Case Report - Interventional Cardiology (2019) Volume 11, Issue 6
The occlutech duct occluder: How to choose the correct device size and refine implantation technique to ensure optimal results in small and large arterial duct closure
- Corresponding Author:
- Christopher Duke
Sheikh Khalifa Medical City
Abu Dhabi, UAE
E-mail: cduke@seha.ae
Received date: November 08, 2019; Accepted date: November 22, 2019; Published date: November 28, 2019
Abstract
Background: Previous studies on the Occlutech Duct Occluder (ODO) have established the safety and efficacy of duct occlusion in small patient cohorts, without clearly defining the technique required for successful device implantation. The objective of this study was to describe in detail how to size and implant the device, evaluating the proposed technique in a large patient cohort, including patients with large ducts (≥4 mm). Methods and findings: Transcatheter arterial duct (PDA) occlusion was attempted in 166 successive patients with a PDA ≥ 1 mm diameter. For PDAs<4 mm, the ODO was oversized by 1-2 mm. For PDAs ≥ 4 mm, the ODO was oversized by 2-4 mm. The device was deployed with light pressure and angiography was used to demonstrate its position. Intention to treat and as treated procedural success rates were 159/166 (96%) and 159/161 (99%). Success rates were 71/73 (97%) and 72/73 (99%) in patients with large ducts. All implants achieved complete PDA occlusion by the following day. At median follow up of 10.6 (range 1.6-13.1) months echocardiography showed no residual shunts. One device embolised immediately and was successfully retrieved by snaring. Devices were median 1.9 (range 1.1-5.2) mm larger than ducts<4 mm and median 3.3 (0.9-6.4) mm larger than ducts ≥ 4 mm. In 46/89 (52%) ducts<4 mm the device was oversized by only 1 mm. Conclusion: Safe and effective occlusion is achieved when the ODO is oversized by 1-2 mm in ducts<4 mm in diameter and 2-4 mm in ducts ≥ 4 mm in diameter. The majority of ducts<4 mm in diameter can be safely occluded with a device oversized by only 1 mm. Correct device position can be determined angiographically, without regard to compression of the device within the duct. The device and techniques are equally safe and effective for large ducts.
Keywords
Transcatheter arterial duct • Echocardiography • Embolisation
Introduction
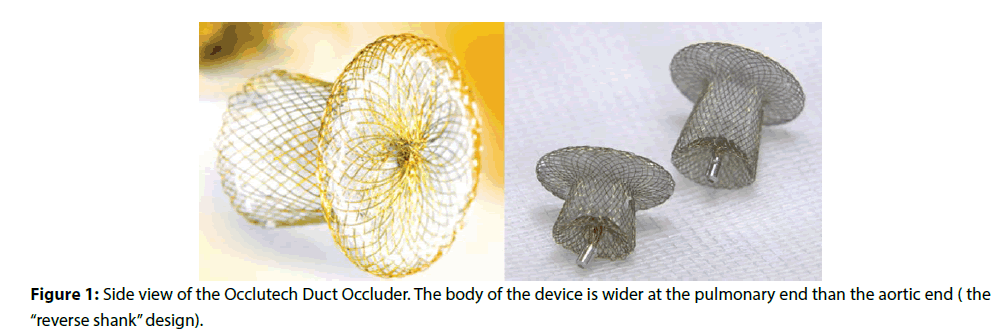
The Occlutech PDA Occluder (ODO) is a new device for patent arterial duct (PDA) occlusion. In common with other PDA devices, it has an expandable nitinol body and an aortic retention disc, but its distinctive feature is that the body of the device is wider at the pulmonary end than the aortic end (Figure 1). This “reverse shank” design is intended to enhance device stability in the duct and minimise the risk of embolisation. The device comes in 2 lengths, the standard device and the “long shank” device, designed for ducts with a long ductal ampulla.
Early publications on the ODO described the technique required to implant the device and demonstrated successful implantation in 96%-100% of patients, with a device embolization rate of up to 4% [1-3]. Although there is often a residual shunt through the device immediately following implantation, excellent occlusion rates have been reported at 1 day (82%-97%), 1 month (96%-100%) and 6 months (96%-100%) follow up [1-5]. However, existing studies include only small numbers of patients and a limited number with large ducts (defined as PDA ≥ 4 mm in diameter).
