Research Article - Clinical Practice (2018) Volume 15, Issue 4
The patient burden of opioidinduced bowel dysfunction
- Corresponding Author:
- Christina Brock
Department of Gastroenterology and Hepatology
Aalborg University Hospital, Aalborg, Denmark
E-mail: christina.brock@rn.dk
Abstract
Orally formulated opioids are commonly prescribed analgesics to treat severe pain. Among severe adverse effects are respiratory depression, sedation and opioid-addiction; less dangerous, however still burdensome for the many treated patients, are symptoms such as nausea, cognitive impairment and bowel dysfunction. It has e.g. been shown that the patient experienced burdens of opioid induced bowel dysfunction (OIBD) and opioid induced constipation (OIC) negatively impact the quality of life of these patients. Consequently, patients often take less medication than prescribed to dampen these adverse effects. This review focuses a thorough description of the underlying mechanistic causes of OIBD, originating from binding of exogenous opioids to the peripheral opioid receptors in the enteric nervous system of the gut. Consequently, the bowel becomes dysfunctional function changes caused by alterations in i) motility, ii) secretion and absorption and iii)sphincter functions. Such alterations lead to a variety of symptoms including gastro-esophageal reflux, abdominal pain, prolongation of transit times and dry hardened stool. Thus, the experienced psychological burden of opioid-induced bowel dysfunction is a net-result of its physical occurrence as well as its negative impact on health-related quality of life. Unfortunately, gastrointestinal dysfunction is considered a taboo and people may refrain to describe and discuss the experienced burdens. This review targets health care professionals, who to a higher degree are encouraged to discuss openly if presence of OIBD/OIC impacts the daily life and social activities of the patients. Finally, different mechanistic treatment approaches are reviewed in order to provide clinicians with different pharmacological options and strategies, which include traditional laxatives, prokinetics and co-administration of opioid antagonists. Even though the opioid-antagonists have proved to be superior to placebo in the relief of OIBD/OIC, further clinical studies are needed in order to optimize treatments for the benefit of future patients.
Keywords
opioids, gastrointestinal induced bowel dysfunction, opioid-induced, opioid induced bowel constipation, OIBD, OIC, burden, symptomatology
Introduction
Despite the originally intended use of opioids, limited to acute severe pain such as e.g. post-operative pain and in terminal palliative treatments, orally formulated opioids are commonly prescribed to treat chronic non-malignant pain in Western countries. Consequently, in the U.S. more than 240 million opioid prescriptions are dispensed per year, with the majority on back pain and other musculoskeletal causes [1,2], and thus the prescription of opioids has quadrupled over the past three decades [1,3,4]. Different explanations have been suggested and prescription behaviour among clinicians varies not only between countries, health-care institutions but also between individuals. Unsurprisingly, the numbers of patients experiencing adverse effects is increasing. Severe adverse effects of opioids are respiratory depression, sedation and opioidaddiction [5]. Less dangerous, however still detrimental and burdensome are the numbers of serious adverse effects experienced by patients in response to long-term opioid use such as sedation, nausea, cognitive impairment and bowel dysfunction.
Opioid induced bowel dysfunction and opioid induced constipation
The opioid drugs, typified by morphine, create their pharmacological actions, including analgesia, by binding on receptors located on neuronal cell membranes in the spine and brain. Empirically, tolerance, which still needs further clarification, develops quickly to most opioidinduced side effects originating in the brain, e.g. such as nausea and cognitive impairment. As in the CNS, opioids also binds to peripherally (outside the CNS) acting mu-opioid receptors, such as those in myenteric and submucosal plexuses of the entire wall of the gastrointestinal (GI) tract. Here, tolerance is not seen and treatment may results in clinically significant adverse effects known as opioid induced bowel dysfunction (OIBD) [6,7]. This potentially interferes with GI motility, secretion and sphincther function [6,8,9], and may can lead to debiliating symptoms.
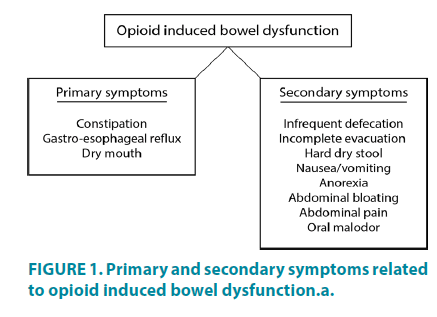
The primary symptomatology of OIBD comprises opioid induced constipation (OIC), however secondary symptoms such as dry mouth, gastro-esophageal reflux, vomiting, bloating, abdominal pain arising from luminal stretch, anorexia, hard and dry stools, and incomplete evacuation are all included in the OIBD (FIGURE 1) [8,10].
