no fractures. The SMART sample displayed fractures at 172,078 cycles (equivalent to 2 months) and complete separation into two parts at 495,008 cycles (equivalent to 6 months) (Figure 7B).
▪ Fatigue discussion
Stent fatigue resistance is inversely correlated with the peak alternating material strains experienced by the stent during deformation [24]. According to the metrics reported by Cheng et al., the FPA experiences significant axial shortening, axial twist and bending deformations [17], and according to the results shown in the SAMBA flexibility/ conformance section, the SAMBA stent is more flexible and conformable in each of those deformation modes compared with the SMART stent and the LifeStent. Not surprisingly, the SAMBA stent performed as well as LifeStent and better than the SMART stent in accelerated durability testing for bending and buckle/twist. In fact, the SAMBA stent went fracture free for the duration of all of the tests equivalent to 10 years.
Extrapolating from a previous study, where similar torsion testing was performed, the SAMBA stent outperformed the ev3 Protégé GPS™, which fractured at approximately 300,000 cycles, or the equivalent of 4 months [20]. In addition, with similar bending testing, the SAMBA stent outperformed the LifeStent (fractured at 21,087 [~8 days] and 92,415 cycles [~1 month]), the SMART stent (fractured at 21,087 cycles [~8 days]), the Cook Zilver 635 (fractured at 1738 cycles [~1 day]) and the ev3 Protégé Everflex (fractured at 21,087 cycles [~8 days]) [20].
SAMBA preclinical experience
▪ Preclinical testing conditions
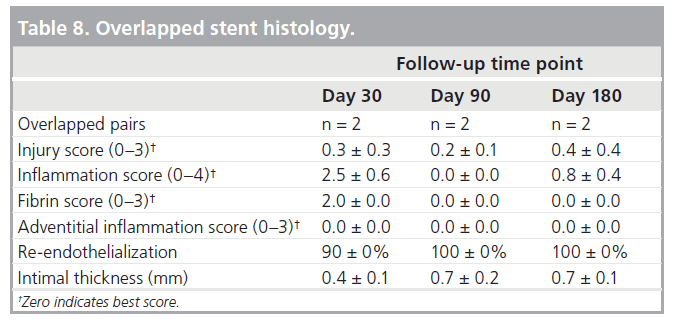
The SAMBA stent was evaluated in two different animal conditions to assess biological response [25]. A total of 11 sheep were implanted with either two single 7 × 50 mm stents placed bilaterally (n = 5) in appropriately sized vessels, or a pair of overlapped 7 × 50 mm stents (n = 6) were placed in a vessel much smaller than the minimum treatment diameter. The antiplatelet regimen was clopidogrel for 30 days and aspirin for the duration of the study. Single stents were placed in native SFAs ranging from 5 to 6 mm in diameter, the appropriate treatment range for the 7-mm SAMBA stent. Angiographic and intravascular ultrasound (IVUS) assessments were performed at an interim time point (30 days) as well as planned sacrifice (90 days). Histopathology was performed on the 90 days specimens by CVPath Institute (Gaithersburg, MD, USA). For the overlapped device evaluation, SAMBA stents were placed in native SFAs measuring less than 5 mm in diameter. Prior to sacrifice at 30, 90 and 180 days (n = 2 per time point), angiography was performed followed by histolopathology evaluation.