To date, there is no clear guidance on how to select the size of an ODO. The relevant diameter of the ODO for sizing purposes is the diameter of the core of the device at the aortic end. Most operators elect to implant devices at least 2 mm larger than the narrowest point of the duct [2,4,5]. This sizing strategy reflects existing experience with the Amplatzer duct occluder (St Jude Medical, St Paul, MN) and may not be correct for the ODO. Kudumula et al. suggested that the ODO remains stable and effective when the device diameter is only 1 mm larger than the duct diameter, but most of the ducts in their series were small. They also suggested that duct length should not exceed device length by more than 4 mm, to ensure that the pulmonary artery end of the device reaches the pulmonary artery, which is essential for device stability [1]. These diameter and length sizing strategies have not been tested in a large patient cohort and remain untested in patients with large ducts.
The technique of ODO implantation remains unfamiliar to some operators, who are used to firm traction to deploy the Amplatzer duct occluder, and rely on device compression within the duct to determine correct position. The success of light traction and evaluation of device position with only angiography requires further evaluation.
The aim of this study was to assess whether selecting an ODO 1-2 mm larger than ducts<4 mm in diameter and 2-4 mm larger than ducts>4 mm in diameter and deploying the ODO with light pressure, using only angiography as guidance, was an effective strategy to achieve safe and effective device closure in a large patient cohort. The secondary aim was to assess the performance of the device and these techniques in closing large ducts.
Methods
Transcatheter PDA occlusion was attempted in 166 successive patients found to have a PDA ≥ 1 mm diameter on angiography. There was an intention to treat all patients with the ODO. Cardiac catheterisation was not offered to patients weighing less than 6 kg or patients with other cardiac problems requiring surgery. Cases were carried out at a single institution over a 32 month period. Results were analysed retrospectively.
Technical Aspects
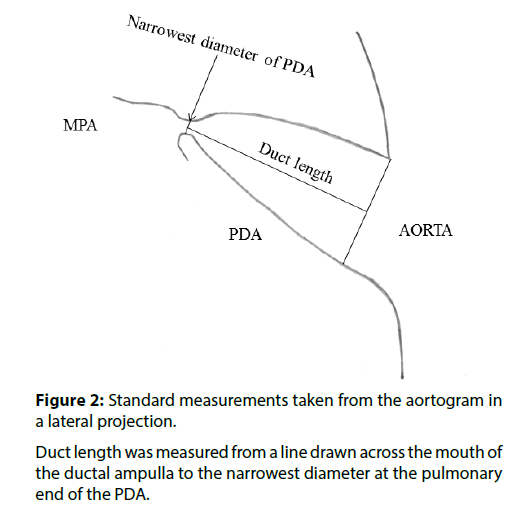
Procedures were carried out under general anaesthesia (age less than 3 years) or local anaesthesia with sedation (age greater than 3 years). A single plane cardiac catheterisation laboratory was used. Vascular access was via the femoral artery and vein. 100 Units/kg of intravenous heparin was administered. An aortogram in lateral projection was performed opposite the duct using a pigtail catheter. Angiographic images were calibrated against catheter size. The minimum diameter and the length of the duct were measured (Figure 2). The duct was crossed from the venous side and device implantation was carried out according to the instructions for use provided with the ODO, using a standard approach [3,6]. A Mullins sheath (William Cook Europe, Bjaeverskov, Denmark) was used to deploy the device. ODO size was chosen according to the guidelines in Table 1. When the minimum diameter of the duct was<4 mm, the ODO was “oversized” by 1-2 mm (i.e. 1-2 mm was added to the minimum diameter of the duct and the ODO that matched or slightly exceeded that size was chosen). When the minimum diameter of the duct was ≥ 4 mm, the ODO was oversized by 2-4 mm. Device implantation and assessment of device position within the duct were carried out according to the guidelines in Table 1. Shunt calculations were not made as they can be inaccurate in the presence of a PDA and did not impact on decision making. The pulmonary artery pressure was measured before device implantation. An aortogram in lateral projection was carried out 1 minute after implantation (Moving Image 1). If the main pulmonary artery was not well opacified from flow through the device during the aortogram, a pulmonary artery angiogram was carried out in lateral projection by injecting contrast through the side arm of the Mullins sheath (Moving Image 2). Once device position was confirmed, the ODO was released from the delivery cable, if necessary, rotating the delivery cable by holding it with mosquito forceps.
| Choosing device size |
|
|
Do not implant if the PDA is>4 mm longer than the long shank device |
| Deploying the device |
|
|
| Checking Position after implantation |
|
|
Table 1: The Principles of Occlutech PDA device Implantation.