OIBD and OIC are under-diagnosed, yet common and debilitating adverse effect of opioid treatment. In placebo trials, OIC occurs in 11% of patients, whereas the chronic constipation frequency ranges from 33 to 94% in non-cancer and cancer opioid-treated patients [9,11-16]. In attempts to avoid the burdens of such bowel dysfunction these patients sometimes decrease their opioid use, and thereby risk to increase their pain. In the Patient Reports of Opioidrelated Bothersome Effects (PROBE) trial, 322 patients with chronic pain in the US and EU were included, and one third of these stated that they had missed doses, decreased the dose of or stopped using opioid medication in order to relieve bowel-related side effects. Subsequently, 92% of the patients experienced increased pain, of whom 86% reported reduction in their Quality of Life (QoL) and daily activities[9,12,17] . These findings are supported by an American survey where more than half of the patients took less opioid than prescribed due to adverse effects [18]. Even though, health care providers seems to underestimate the burden of OIBD, it is obvious that OIC leads to decreased QoL, hospitalizations and more absence from work, which prevents effective clinical management and increases socio-economic burdens.
Mechanisms of opioids in general on the enteric nervous system
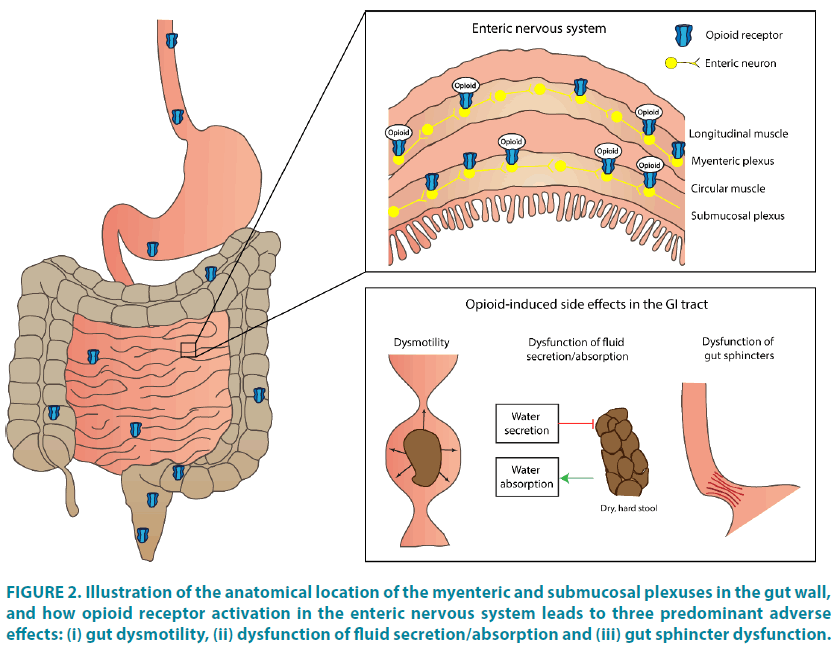
The GI tract is composed of smooth muscle arranged in two layers: the longitudinal and circular layer, both innervated through the enteric nervous system (ENS). The ENS is organized in two plexuses; the myenteric plexus which lies between the two muscular layers, and the submucosal plexus which lies within the submucosa. Three classes of opioid receptors are expressed in the ENS; δ-, κ-, and μ-opioid receptors, all being G-protein-coupled receptors. Of these, the μ-opioid receptor is considered the most widely distributed, and the principal mediator of opioid effects on the GI tract in humans [9]. The main effect of opioid receptor activation is decreased formation of cyclic adenosine monophosphate (cyclic AMP), which inhibits the release of excitatory and inhibitory neurotransmitters and thus, inhibits cell activity[19] Under normal conditions, endogenous opioids such as enkephalins and endorphins, coordinate sensory, motor and secretory activities in the gut, and suppress intestinal motility when required. However, exogenous opioids also influence the enteric neurons and shift the natural balance between transmitters. Therefore opioid administration can lead to GI motility dysfunction, decreased GI secretion and increased absorption, and GI sphincter dysfunction, as depicted in FIGURE 2 [8]. These synergistic effects may influence and enhance each other in a complex interplay, and all contribute to the multifaceted symptomatology of OIBD.
Figure 2: Illustration of the anatomical location of the myenteric and submucosal plexuses in the gut wall, and how opioid receptor activation in the enteric nervous system leads to three predominant adverse effects: (i) gut dysmotility, (ii) dysfunction of fluid secretion/absorption and (iii) gut sphincter dysfunction.
