Research Article - Diabetes Management (2020) Volume 10, Issue 2
The potential role of male bone marrow mesenchymal stem cells of diabetic female rats
- *Corresponding Author:
- Biochemistry Unit
Chemistry Department
Faculty of Science
Tanta University, Egypt
E-mail: amiramaram52@yahoo.com
Abstract
Background: Diabetes Mellitus is a metabolic defect that has the ability to produce an irreversible injury, abnormality of function and defect of several organs. Stem cell treatment posses a fantastic hope for the remedy damaged tissues or organs, and it is one of the utmost hopeful remedy for diabetic people. Thus, the current research direct to highlights of the potency function of MSCs of diabetic female rats. Methodology: Male albino bone marrow mesenchymal stem cells have been isolated and confirmed by their adhesion, spindle shape and also by cluster differentiation marker. Streptozotocin-induced diabetic female rats were intravenous injected by 1 x 105 MSCs (cell/rat). Results: MSCs group is capable of lowering blood glucose level, the activities of α-amylase, and S. L-MDA. However, hepatic glycogen contents showed an incredible increase when compared with the STZ-diabetic female nontreated rats. The histopathological and immuno-histochemical photo results confirmed that MSCs group noteworthy diminutive the degenerative alteration in pancreatic β-cells islet. Conclusions: Using bone marrow MSCs in remedy of STZ-diabetic female rats have the ability to recover odd biochemical parameters to normal or near normal level in STZ-induced diabetes model. But, MSCs needs more research and it may be more effective by injection more than one dose.
Keywords
streptozotocin, diabetes, stem cell, Oxidative stress
Introduction
Diabetes is a metabolic defect which appear as a result of a deficiency in insulin secretion, action, or together. The incurable blood glucose level, which produced as a result of diabetes, has the ability to produce an irreversible injury, abnormality of function and defect of several organs [1]. Oxidative stress plays a considerable role in the diabetic pathophysiology [2]. Likewise, oxidative stress is the main factor Oxidative stress sport a considerable role in the diabetic pathophysiology [2]. Likewise, oxidative stress is the main factor the advancement of diabetic complications, that relate to insulin resistance and reduce insulin secretion that cause the evolution of diabetes mellitus [3]. Oxidative stress creates reactive oxygen species (ROS) that generate toxic effect on cell growth [4]. In hyperglycemia, oxidative stress is generated by the forming of proceeding glycation end (AGE) product that has a potential relation of the complications of diabetes like nephropathy, neuropathy, retinopathy and cardiovascular diseases [5]. Insulin medication has improved serum glucose concentration in type 1 diabetic person. However, the method does not totally monitoring the moment-to-moment alteration in systemic blood glucose [6]. Moreover, involving cells that are genetically dissimilar and hence immunologically incompatible has various hurdles, requirement for numerous donor of pancreas and transplants, hardness preserve insulin independence, and injurious side effects from immune suppressants [7]. These limitations have led to the studding of other sources of β -cells. So, many studies have been aiming to treat diabetic people with cellular therapies which circumvent the need for exogenous insulin delivery by traditional injection or via newest pump technology [8]. Stem cell treatment possess a fantastic hope for the remedy damaged tissues or organs, and it is one of the utmost hopeful remedy for diabetic people [9]. Stem cells can improve the supplying of pancreatic islet cells [10]. It could be differentiated to pancreas β cells to raise β cell supply ameliorative the microenvironment of islet to support β cell function and survival [11]. So, this research aimed to discuss the role of MSCs that has been harvested from the male albino Wistar bone marrow on STZ-induced-diabetic female rats.
Methodology
▪ Protocol of BMSCs
Male albino rat’s bone marrow has been harvested as the method described by [12] with some modification. The tibiae and femurs of male albino rats has been flushed with dulbecco’s modified eagle’s medium (Gaithersburg) completed with foetal bovine serum (GIBCO/ BRL). Mononuclear cell has been separated and recultured in complete medium. Then, the culture cells were incubated in CO2 incubator for 14 days. The cultures were washed two times with saline, and then the cells were treated with trypsin. After that, cells have been collected and centrifuge and it resuspended again in serum supplemented medium. Mesenchymal stem cells were confirmed by adhesiveness and fusiform form [13], likewise, cluster of differentiation.
▪ Experimental animals
White female Wistar albino rats, 3-4 months old and average body weight 200-230 g were purchased from academy of Ophthalmology, Nasser Eye institution, Egypt. Rats were live in a chamber, temperature has been controlled, a 12 h light-dark cycle without control their water drinking and chow. Rats were handled in accordance to the suggested National ethical guidelines instruction as the Animal Ethics Committee (IAEC) of Faculty of Science, Tanta University, Tanta, Egypt. Diabetes in female albino rats was induce via injection of streptozotocin.