Dataset
The medical records were reviewed for demographic characteristics, clinical details, echocardiographic findings, complications related to the procedure and follow up information. The initial aortogram was used to assess ductal morphology and to measure the minimum diameter of the PDA. A duct was defined as large if its minimum diameter was ≥ 4 mm. The size of Mullins sheath used, the size of device implanted and any technical difficulties encountered during the procedure were also noted. Patients were evaluated clinically and echocardiographically 1 day, 2 days, 2 weeks and 3 months following device implantation. Earlier patients in the series have also been assessed 1 year after device implantation. Echocardiographic evaluation included assessment of residual shunting, left pulmonary artery patency and descending aorta patency.
Statistics
Parametric data is expressed as mean +/- standard deviation and non-parametric data as median and range. Success rates were compared using a Pearson Chi Square test, with p<0.05 indicating statistical significance. Statistical analyses were performed with SPSS 19.
Results
Efficacy and safety
Patient demographics, clinical details and procedural data are summarised in Table 2. On an intention to treat basis, procedural success rate was 159/166 (96%). On an as treated basis success rate was 159/161 (99%). Every patient who had successful device implantation had complete occlusion of the duct on echocardiography the following day. On follow-up, there were no clinical problems and echocardiography demonstrated no residual shunts. There was no left pulmonary artery or descending aorta obstruction. The median follow-up time was 10.6 (range 1.6-13.1) months.
| Number of patients | 166 |
|---|---|
| Number of ODO devices successfully implanted | 159 (95%) |
| Age (years) | Median 5.5 (range 1-52) |
| Weight (kg) | Median 16 (range 7-96) |
| Associated cardiac abnormalities | 1 (0.7%) |
| Clinical evicence of cardiac failure | 166 (100%) |
| Echocardiographic evicence of left heart volume overload | 166 (100%) |
| PDA narrowest diameter (mm) | Median 3.7 (range 1-12) |
| PDA<4 mm diameter | 93/166 (56%) |
| Large PDA ≥ 4 mm diameter | 73/166 (44%) |
| Krichenko A morphology | 161 (96.4%) |
| Krichenko B morphology | 1 (0.6%) |
| Krichenko C morphology | 4 (2.4%) |
| Krichenko D morphology | 1 (0.6%) |
| Mean PA pressure before device occlusion (mmHg) | Median 26 (range 11-69) |
| Local anaesthesia and sedation | 96 (58%) |
| General anaesthesia | 70 (42%) |
| Device embolisation | 1 (0.6%) |
| Transient femoral artery occlusion | 2 (1.2%) |
Table 2: Patient demographics, clinical details and procedure related data.
73/166 (44%) patients had a large duct. In this subgroup, median age was 10 (range 1-52) years, median weight 29 (range 8-96) kg and median PDA size 5.2 (range 4-12) mm. On an intention to treat basis, procedural success rate was 71/73 (97%). On an as treated basis success rate was 72/73 (99%). There was no significant difference between success rates in the subgroup with large ducts and the subgroup with ducts<4 mm in diameter. All patients who had successful closure had a Krichenko type A duct [7].
In 5 cases there was no attempt to implant an ODO. Three of these patients had a small long PDA (minimum diameters: 1.0, 1.2, 1.6 mm). Closure was carried out with a single Mreye Flipper detachable embolization coil (William Cook Europe, Bjaeverskov, Denmark). One patient had a large, 6.8 mm diameter, Krichenko Type B duct (window morphology). The operator chose to implant an 8 mm Amplatzer muscular VSD device (St Jude Medical, St Paul, MN) as he was unsure whether an ODO would be stable in a very short duct. One patient had a 3.9 mm diameter Krichenko Type C duct (tubular morphology). As the length of the duct exceeded the 8/10 “long shank” device length by more than 4 mm, the operator considered that the ODO was unlikely to reach the pulmonary artery. The procedure was therefore abandoned and a plan was made to close the PDA at a later date with an alternative device.