Opioid effects on motility
Normal coordination of gut motility is controlled by a delicate balance between the release of acetylcholine, nitric oxide and vasoactive intestinal peptide from the enteric neurons onto the longitudinal and circular layers. In addition, the primary function of the “pacemaker” cell-network in the gut wall, the so-called interstitial cells of Cajal, are to generate spontaneous electrical activity. Thereby peristaltic movements in the two muscle layers are induced, which again are regulated through hormonal and neurotransmitter influence, causing normal motility [20,21]. As opioids in the ENS, reduce neuronal excitability and neurotransmitter release, administration of these drugs exerts significant influence on gut motility. Consequently, stronger and more frequent phasic, non-migrating muscle contractions (known as “spike bursts”) occurs in the circular muscle layer, adjacent to an increase in resting contractile tone (less relaxation). This condition is further accompanied by a reduction in propulsive contractions of the longitudinal muscle layer. Taken together, these effects cause increased segmental contraction combined with decreased propulsive forward peristalsis [8,22]. The exact mechanism of these altered motility patterns have yet to be fully explored, however, prolongation of GI transit time may reflect underlying dysmotility [6]. Thus, several in vivo human studies have confirmed that opioids delay gastric emptying, oro-cecal transit and colonic transit time [10,23-25]. In addition, a recent study indicated that opioids reduce the number of colonic mass movements [26]. The most apparent consequence of opioid-induced GI dysmotility and subsequent prolonged transit, is constipation.
Opioid effect on fluid secretion and absorption
Intestinal fluid secretion and absorption is controlled by the submucosal plexus, and are important factors in ensuring optimal conditions for digestion, and not at least, local reflexes resulting in propulsive motility. Opioids reduce gut secretion as a direct result of inhibited formation of cyclic AMP, leading to less acetylcholine, and vasoactive intestinal peptide production. This ultimately causes an inactivation of epithelial chloride channels and reduce chloride and water transport from enterocytes into the gut lumen [27]. This mechanism is not limited to the small and large intestine: opioids also cause less gastric secretion, pancreatico-bilary secretion, and saliva production[28]. Such alterations may lead to altered bioavailability of food contents and absorption of pharmacologically compounds. Furthermore, as gut motility is dependent on luminal volume, mainly via local stretch reflexes, such decreased secretion (and volume) will also dampen propulsive peristalsis[29]. In addition, opioid-induced delayed transit will increase the contact time of fecal matter to the gut wall, thereby allowing more water absorption in the colonic regions. Accordingly, opioid effects on fluid secretion and absorption leads to prolonged transit and passage of dry and hardened stool.
Opioid effect on gut sphincter function
Six sphincters throughout the GI tract control the passage of chyme, regulate propulsive peristaltic movements between GI segments and control defecation. The pathophysiological mechanism behind opioid-induced gut Six sphincters throughout the GI tract control the passage of chyme, regulate propulsive peristaltic movements between GI segments and control defecation. The pathophysiological mechanism behind opioid-induced gut sphincter dysfunction is not completely understood, and more knowledge within this area is needed. However, opioids may induce increased esophageal sphincter tone, which can cause symptoms of achalasia and increase incidence of gastro-esophageal reflux [30]. In addition, opioid effects on the sphincter of Oddi is a well-recognized consequence of opioid administration. This is characterized by increased muscular tone, assessed as increased contraction amplitudes, and episodes which may generate colics and epigastric pain [31,32]. Opioids may also affect the internal anal sphincter. This is clinically manifested as straining during evacuation, sensation of anal blocking and the need for digital manoeuvres to facilitate bowel movements [33]. The incomplete bowel evacuations of dry hard stool further burdening the experience of opioid adverse effects. Studies regarding opioid-induced anal dysfunction are however scarce and contradictory. Most studies report that opioids increase anal basal tone [34,35], whereas or find no effect [36-38]. Furthermore, two randomized controlled trials recently addressed the effect of opioids on anal canal distensibility, and found no apparent influence on dynamic resistance to sphincter distension [39,40]. However, the recto-anal inhibitory reflex (an important intrinsic reflex causing the internal anal sphincter to relax upon rectal distension) is diminished by opioids, and this effect was found to be reversible by coadministration of naloxone and naloxegol. In contrast, some older studies have shown that the recto-anal inhibitory reflex was unaffected by opioids, however the differences may be caused by different methodologies [13,36,37].