▪ Single stent results
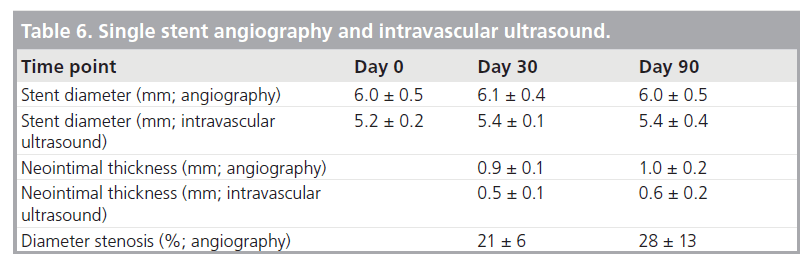
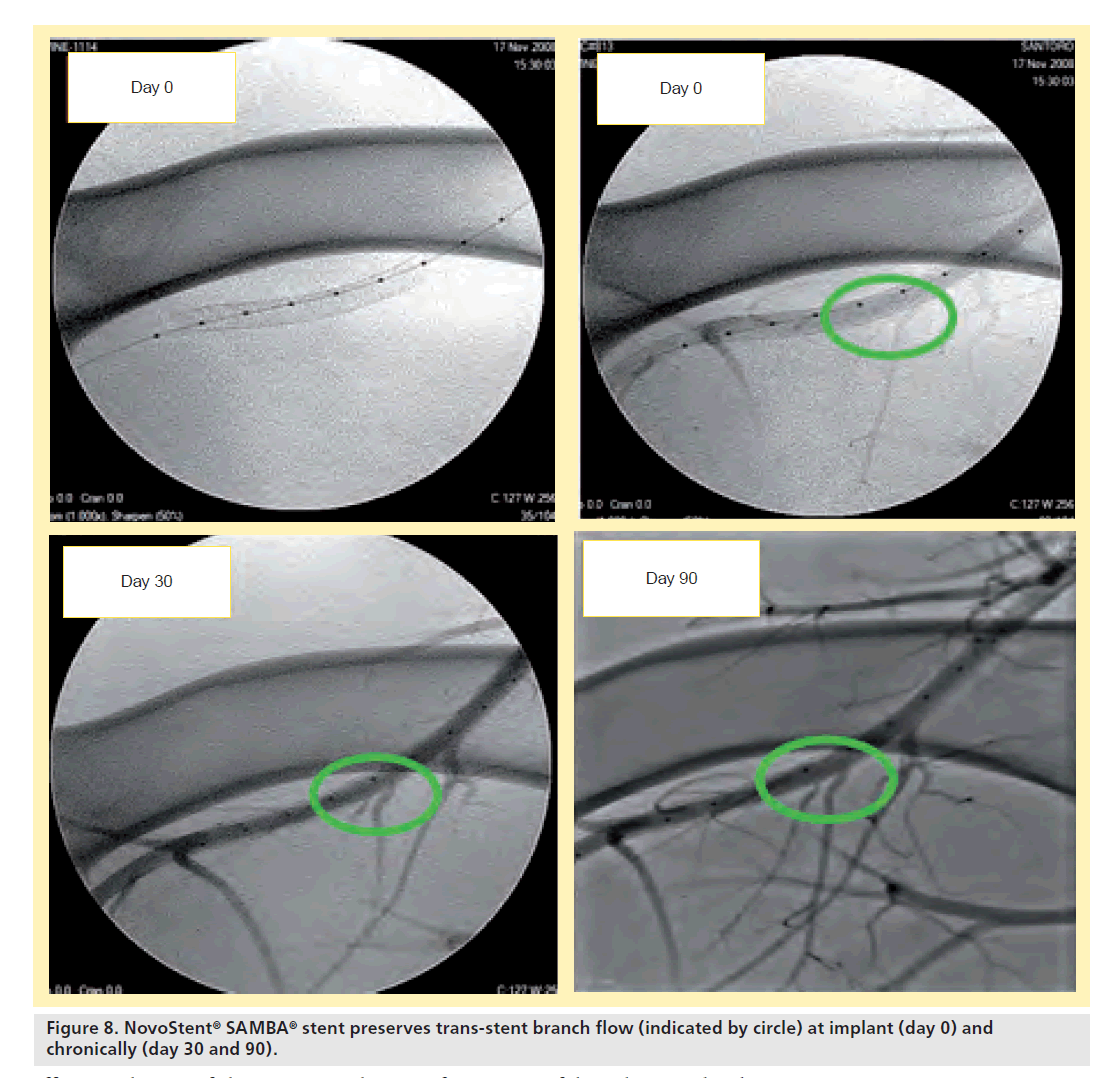
For the single stents, all devices were patent at follow-up and no stent fractures occurred. Diameter stenosis via IVUS and angiography were strongly correlated and the stent diameter was stable over the follow-up period (Table 6). Trans-stent blood flow and demonstrated branch vessel preservation were observed at implant as well as during the follow-up course (Figure 8).