In 2 cases ODO implantation was attempted, but was unsuccessful. One patient, aged 6 years, weighing 22 Kg, had a large, 7.8 mm diameter, Krichenko Type C duct. A 12/15 ODO pulled straight through the PDA during deployment with very light pressure. As the 12/15 ODO was the largest device in stock at that time and the PDA seemed expansile, the device was removed on its cable and the patient was sent for surgical duct ligation. The other patient had an ODO implanted, but the device embolised to the descending aorta. The ODO was successfully retrieved and the patient had surgical duct ligation. This was the only device related complication. Two patients had complications related to vascular access; reduced limb perfusion following the procedure resolved after a heparin infusion in one and streptokinase in the other.
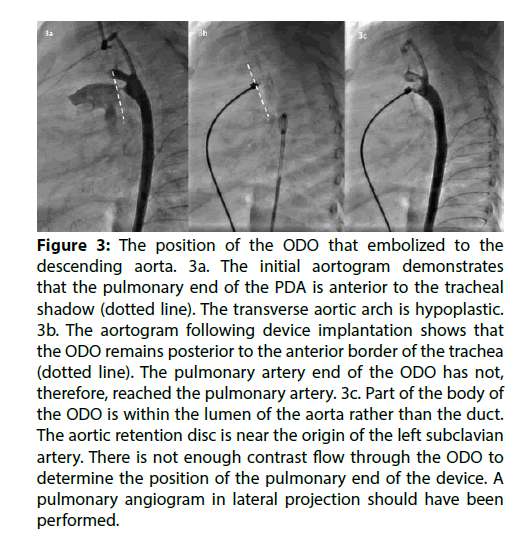
The patient in whom the ODO embolised was 2.5 years old weighing 7.8 kg. The duct was Krichenko Type A morphology, with a minimum diameter of 1.5 mm. It was closed with a 3.5/5 ODO. The device embolised to the descending aorta immediately following release from the delivery cable. It was retrieved from a femoral artery approach. The ODO was moved with a catheter until its orientation was favourable, then the screw thread of the device was captured with an EN Snare (Merit medical Systems Inc., South Jordan, UT, USA) using a retrograde approach. The device was retracted into a 6 French sheath, without vascular damage. The retrieval took approximately 20 min. Retrospective review of the angiograms revealed that the pulmonary artery end of the ODO had not reached the pulmonary artery during implantation (Figure 3 and Moving Image 3).
Figure 3: The position of the ODO that embolized to the descending aorta. 3a. The initial aortogram demonstrates that the pulmonary end of the PDA is anterior to the tracheal shadow (dotted line). The transverse aortic arch is hypoplastic. 3b. The aortogram following device implantation shows that the ODO remains posterior to the anterior border of the trachea (dotted line). The pulmonary artery end of the ODO has not, therefore, reached the pulmonary artery. 3c. Part of the body of the ODO is within the lumen of the aorta rather than the duct. The aortic retention disc is near the origin of the left subclavian artery. There is not enough contrast flow through the ODO to determine the position of the pulmonary end of the device. A pulmonary angiogram in lateral projection should have been performed.
Device sizing
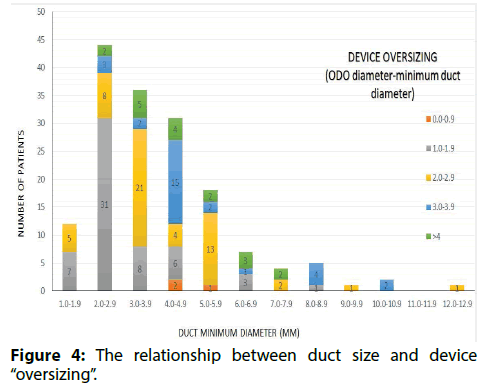
Figure 4 shows the relationship between duct size and device oversizing. In 137/161 (85%) ODO implants the operator adhered to the sizing strategy in Table 1. Devices were a median of 1.9 (range 1.1-5.2) mm larger than ducts<4 mm in diameter and 3.3 (0.9-6.4) mm larger than ducts ≥ 4 mm in diameter. In 46/89 (52%) small ducts the operator oversized the device by only 1 mm. In 12/72 (17%) large ducts the device was oversized by less than 2 mm (in this subgroup device size was a mean of 1.4 +/- 0.4 mm larger than duct size). A long shank device was implanted in 1 case, where the length of the duct exceeded the length of the standard device by more than 4 mm.