The patient burden of OIBD and OIC
Gastrointestinal dysfunction is considered a taboo in the Western World, the subject is deemed inappropriate and consequently it is not discussed openly Furthermore, people do not possess the suitable vocabulary to describe and discuss the subject, mainly because they are afraid of offending the surroundings; coming across as being offensive [41]. This is particularly relevant to address in cases of opioid induced bowel dysfunction [42,43]. Examples of such embarrassing and unacceptable topics are bowel noise, flatus and changes in toilet habits, however the most common and often most debilitating adverse effect associated the opioid therapy is constipation. Thus, the psychological burden of opioid-induced bowel dysfunction is a net-result of its physical occurrence as well as its negative impact on health-related quality of life [44]. In a survey the impact of OIC on QoL was investigated and 38% responded that they had impaired work performances, 49% were prevented from daily activities, 45% said they had limited social interactions, 45% reported their sex lives were affected and 43% limited their ability to leave the house [18]. Finally, the fear of being stigmatised (the impact of the taboo and the perception of the person being “not fully social acceptable”) associated with impaired bowel conditions often leads to underreporting of symptoms and preventing health seeking by those affected [45]. The Western culture may however change in the upcoming years, which may make it more socially acceptable to talk and share experiences of OIC in e.g. patient fora and targeted groups on social media. Another excellent example was Astra Zeneca who spent $5 million USD on an ad for Super Bowl, with the purpose of educating potential patients and their relatives on treatment options for OIBD/ OIC, and thereby contribute to the social acceptance of openly target and discuss the burden [46]. Another example is Giulia Enders bestseller “Darm mit charme”, highlighting that the GI tract for too long has been the body’s least appreciated and most ignored organ, and emphasizing that our gut is at the core of who we are both physically and mentally. Therefore, health care professionals should proactively target possible interventions to relief OIC, and guide patients in order to reduce the experienced stigmatization for gastrointestinal disorders and thereby emphasize the need for further debate, investigation, and research within the topic.
Prevalence and bodily signs and symptoms of OIBD/OIC
The various effects that exogenous opioids exert on the GI tract is mirrored in the variety of bodily symptoms and signs experienced by the patients. A prospective survey of incidence, studied prevalence and severity of morphine adverse effects during repeated individualized dosing for chronic cancer pain. Herein 95% of the patients reported dry mouth, 88% reported sedation and OIC, whereas fewer than 50% reported nausea [47]. In comparison several studies have investigated the prevalence of GI symptoms in patients treated with opioids for non-cancer pain, which reported OIC in 40 -63% of patients, chronic abdominal pain in 58%, gastro-oesophageal reflux-related symptoms in 33%, nausea in 27% and vomiting in 9% of patients[11,48]. Similar findings were reported in a population-based survey, where increased frequency of constipationrelated symptoms (including straining, hard stools and infrequent bowel movements) were seen in opioid-treated patients [49]. However, diarrhoea-related symptoms, including urgency and loose or frequent bowel movements have also been reported in patients treated with opioids, which may be related to overflow diarrhea [50].
While OIC may present as the dominant symptom in most cases, it is important to remember the multifaceted symptomatology of OIBD when evaluating patients in the clinic. TABLE 1 lists the clisymptoms seen in OIBD, which mirrors the diversity of Gl-related adverse effects associated with chronic opioid consumption.
| Clinical symptoms | Pharmacological mechanism | |
| Decreased secretion | Dry mouth Oral malodor Constipation Hard dry stool Delayed absorption of medication, upper abdominal discomfort |
Decreased saliva production Increased bacterial fermentation Disturbed fluid secretion and absorption Diminished intestinal, pancreatic and biliary secretion Decreased gastric secretion, emptying and motility |
| Dyscoordinated GI-sphincters | Gastro-oesophageal reflux (or rarely dysphagia) Biliary colic, epigastric discomfort and pain Evacuation disorders |
Dysmotility of the lower oesophageal sphincter Constriction of sphincter of Oddi Increased anal sphincter tone and impaired reflex relaxation during rectal distension |
| Dysmotility | Spasm, abdominal cramps and pain, stasis of luminal contents and
Bloating, abdominal distension, constipation, straining, incomplete bowel evacuation Hard dry stools Bowel noise and flatus Chronic visceral pain Anorexia, nausea and vomiting |
Increased amplitudes of non-propulsive segmental bowel contractions Abnormal bowel motility, increased resting contractile tone in the small and large intestinal circular muscles and sphincter dysfunction Abnormal bowel motility, increased fermentation and meteorism Increased bacterial fermentation Opioid-induced hyperalgesia Central effects of opioids |
Table 1. Symptoms and underlying pharmacological mechanisms are displayed. .