Table 6. Single stent angiography and intravascular ultrasound.
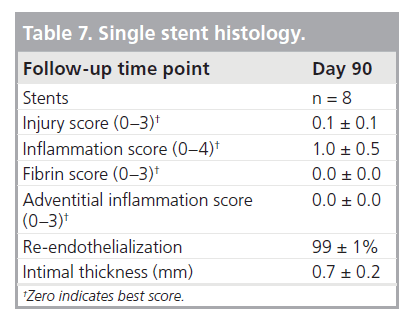
Histology revealed 99% re-endothelialization at 90 days, well-organized smooth muscle neointimal growth, and low injury and inflammation scores (Table 7). Inflammation was localized around stent struts and consisted mostly of macro-phages with occasional multinucleated giant cells.
▪ Overlapped stent results
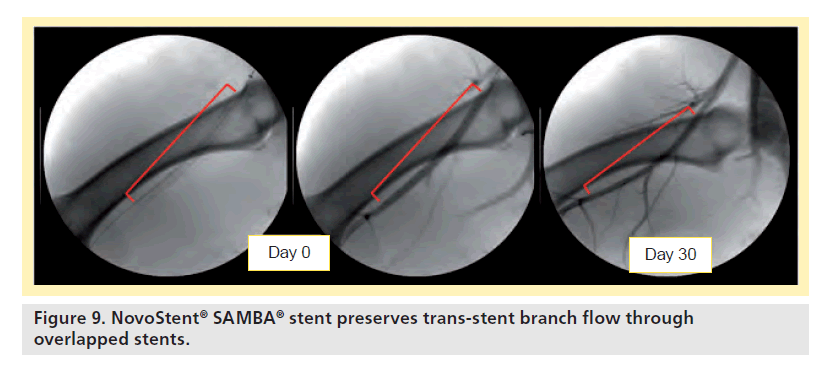
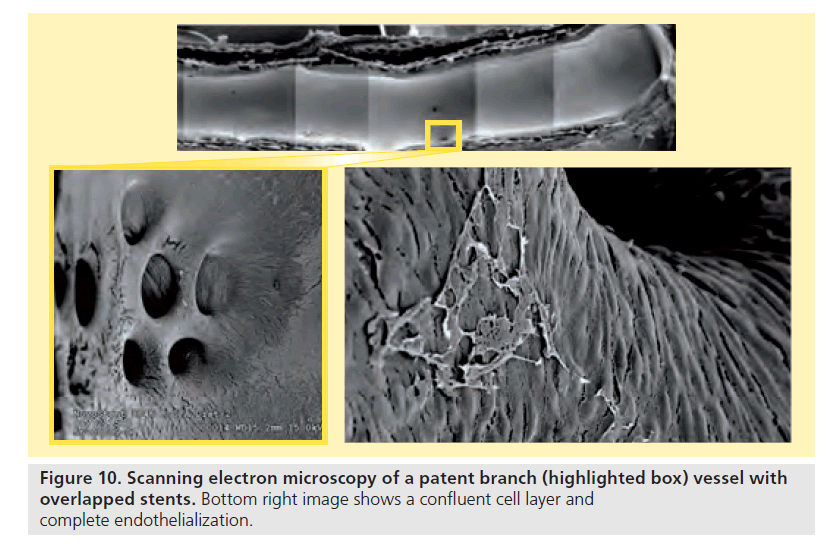
For the overlapped devices, all stents were patent at all follow-up time point, and no stent fractures were observed. Branch vessel preservation was demonstrated by angiography (Figure 9) as well as scanning electron microscopy (Figure 10).