Procedural issues
In every case where an ODO was successfully implanted, angiography showed whether the device was well applied to the walls of the duct and whether the pulmonary artery end of the ODO had reached the pulmonary artery. Shunt around the device was not observed in any case, though shunt through the body of the device was almost universal on the initial check angiogram. Minor technical difficulties were encountered. A standard short introducer sheath was used as a loader for the device. If the tip of the short sheath was pushed too far through the bung of the Mullins sheath, it became longitudinally crimped, making it impossible to advance the device into the long sheath. This problem was overcome with experience, by advancing the short sheath only about 5 mm through the bung of the Mullins sheath. Sometimes there were problems with the delivery cable. Either the vise at the distal end of the delivery cable would not grip the cable or rotational torque would not pass down the cable during attempts release the device. In these cases, the device was released by holding the cable near the sheath with mosquito forceps and spinning the forceps around the axis of the cable. In one case, when the ODO was pulled forcefully into the PDA, the aortic retention disc took the shape of a cone rather than a disc. The body of the device did not expand completely. When the device was recaptured and deployed with less traction, allowing the aortic retention disc to remain flat, the body of the device expanded normally (Moving Images 4 and 5).
Discussion
In 85% of the ODO implants device size was selected according to the principles in Table 1.
In certain cases, the strategy was not followed, owing to operator preference or the availability of device sizes. Our results demonstrate that the sizing strategy is safe and effective. The observation that devices only needed to be oversized by 1 mm in the majority of ducts<4 mm in diameter is significant, as it allows smaller devices to be implanted in infants, where the size of the aortic retention disc should be minimised to avoid aortic obstruction. It is also possible that devices implanted into ducts ≥ 4 mm in diameter do not need to be oversized by as much as 2-4 mm. In this regard, it is important to bear in mind that the pulmonary artery end of the ODO is 2 mm wider than the aortic end, so a 1 mm oversizing strategy means that the pulmonary artery end of the device is 3 mm larger than the narrowest part of the duct, which may be adequate for stability. Our cohort includes 12 large ducts successfully occluded by devices oversized by less than 2 mm. In a recent series of 8 patients with very large ducts, 5 were successfully closed with an ODO only 1 mm larger than the minimum ductal diameter, even though 3 of those patients had a PDA>10 mm in diameter [8]. Although this data suggests that 1 mm oversizing might be adequate even for large ducts, it may prove difficult to evaluate such a sizing strategy in practice, as operators may be reluctant to use a small device when the aorta can accommodate the retention disc of a larger one, because it is not yet known whether minimal oversizing increases the risk of embolization. Indeed, device embolization has already been described in a patient with a 10.5 mm duct who received a 12/15 ODO [3].There may also be pressure to avoid the additional expense that can occur if a second larger device has to be opened after a smaller device pulls through.
Some operators in the early phase of their experience with the ODO have expressed concern that they find it difficult to determine whether the device is correctly positioned [9]. This problem arises because operators familiar with the Amplatzer Duct Occluder look for “apple core” compression of the body of the device to evaluate whether it is across the narrow point of the duct. This apple core sign cannot be used to check the position of the ODO, because it already has an apple core shape before it is implanted. Our study has demonstrated that the apple core sign is not required. Angiography is sufficient to demonstrate that the device is in a stable position, using the principles in Table 1. Our case where the device embolised to the aorta emphasises the importance of checking that the pulmonary artery end of the device has reached the pulmonary artery. The authors contend that this is the single most important factor in determining whether the device has been correctly implanted. Operators should use contrast injection through the side arm of the long sheath to evaluate the pulmonary artery end of the device if there is any doubt following the post implantation aortogram. Previous studies evaluating the Occlutech Ductal Occluder reported procedural success rates of 96%-100% and occlusion rates of 95%-100% on follow up [1-5]. These findings have been confirmed in our larger cohort. Although some studies have described high occlusion rates only at 1 month follow up, we observed complete occlusion in all cases 1 day following ODO implantation, as described in another recent series [3]. The shunt seen through the device immediately following implantation was of no clinical importance. Our results demonstrate that the device is equally effective in patients with large ducts. The number of large ducts in this cohort is greater than in the other published series. This may be because patients attending the authors’ hospital for PDA closure are older than those seen in more developed health care systems and more likely to present with symptoms rather than a murmur detected on routine examination.