Impact of OIC on well-being and social activities
During OIC, a commonly reported symptom is abdominal pain, which typically is described spasmodic, convulsive and severe. During periods of constipation, the altered propulsive motility causes that patients often experiences what they call “false alarms” [51], where they feel the pressure of a spontaneous bowel movement. However, often these are were unproductive and unsuccessful, and therefore when patients describe that they need to be close to toilet facilities acted accordingly and went to the toilet. Moreover, patients typically describe that the only relief to the OIC symptoms was having a bowel movement. Thus, ultimately, the defecation procedure in itself challenged some of the constipated patient’s strength, who described themselves as exhausted at the end [51]. Finally, the psychological fear of being constipated may lead to anorexia, because patients avoid to fill up their bowels again, in order to prevent subsequent OIC [52]. Furthermore, these patients typically feel fatigue and exhaustion which explains why they often avoid social activities and express the burden of their need to constantly stay close to toilet facilities [51]. Accordingly, it has been shown in other patients that during periods with constipation, quality of life is negatively affected as a result of experiencing spoiled optimism, depleted energy, general well-being, and negative emotions leading to worry or even a kind of desperation [51]. Unsurprisingly, the QoL in response to constipation correlated negatively with age and especially constipated women experienced reduced QoL in comparison to constipated men [53]. In a Swedish small interview study of constipated elderly women, patients ultimately described themselves as being left alone with a feeling of either tormented or released, depending on the condition of the bowel. Subsequently, they felt to a high degree neglected by the healthcare system, which evoked further feelings of stress and anxiety [54]. Similar experiences may be expected in chronic pain patients who develop OIC. For the majority of patients constipation is a transcedent and regarded as a highly private subject. For example, constipated patients described that during hospitalization the use of hospital toilets was an experience of insecurity and stress due to the fact that the hospital was not their home [51]. Unfortunately, a discordance between the perceptions of health care providers and patients is found in several areas of primary, secondary and tertiary medical centers, which may compromise patient care, medical compliance and disease management [14]. Therefore, a proactive approach from the healthcare personnel would allow the patient to discuss bowel habits, constipation and stool consistency more openly. Such communication about the burdens of OIBD/OIC may ultimately contribute to patient satisfaction, as it would have positive impact on the patient´s experiences of being heard and their treatment the potential of having their treatment adjusted - also during OIC.
Treatment
When it comes to treatment of OIBD the usual recommendation is to initiate prophylaxis against OIBD in all patients receiving opioid therapy. There is little evidence that lifestyle changes and fibres improve constipation in general and many patients cannot exercise due to pain or increase their fluid intake because of comorbidity [55]. Laxatives are therefore recommended as first line treatment in all patients for whom opioids are prescribed [56]. Laxatives can be divided into different subgroups, including osmotic agents (magnesium, lactulose, polyethylene glycol), stimulants (bisacodyl, senna), bulking agents (methylcellulose, psyllium) and stool softeners (anionic surfactants). Although it is still disputed how effective laxatives are in classical [57,58], their efficacy in OIBD is, overall, insufficient [59]. This may be explained by the fact that the key symptoms related to blockade of opioid receptors in the GI tract are not targeted by laxatives, which exert their main effect limited to the colon [8]. Hence, laxatives will not have any effect of the sphincter tone, and motor and secretory changes of the upper intestine. Treatment with laxatives may itself cause side effects such as bad taste, bloating, gas and reflux, and together with the lack of efficacy this may explain why about a third of the patients omit, reduce, or even discontinue their opioid therapy in order to relieve adverse effects [60]. Although the evidence is sparse, opioid rotation may also be helpful in reducing OIBD [61]. Some opioids such as tapentadol with effects on the noradrenergic system may also preserve the analgesic effects with less adverse effects on the gut [62]. Novel pharmacological approaches are the 5-HT4 receptor agonist prucalopride and the secretagogue lubiprostone (activator of the type 2 chloride channel). For both drugs, it has been demonstrated that spontaneous complete bowel movements increased in patients with opioidinduced constipation. However, prucalapride is only effective for the first few weeks of treatment and lubiprostone is not marketed in Europe [63,64].