Histology showed 90% re-endothelialization at 30 days and 100% at 90 and 180 days, well-organized smooth muscle neointimal growth, and generally low injury and inflammation scores (Table 8). Inflammation was localized around stent struts and consisted mostly of macrophages with occasional multinucleated giant cells. Despite the aggressive oversizing (mean 61% relative to native diameter), there was no adventitial or granulomatous inflammation present.
▪ Preclinical: discussion
The healing observed for the implanted stent was excellent, especially noteworthy in the case of the overlapped stents where the oversizing was aggressive. Both fluoroscopy and IVUS showed stable stent diameter, which indicates a very low chronic outward acting force. This is in direct contrast to slotted-tube stents that slowly grow diametrically and approach the free state manufactured diameter. Endothelialization, histological scoring, and intimal thickness were favorable and similar between single and overlapped stents.
SAMBA clinical experience
The safety and performance of the SAMBA stent and delivery system in the treatment of femoro–popliteal lesions is assessed in a nonrandomized, prospective evaluation at two German centers (Herz-Zentrum Bad Krozingen and Park Krankenhaus Leipzig). The primary safety end point is freedom from all-cause death, unplanned index limb amputation, and target lesion revascularization (TLR) through 1 month. The primary efficacy end point of this interim analysis is 3-month stent patency determined by color duplex ultrasound with a peak systolic velocity ratio threshold of 2.5. Patients are followed through 12 months where other performance metrics are evaluated such as stent fracture, clinically driven TLR and change in Rutherford Classification.
▪ Patient selection
The study is designed to treat up to 40 patients with symptomatic atherosclerotic disease due to stenotic or occlusive femoro–popliteal lesions. Following informed consent, a patient is eligible for treatment if the inclusion and exclusion criteria are met. The major enrollment criteria are highlighted below (Box 1):
▪Target lesion over 50% diameter stenosis as demonstrated by angiography
▪ Lesion length not exceeding 15 cm
▪Reference vessel diameter between 5 and 6 mm
▪At least one vessel run-off prior to treatment
Methods
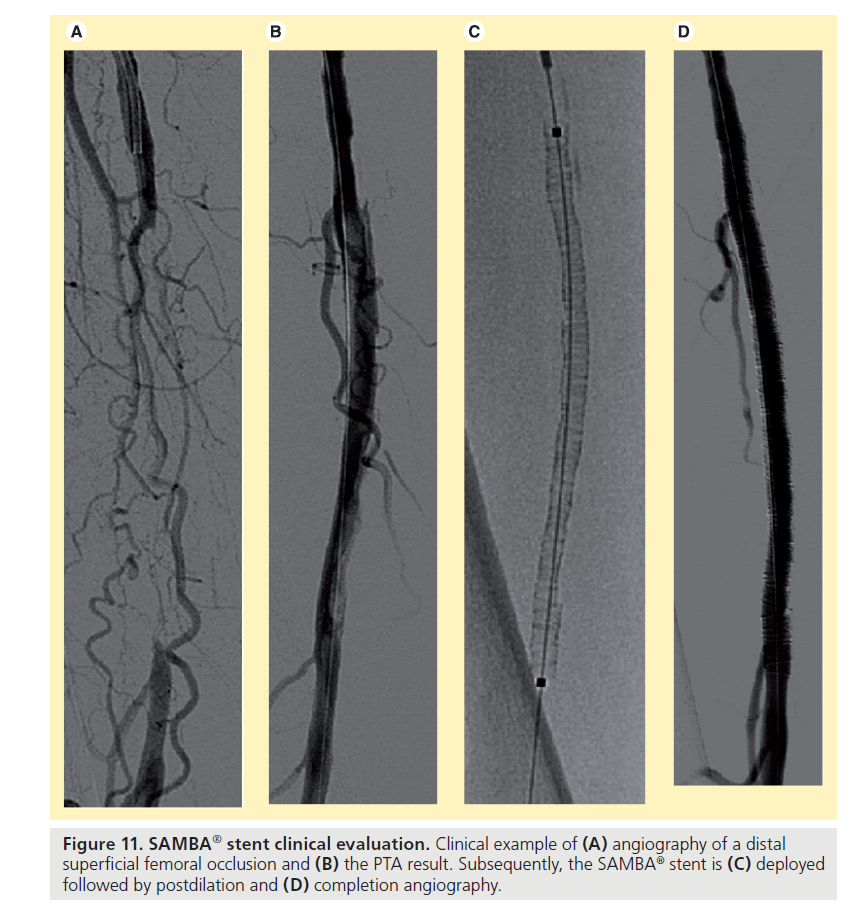
For arterial access a 7F sheath has to be placed. Placement of a guidewire across the lesion is achieved using standard interventional techniques. After careful predilation of the lesion with a balloon catheter matching the reference vessel diameter, the SAMBA stent and delivery system is inserted into the patient over a 0.018’’ guidewire, the stent is deployed, and the delivery system withdrawn. The stent is then postdilated segment wise with slowly increasing inflation pressure to assure good wall apposition and completion angiography confirms patency (Figure 11). Additional stents can be deployed as needed to cover the lesion with at least 1 cm extension, proximally and distally, into the healthy arterial segment. Stent sizes used had a maximum diameter of 7 mm and lengths of 5, 10 and 15 cm. For antiplatelet therapy, patients are administered 100 mg aspirin daily for an indefinite period and either ticlopidine (250 mg) or clopidogrel (75 mg) daily for 3 months following a loading dose.