While PDA size is not a significant factor in procedural success, the morphology of the duct may be important. The data in this study demonstrates that the ODO is effective in closing Krichenko type A ducts, but it does not show whether the device is effective in closing ducts with alternative morphologies, as there were very few such ducts in this study group. Other publications have described successful closure of Krichenko type B, C, D and E ducts using the ODO, but the numbers were too limited to analyse procedural success rates according to PDA morphology [1-5,10]. The published series also document unsuccessful ODO implantation in 3 cases with type C morphology. In one case an ODO had to be withdrawn because it protruded into the aorta and in 2 cases devices embolised to the pulmonary artery [3-5]. In one of the embolisation cases, the entire device was implanted inside the duct. Our 2 cases with type C ducts were also unsuccessful. In one case the ODO pulled through the duct and in the other an ODO was not implanted because the duct was so long that it was thought the ODO would not reach the pulmonary artery. We suggest that this device is not suitable for long Krichenko type C ducts. If the ODO is retracted into the PDA with minimal force, so that its retention disc remains in the aorta, the core of the device may not reach the pulmonary artery, which carries a risk of embolisation to the aorta. If the ODO is firmly retracted into the PDA, so that the aortic retention disc is distorted or constrained within the duct, then the core of the device may fail to expand, which carries a risk of residual shunting and device embolisation to the pulmonary artery.
The screw of the Amplatzer Duct Occluder cannot usually be snared when the device needs to be retrieved, as it is recessed into the body of the device [11]. This series documents successful retrieval of an ODO and demonstrates that the screw, which is not recessed, can be easily snared to extract the device. This resulted in a quick retrieval, though others have described difficulty when the screw is not in an accessible position, particularly when the device embolizes to the right pulmonary artery with the screw in a distal position and the device cannot be turned [3,5].
Conclusion
When selecting the size of the ODO, safe occlusion is achieved when the device is oversized by 1-2 mm in ducts<4 mm in diameter and 2-4 mm in ducts ≥ 4 mm in diameter. The majority of ducts<4 mm in diameter can be safely occluded with a device oversized by only 1 mm. Correct device position can be determined angiographically, without regard to compression of the device within the duct. It is particularly important to check that the pulmonary artery end of the device has reached the pulmonary artery. Using these strategies, implantation was successful in 96% cases and in these cases 100% occlusion was observed. The device and techniques are equally effective for large ducts.
References
- Kudumula V, Taliotis D, Duke C. The new occlutech duct occluder: immediate results, procedural challenges, and short-term follow-up. J Invasive Cardiol. 2015;27(5):250-7.
- Abdelbasit MA, Alwi M, Kandavello G, et al. The new Occlutech(R) PDA occluder: initial human experience. Catheter Cardiovasc Interv. 2015;86(1):94-9.
- Pepeta L, Greyling A, Nxele MF, et al. Patent ductus arteriosus closure using occlutech(R) duct occluder, experience in Port Elizabeth, South Africa. Ann Pediatr Cardiol. 2017;10(2):131-6.
- Dedeoglu R, Bilici M, Demir F, et al. Short-term outcomes of patent ductus arteriosus closure with new occlutech(R) duct occluder: A multicenter study. J Interv Cardiol. 2016;29(3):325-31.
- Boudjemline Y. The new occlutech((R)) patent ductus arteriosus occluder: single centre experience. Arch Cardiovasc Dis. 2016;109(6):384-9.
- Masura J, Walsh KP, Thanopoulous B, et al. Catheter closure of moderate to large-sized patent ductus arteriosus using the new Amplatzer duct occluder: immediate and short-term results. J Am Coll Cardiol. 1998;31(4): 878-82.
- Krichenko A, Benson LN, Burrows P, et al. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. 1989;63(12):877-80.
- Lehner A, Ulrich S, Happel CM, et al. Closure of very large PDA with pulmonary hypertension: Initial clinical case-series with the new Occlutech(R) PDA occluder. Catheter Cardiovasc Interv. 2017;89(4):718-25.
- Rios-Mendez RE. The new occlutech duct occluder. J Invasive Cardiol. 2015;27(10):229.
- Bilici M, Demir F, Akin A, et al. Transcatheter closure of patent ductus arteriosus in children with the occlutech duct occluder. Pediatr Cardiol, 2017.
- Duke C, Chan KC. Aortic obstruction caused by device occlusion of patent arterial duct. Heart. 1999;82(1):109-11.