A more logical approach to treatment is to use opioid antagonists with restricted effects in the gut and their effectiveness has been documented in a series of studies including a meta-analysis [59,64,65]. A combined prolonged release formulation of oxycodone and naloxone in a 2:1 ratio is available. The slow release of naloxone ensures that most is metabolized in the liver and less than 2% is released to the systemic circulation so analgesia is preserved [66]. Hence, the antagonist is mainly exerting its effect in the “gut compartment”. As naloxone is primarily metabolized in the liver, there is a potential risk of increased bioavailability in patients with hepatic impairment, and the fixed combination with oxycodone can make treatment difficult in cases where opioid rotation is necessary. PAMORAs are drugs that do not have any major effect on the central nervous system, but are present in the “peripheral compartment”. 1: Poulsen JL, Brock C, Olesen AE, Nilsson M, Drewes AM. Evolving paradigms in the treatment of opioid-induced bowel dysfunction. Therap Adv Gastroenterol. 2015 Nov;8(6):360- 72. doi: 10.1177/1756283X15589526. Review. PubMed PMID: 26557892; PubMed Central PMCID: PMC4622283. Methylnaltrexone does not pass the blood-brain-barrier due to its ammonium group. It has been shown to relieve OIBD and induce laxation [59]. Although an oral formulation may be launched in near future, it is currently only available in subcutaneous formulation. The drug shall be used carefully especially in patients with preexisting gastrointestinal diseases as the strong effect can lead to ruptures of the gut. Alvimopan is another orally administered PAMORA, which does not cross the blood-brain, but cardiovascular safety concerns (increased risk of myocardial infarction) halted further development and the drug is not registered in Europe [7]. Naloxegol is a PEGylated naloxone molecule that cannot cross the blood-brain barrier [67]. It has an acceptable safety profile and can be used as add-on to existing opioid treatment [61,68]. Consequently, opioid rotation is not necessary, potentially making it easier to use than current drugs. However, it has only been tested in few studies in non-cancer pain, and clinical experience is in different patient groups is needed [69].
Conclusion
Orally formulated opioids are commonly prescribed analgesics to treat severe pain and hence many patients experiences concomitantly adverse effects such as OIBD/OIC. This condition, possesses major negative impact on the health related QoL and limits the patients in their daily work and social activities. Thus OIBD/OIC challenges the patients and their healthcare professionals and the physicians are encouraged to pro-actively and openhearted discuss the physical and mental burdens of OIBD/OIC in order to find individual solutions such as initiating targeted treatment.
References
- Paulozzi LJ, Jones C, Mack KRR. Vital signs: overdoses of prescription opioid pain relievers. United States, 1999-2008. MMWR. Morb. Mortal. Wkly. Rep. 60, 1487-1492 (2011).
- Bartholow M. Top 200 Drugs of 2012. Pharm Times 79, 42-44 (2013).
- CDC. Prescription opioid overdose data. Atlanta: Centers for Disease Control and Prevention, 2016. (2017).
- National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse.
- Bohnert ASB, Valenstein M, Bair MJ. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA - J. Am. Med. Assoc. 305, 1315-1321 (2011).
- Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol. Motil. 16, 17-28 (2004).
- Thomas J. Opioid-Induced Bowel Dysfunction. J. Pain Symptom Manage. 35, 103-113 (2008).
- Brock C, Olesen SS, Olesen AE, et al. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs. 72, 1847-1865 (2012).
- Holzer P. Opioid receptors in the gastrointestinal tract. Regul. Pept. 155, 11-17 (2009).
- Poulsen JL, Nilsson M, Brock C, et al. The impact of opioid treatment on regional gastrointestinal transit. J. Neurogastroenterol. Motil. 22, 282-291 (2016).
- Tuteja AK, Biskupiak J, Stoddard GJ. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil. 22, 424-430 (2010).
- Cook SF, Lanza L, Zhou X. Gastrointestinal side effects in chronic opioid users: Results from a population-based survey. Aliment. Pharmacol. Ther. 27, 1224-1232 (2008).
- Musial F, Enck P, Kalveram KT, Erckenbrecht JF. The effect of loperamide on anorectal function in normal healthy men. J. Clin. Gastroenterol. 15, 321-324 (1992).
- LoCasale RJ, Datto C, Wilson H. The Burden of Opioid-Induced Constipation: Discordance Between Patient and Health Care Provider Reports. J. Manag. Care Spec. Pharm. 22, 236-245 (2016).
- Coyne KS, LoCasale RJ, Datto CJ, et al. Opioid-induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany, and the UK: descriptive analysis of baseline patient-reported outcomes and retrospective chart review. Clinicoecon. Outcomes Res. 6, 269-281 (2014).
- LoCasale RJ, Datto C, Margolis MK. Satisfaction with Therapy among Patients with Chronic Noncancer Pain with Opioid-Induced Constipation. J. Manag. Care Spec. Pharm. 22, 246-253 (2016).
- Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med. 10, 35-42 (2009).
- Rauck RL, Hong KSJ, North J. Opioid-Induced Constipation Survey in Patients with Chronic Non-cancer Pain. Pain Pract. 17, 329-335 (2017).
- Sharma SK, Nirenberg M, Klee WA. Morphine receptors as regulators of adenylate cyclase activity. Proc. Natl. Acad. Sci. U.S.A. 72, 590-594 (1975).