Figure 11. SAMBA® stent clinical evaluation. Clinical example of (A) angiography of a distal superficial femoral occlusion and (B) the PTA result. Subsequently, the SAMBA® stent is (C) deployed followed by postdilation and (D) completion angiography.
Prior to discharge, at 1 and 3 months after the index procedure the patient received a physical examination, ankle-brachial index (ABI) measurement, and color duplex ultrasound to gather post-treatment data.
▪ Results
Baseline patient characteristics
During the trial, treatment with the device was attempted in 39 limbs in 38 patients. One patient was treated in both limbs, with approximately a 1‑month interval separating the treatment in each limb. Patients were evenly split with regard to gender with a mean age of 70 years. Hypertension, hypercholesterolemia and smoking were frequent comorbidities for the study population. Prior to treatment, the patients were Rutherford Category 2, 3, 4, and 5 with 97% of the patients in categories 3, 4, and 5. The target limb ABI prior to treatment was 0.62 ± 0.30.
Baseline lesion characteristics
The estimated reference vessel diameter was 5.3 ± 0.5 mm. The percentage diameter stenosis of the lesions was 91 ± 8% and the mean lesion length was 87 ± 41 mm. While the majority of lesions were located in the middle or distal superficial femoral artery, a substantial number of patients (21%) had lesions in the popliteal artery treated. In total, 66% of the patients had moderate or severe calcium in the target lesion.
Acute results
Technical success was achieved in 97% of limbs. A total of 49 stents were deployed in the 38 limbs, ranging from one to three stents per limb and yielding an average number of 1.3 stents per limb. At discharge, 79% of patients had an ABI increase of at least 0.1.
There were no major adverse events (i.e., death, stroke, periprocedural myocardial infarction [Q wave or non-Q wave], ipsilateral amputation, renal failure, abrupt lesion re-closure, need for an ipsilateral bypass graft, or TLR) during the procedure and prior to discharge. Additionally, through the 1-month period for the safety primary end point assessment, there were no major adverse events.
30-day results
The primary patency rate was 100% and the freedom from TLR was also 100%. Two patients did not return for 1-month follow-up. The mean ABI increased from 0.62 to 0.99. For the 30 patients for whom ABI data was available at 1 month, an increase of at least 0.1 relative to pretreatment was observed in 83% of patients. Nearly half of the patients were Rutherford Category 0, and two-thirds of the patients were Rutherford Category 0 or 1. Relative to pretreatment, two to four categories improvement was noted in 66% and at least one category improvement was achieved in 83% of the patients.
3-month results
The primary patency rate was 94%, and the freedom from TLR was 100%. 3-month follow-up data was unavailable for five patients. The mean ABI was 0.95, an increase of 0.33 relative to the pretreatment ABI. The proportion of patients with ABI increases of at least 0.1 relative to pretreatment was 81 and 81% of the patients were Rutherford Category 0 or 1. Two to four categories improvement was noted in 78% of patients. At 3 months, 88% of the patients had improvement of at least one Rutherford Category.
▪ Preliminary clinical
experience summary
The SAMBA stent demonstrated an excellent safety profile. A substantial number of patients experienced improvement in Rutherford Category and ABI relative to their condition pretreatment, and that improvement was sustained for at least 3 months. The patency rate at 3 months was comparable to the rate achieved by other stents that have received the CE Mark. Based on this successful clinical data and other supporting information, the SAMBA stent and delivery system has received the CE Mark.
Future perspective
Major challenges of acute success of femoro–popliteal interventions are resolved by using dedicated access techniques, crossing- and re-entry devices and durable stent designs. The major challenge to date is the durability of the clinical improvement following the endovascular procedure, which is still affected by a high restenosis rate. Recently, first promising data on stent based short time paclitaxel release has been presented [26]. The NovoStent SAMBA stent design is well-suited for serving as a drug carrier due to its high vessel coverage and with this homogenous drug distribution into the vessel wall. Moreover, the stent could be easily covered with two different drugs, one on each side, for example with an antiproliferative agent on the vessel wall opposed side and an endothelium cell growth stimulating drug on the vessel lumen opposed side. It is very likely that with different drug-releasing concepts – whether balloon based or stent based – the currently only remaining major challenge of endovascular therapy of femoro–popliteal lesions, restenosis/reocclusion will be solved within the next decade.
Conclusion
The dramatic improvements in axial, twist, and bending flexibility of the NovoStent SAMBA stent design over slotted-tube designs enable better conformance to the natural deformations of the FPA during normal body movements. In fact, even two completely overlapped SAMBA stents exhibit significant f lexibility advantages over slotted-tube designs. In addition, the SAMBA stent is more flexible and would exhibit much lower reactive forces to vascular deformations, potentially minimizing trauma and other mechanobiologic reactions. Also, although the SAMBA stent design is of comparable mass to slotted-tube designs, it exhibits dramatically greater radial strength and its resistance to radial constriction increases at smaller diameters, when it is needed most.
This increased f lexibility is the result of decreased material strains for the same associated anatomic deformations, which leads to superior fatigue resistance, as demonstrated by this and previous studies [20], compared with most slotted- tube stent designs. The SAMBA stent design survived 10 million cycles in both the bending and buckle/twist fatigue testing presented here and should therefore demonstrate a very low rate of clinical fracture.
Animal results have been encouraging (i.e., patency, healing and branch preservation). Higher metal surface area (over 50%) holds promise as a barrier to disease prolapse potentially reducing distal embolization while maintaining side branch patency.
An initial clinical evaluation in a single-arm trial is in the stage of collecting 12 months follow-up data and a postmarket registry is currently enrolling patients. Short-term data analysis revealed promising short-term safety and performance outcomes.
Executive summary
SAMBA® flexibility/conformability
▪ The stent masses of the SAMBA and slotted-tube designs are similar.
▪ The SAMBA stent has approximately one third the strut thickness and approximately three-times the area coverage of slotted-tube stents.
▪ Axial elongation:
- The SAMBA stent is 37-times and 207-times as flexible as the LifeStent® and SMART® stents, respectively.
▪ Axial compression:
- The SAMBA stent is 40-times and 184-times as flexible as the LifeStent and SMART stents, respectively.
▪ Axial twist:
- The SAMBA stent is 7.2-times and 15-times as flexible as the LifeStent and SMART stents, respectively.
▪ Bending:
- The SAMBA Stent is 6.9-times and 32-times as flexible as the LifeStent and SMART stents, respectively.