- Drumm BT, Baker SA. Teaching a changing paradigm in physiology: a historical perspective on gut interstitial cells. Adv. Physiol. Educ. 41, 100-109 (2017).
- Kim HJ, Kim H, Jung MH, Kwon YK, Kim BJ. Berberine induces pacemaker potential inhibition via cGMP-dependent ATP-sensitive K+ channels by stimulating mu/delta opioid receptors in cultured interstitial cells of Cajal from mouse small intestine. Mol. Med. Rep. 14, 3985-3991 (2016).
- Kurz A, Sessler DI. Opioid-induced bowel dysfunction: Pathophysiology and potential new therapies. Drugs. 63, 649-671 (2003).
- Yuan CS, Foss JF, O’Connor M, Roizen MF, Moss J. Effects of low-dose morphine on gastric emptying in healthy volunteers. J. Clin. Pharmacol. 38, 1017-1020 (1998).
- Hawkes ND, Rhodes J, Evans BK, et al. Naloxone treatment for irritable bowel syndrome--a randomized controlled trial with an oral formulation. Aliment Pharmacol. Ther. 16, 1649-1654 (2002).
- Smith K, Hopp M, Mundin G. Naloxone as part of a prolonged release oxycodone/naloxone combination reduces oxycodone-induced slowing of gastrointestinal transit in healthy volunteers. Expert Opin. Investig. Drugs. 20, 427-439 (2011).
- Mark EB, Poulsen JL, Haase AM, et al. The effect of opioid treatment on colorectal motility assessed by electromagnetic capsules. Neurogastroenterol. Motil. 106 (2017).
- Galligan JJ, Akbarali HI. Molecular Physiology of Enteric Opioid Receptors. Am. J. Gastroenterol. 17-21 (2014).
- Kromer W. Endogenous and exogenous opioids in the control of gastrointestinal motility and secretion. Pharmacol. Rev. 40, 121-162 (1988).
- Huizinga JD, Lammers WJEP. Gut peristalsis is governed by a multitude of cooperating mechanisms. AJP Gastrointest. Liver Physiol. 296, 1-8 (2008).
- Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol. Ther. 31, 601-606 (2010).
- Sharma SS. Sphincter of Oddi dysfunction in patients addicted to opium: An unrecognized entity. Gastrointest. Endosc. 55, 427-430 (2002).
- Torres D, Parrinello G, Trapanese C, Licata G. Sudden severe abdominal pain after a single low dose of paracetamol/codein in a cholecystectomized patient: Learning from a case report. Am. J. Ther. 17, 133-134 (2010).
- Tuteja AK, Biskupiak J, Stoddard GJ, et al. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol. Motil. 22 (2010).
- Chowdhury AR, Lorber SH. Effects of glucagon and secretin on food- or morphine-induced motor activity of the distal colon, rectum, and anal sphincter. Am. J. Dig. Dis. 22, 775-780 (1977).
- Rattan S, Culver PJ. Influence of loperamide on the internal anal sphincter in the opossum. Gastroenterology. 93, 121-128 (1987).
- Göke M, Ewe K, Donner K, Meyer zum Büschenfelde KH. Influence of loperamide and loperamide oxide on the anal sphincter. Dis. Colon Rectum. 35(9), 857-861(1992).
- Read M, Read NW, Barber DC, Duthie HL. Effects of loperamide on anal sphincter function in patients complaining of chronic diarrhea with fecal incontinence and urgency. Dig. Dis. Sci. 27, 807-814 (1982).
- Wilder-Smith CH, Bettiga A. The analgesic tramadol has minimal effect on gastrointestinal motor function. Br. J. Clin. Pharmacol. 43, 71-75 (1997).
- Grønlund D, Poulsen JL, Krogh K, et al. The impact of naloxegol on anal sphincter function - Using a human experimental model of opioid-induced bowel dysfunction. Eur. J. Pharm. Sci. 117, 187-192 (2018).
- Poulsen JL, Brock C, Grønlund D, et al. Prolonged-Release Oxycodone/Naloxone Improves Anal Sphincter Relaxation Compared to Oxycodone Plus Macrogol 3350. Dig. Dis. Sci. 62, 3156-3166 (2017).
- Chelvanayagam S. Stigma, taboos and altered bowel function. Gastrointest. Nurs. 12, 1-10 (2014).
- Thompson AI. “Sometimes, I think i might say too much”: Dark secrets and the performance of inflammatory bowel disease. Symb. Interact. 36, 21-39 (2013).
- Norton C. Nurses, bowel continence, stigma, and taboos. J. Wound Ostomy. Continence Nurs. 31, 85-94 (2004).