▪ Conformability:
- The SAMBA stent remains planar and circular in cross-section for 90° bend/180° twist and 180° bend/180° twist, while the SMART and LifeStent designs take on out-of-plane curves and/or noncircular cross-sections.
SAMBA strength:
▪ Diametric crush:
- For 25% diametric crush, the SAMBA stent is 2.2-times and 2.1-times as strong as the SMART and LifeStent stents, respectively.
- For 83% diametric crush, the SAMBA Stent is 2.3-times and 2.1-times as strong as the SMART and LifeStent stents, respectively.
▪ Radial force:
- At a diameter of 6 mm (for these 7 mm stents), the force that the SAMBA stent resists radial constriction is 2.4-times and 2.3-times that of the SMART and LifeStent stents, respectively.
- At a diameter of 5 mm, the SAMBA stent resists radial constriction 3.3-times and three-times as much as the SMART and LifeStent stents, respectively.
SAMBA fatigue resistance
▪ Twist:
- SAMBA stent endured 10 million cycles with no fractures.
▪ Bending:
- The SAMBA stent and LifeStent samples endured 10 million cycles (equivalent to 10 years) with no fractures. The SMART sample displayed fractures at 17,045 cycles (equivalent to 6 days).
▪ Buckle and twist:
- The SAMBA stent and LifeStent samples endured 10 million cycles (equivalent to 10 years) with no fractures. The SMART sample displayed fractures at 172,078 cycles (equivalent to 2 months) and complete separation into two parts at 495,008 cycles (equivalent to 6 months).
SAMBA preclinical experience
▪ Single stents:
- All devices were patent at follow-up.
– Percent stenosis via intravascular ultrasound and angiography were strongly correlated and the stent diameter was stable over the follow-up period.
- Angiography demonstrated branch vessel preservation at implantation and chronically.
- Histology showed 99% re-endothelialization at 90 days, well-organized smooth muscle neointimal growth, and low injury and inflammation scores.
- No stent fractures.
▪ Overlapped stents:
- All stents were patent, no fractures were observed.
- Branch vessel preservation was demonstrated by angiography and scanning electron microscopy.
- Histology showed 90% re-endothelialization at 30 days and 100% at 90 and 180 days, well-organized smooth muscle neointimal growth, and generally low injury and inflammation scores.
SAMBA clinical experience
▪ Clinical use of the SAMBA stent is safe and was technically successful in 97% of the cases despite a challenging patient population.
▪ All but one device were patent at 3‑month follow-up.
▪ The technical success was translated in a substantial improvement of clinical status of the patients at 3‑month follow-up regarding the change in Rutherford Category with a 0% target lesion revascularization rate.
Financial & competing interests disclosure
M Waldo and C Cheng are employees of NovoStent Corporation and receive salary compensation. D Scheinert has been granted a minor number of stock options. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Norgren L Hiatt WR, Dormandy JA et al.: Inter-society consensus for management of peripheral arterial disease (TASC II). Eur. J. Vasc. Endovasc. Surg. 33(Suppl.), S1–S75 (2007).
- Hirsch AT, Haskal ZJ, Hertzer NR et al.: ACC/AHA 2005 Guidelines of the Management of Patients with PAD (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J. Am. Coll. Cardiol. 47(6), 1239–1312 (2006).
- Duda SH, Bosiers M, Lammer J et al.: for the SIROCCO study group. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. J. Vasc. Intervent. Radiol. 16, 331–338 (2005).
- Krankenberg H, Schlüter M, Steinkamp HJ et al.: Nitinol stent implantation vs. percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation 116, 285–292 (2007).
- Zeller T, Saratzis N, Scheinert D et al.: Non-randomized, prospective, multi-centre evaluation of the ABSOLUTE™ .035 Peripheral Self-Expanding stent system for occluded or stenotic superficial femoral or proximal popliteal arteries (ASSESS Trial): Acute and 30-day results. J. Cardiovasc. Surg. (Torino) 48, 719–726 (2007).
- Zeller T, Tiefenbacher C, Steinkamp HJ et al.: Nitinol stent implantation in TASC A and B superficial femoral artery lesions: the Femoral Artery Conformexx Trial (FACT). J. Endovasc. Ther. 15, 390–398 (2008).
- Schillinger M, Sabeti S, Dick P et al.: Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation 155, 2745–2749 (2007).
- Laird JR, Katzen BT, Scheinert D et al.: Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery. Cardiovasc. Interv. 3, 267–276 (2010).
- Schillinger M, Sabeti S, Loewec et al.: Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N. Engl. J. Med. 354, 1879–1888 (2006).
- Bosiers M, Torsello G, Giler H-M et al.: Nitinol stent implantation in long superficial femoral artery lesions: 12-month results of DURABILITY I study. J. Endovasc. Ther. 16, 261–269 (2009).
- Duda SH, Pusich B, Richter G et al.: Sirolimus-eluting stents for the treatment of obstructive superficial femoral artery disease Circulation 106, 1505–1509 (2002).
- Duda SH, Bosiers M, Lammer J et al.: Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. J. Vasc. Interv. Radiol. 16, 331–338 (2005).
- Scheinert D, Scheinert S, Sax J et al.: Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J. Am. Coll. Cardiol. 45, 312–315 (2005).
- Schlager O, Dick P, Sabeti S et al.: Longsegment SFA stenting-the dark sides: in-stent restenosis, clinical deterioration, and stent fractures. J. Endovasc. Ther. 12, 676–684 (2005).
- Lugmayr HF, Holzer H, Kastner M et al.: Treatment of complex arteriosclerotic lesions with nitinol stents in the superficial femoral and politeal arteries: a midterm follow-up. Radiology 222, 37–43 (2002).
- Cheng CP, Wilson NM, Hallett RL, Herfkens RJ, Taylor CA: In vivo MR angiographic quantification of axial and twisting deformations of the superficial femoral artery resulting from maximum hip and knee flexion. J. Vasc. Interv. Radiol. 17, 979–987 (2006).
- Cheng CP, Choi G, Herfkens RJ, Taylor CA: The effect of aging on deformations of the superficial femoral artery due to hip and knee flexion: potential clinical implications. J. Vasc. Interv. Radiol. 21(2), 195–202 (2010).
- Nikanorov A, Smouse HB, Osman K, Bialas M, Shrivastava S, Schwartz LB: Fracture of self-expanding nitinol stents stressed in vitro under simulated intravascular conditions. J. Vasc. Surg. 48(2), 435–440 (2008).
- Mechanical properties of nitinol stents and stent-grafts. Gore Brochure 1–11 (2007).
- Walker CM: Wire-interwoven nitinol. Endovascular Today 21–24 (2007).
- Smouse HB, Nikanorov A, Osman K, Bialas M, Shrivastava S, Schwartz LB: Fracture of self-expanding nitinol stents stressed in vitro under simulated intravascular conditions. Presented at: Transcatheter Therapeutics (TCT) 20–25 October, 2007.
- Timmins LH, Meyer CA, Moreno MR, Moore JE: Effects of stent design and atherosclerotic plaque composition on arterial wall biomechanics. J. Endovasc. Ther. 15, 643–654 (2008).
- Iida O, Nanto S, Uematsu M et al.: Effect of exercise on frequency of stent fracture in the superficial femoral artery. Am. J. Cardiol. 98, 272–274 (2006).
- Pelton AR, Gong XY, Duerig T: Fatigue testing of diamond-shaped specimens. SMST-2003. Proc. Int. Conf. Shape Memory Superelastic Technol. 293–302 (2004).
- Zeller T, Johnson A, Cheng CP, Martin GR: Evaluation of Novostent’s SAMBA stent. Am. J. Cardiol. 104, 220D (2009).
- Dake MD: The Zilver PTX randomized trial: 12-month results of a paclitaxel-eluting stent versus balloon angioplasty and bare metal stenting in patients with femoropopliteal artery disease. Presented at: Transcatheter Cardiovascular Therapeutics (TCT). Washington DC, USA 21–25 September, 2010.