- Panchal SJ, Müller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: Prevalence, pathophysiology and burden. Int. J. Clin. Pract. 61, 1181-1187 (2007).
- Yarde D. Common bladder and bowel problems. Pract. Nurs. 24, 43-46 (2013).
- Astra Zeneca. OIC is different (1972).
- Glare P, Walsh D, Sheehan D. The Adverse Effects of Morphine: A Prospective Survey of Common Symptoms During Repeated Dosing for Chronic Cancer Pain. Am. J. Hosp. Palliat. Med. 23, 229-235 (2006).
- Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am. J. Surg. 182, 11S-18S (2001).
- Choung RS, Locke GR, Zinsmeister AR, Schleck CD, Talley NJ. Opioid bowel dysfunction and narcotic bowel syndrome: A population-based study. Am. J. Gastroenterol. 104, 1199-1204 (2009).
- Bril S, Shoham Y, Marcus J. The “mystery” of opioid-induced diarrhea. Pain Res. Manag. 16, 197-199 (2011).
- Munch L, Tvistholm N, Trosborg I, Konradsen H. Living with constipation-older people’s experiences and strategies with constipation before and during hospitalization. Int. J. Qual. Stud. Health Well-being. 11, 1-8 (2016).
- Friedrichsen M, Erichsen E. The lived experience of palliative hospital-based home care. 10, 321-325 (2004).
- Wald A, Scarpignato C, Kamm MA, et al. The burden of constipation on quality of life: Results of a multinational survey. Aliment Pharmacol. Ther. 26, 227-236 (2007).
- Lämås K, Anundsson E, Stare AC, Jacobsson C. An interview study of the experiences of middle-aged women living with constipation. Clin. Nurs. Stud. 3, 1-7 (2014).
- Dorn S, Lembo A, Cremonini F. Opioid-Induced Bowel Dysfunction: Epidemiology, Pathophysiology, Diagnosis, and Initial Therapeutic Approach. Am. J. Gastroenterol. 2, 31-37 (2014).
- Twycross R, Sykes N, Mihalyo M, Wilcock A. Stimulant laxatives and opioid-induced constipation. J. Pain Symptom Manage. 43, 306-313 (2012).
- Jones MP, Talley NJ, Nuyts G, Dubois D. Lack of objective evidence of efficacy of laxatives in chronic constipation. Dig. Dis. Sci. 47, 2222-2230 (2002).
- Ford AC, Suares NC. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: Systematic review and meta-analysis. Gut. 60, 209-218 (2011).
- Brenner DM, Chey WD. An Evidence-Based Review of Novel and Emerging Therapies for Constipation in Patients Taking Opioid Analgesics. Am. J. Gastroenterol. 2, 38-46 (2014).
- Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med. 10, 35-42 (2009).
- Drewes AM, Munkholm P, Simrén M, et al. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction-Recommendations of the Nordic Working Group. Scand. J. Pain. 11, 111-122 (2016).
- Xu XS, Etropolski M, Upmalis D, et al. Pharmacokinetic and pharmacodynamic modeling of opioid-induced gastrointestinal side effects in patients receiving tapentadol IR and oxycodone IR. Pharm. Res. 29, 2555-2564 (2012).
- Sloots CEJ, Rykx A, Cools M, Kerstens R, De Pauw M. Efficacy and Safety of Prucalopride in Patients with Chronic Noncancer Pain Suffering from Opioid-Induced Constipation. Dig. Dis. Sci. 55, 2912-2921 (2010).
- Ford AC, Brenner DM, Schoenfeld PS. Efficacy of Pharmacological Therapies for the Treatment of Opioid-Induced Constipation: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 108, 1566-1574 (2013).
- Diego L, Atayee R, Helmons P, et al. Novel opioid antagonists for opioid-induced bowel dysfunction. Expert. Opin. Investig. Drugs. 20, 1047-1056 (2011).
- Leppert W. Oxycodone/naloxone in the management of patients with pain and opioid-induced bowel dysfunction. Curr. Drug Targets. 15, 124-135 (2014).
- Poulsen JL, Brock C, Olesen AE, Nilsson M, Drewes AM. Clinical potential of naloxegol in the management of opioid-induced bowel dysfunction. Clin. Exp. Gastroenterol. 7, 345-358 (2014).
- Müller Lissner S, Bassotti G, Coffin B, et al. Opioid-Induced Constipation and Bowel Dysfunction: A Clinical Guideline. Pain Med. 7, 121-34 (2016).
- O’Brien T, Christrup LL, Drewes AM, et al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur. J. Pain. 21, 3-19 (2017).