▪ Experimental design
Rats were divided into control normal group, group (CN), diabetic group (CD), which injected once by STZ,40 mg kg-1B.wt. Diabetic group which treated with MSCs (ST), that injected via tail veins with (1 × 105 cells/rat). MSCs group treatment was started after thirtyfive days from STZ-induction once. Serum has proceeded for biochemical analysis. Liver tissues were homogenized (10% w/v) in 0.9% saline, centrifugation at 4000 rpm for 20 min, and the homogenate were used for analysis.
▪ Biochemical parameters in serum
Blood glucose level was enzymatically measured and the activity of S. α-amylase was determined by using kits obtained from spectrum diagnostics, Egypt [14,15]. Lipid profile, S. total cholesterol and S. triacylglycerol were enzymatically measured [16,17]. Serum VLDL-C values has been calculated using the formula described by Bauer [18]. Also, serum urea levels was determined enzymatically [19] and S. creatinine was measured by kinetic method [20] using kit of Spectrum diagnostics, Egypt. Serum L-MDA were determined according to the method adapted by Mesbah [21].
▪ Liver and pancreas
The liver tissue has been collected at the end of duration four and six weeks. Liver homogenate (10%) was prepared by using a chilled glass- Teflon porter-Elvehjem tissue grinder tube. Then, centrifugation has been done at 4ºC to separate supernatant which has been used to determine the liver catalase activity. Moreover, pancreas tissue were taken from all groups and directly fixed in 10% formalin for 24 hours.
▪ Biochemical parameters in tissues
Catalase activity has been determined in liver homogenate, briefly, 10 μl of liver homogenate were added to the working buffer, the zero time was recorded and the decrease of the activity after one min. at 250 nm [22]. Glycogen has been determined in hepatic according to [23].
▪ Histological examination
The pancreas tissues were promptly fixed in neutral formalin for 24 hours. The tissue were dried, clarfied and estiblish in paraffin and cut. Some of the slices were preserved in wax, rehydrated and stained with hematoxylin and eosin.
▪ Immunohistochemistry
Pancreas portion were washed with distilled water, after that it washed with phosphate buffered saline for 10 minutes. Then the sections were incubated with insulin guinea pig-human antiserum for 120 min. at room temperature then it rinsed again in phosphate buffered saline. After that, the sections were incubated with guines pig immunoglobulin conjugated with peroxidase for 60 min. at room temperature then rinsed again in phosphate buffered saline. The reaction was developed as a brown color using 3-3ˋ diaminobenzidine tetrahydrochloride in 40 ml phosphate buffered saline, pH 7.2 that containing hydrogen peroxide in a dark place. The pancreas section was rinsed in distilled water and countered stained with Mayer’s HX, hydrated in ascending grades of alcohol, cleared and mounted [24].
▪ Statistical analysis
The acquired results were analyzed by oneway analysis of variance followed by Duncan multiple tests All analyses were performed using the statistical package for social science. Values of P<0.05 were theorize significant.
Results
▪MSC culture and identification
The isolated undifferentiated MSCs that reached 70%–80% confluent after fourteen days of culturing was confirmed by inverted microscope. MSCs were identified by their adhesion and spindle shaped, in addition to surface markers CD34−ve n CD105 +ve which are identified by immune staining
▪ Serum biochemical parameter in different group
The acquired results in Table 1 showed an increment in blood glucose levels in control diabetes female rats (CD) after duration four and six weeks in comparison with (CN) control group. Also, S. glucose level has been increased after duration four week in MSCs (ST) group as compared to both diabetic rats (CD) and normal rats (CN). Otherwise, diabetic MSCs (ST) treated-group reveled a lowering S. glucose levels after experimental six weeks of in comparison with the STZ-diabetic female group (CD), however, normal level not achieved in comparing with normal group (CN) (Table 1). The results of serum α -amylase activity exhibit a nochange in the activity of diabetic groups (CD), and MSCs (ST) group after experimental four when compared to normal group (CN). in case, duration of six weeks a significant increase in diabetes female rats (CD) as compared to the normal group (CN). Conversely, MSCs group (ST) significantly decrease the activity of S. α-amylase activity as compared to diabetic group (CD) and a non-significant change as compared to control normal group (CN) (Table 1). Hepatic glycogen in all groups had a non-significant alteration in STZ-diabetes female group (CD) all over the duration period of the experiment when compared with normal group (CN). Meanwhile, diabetic MSCs (ST) group had an increment of hepatic glycogen level in comparing with the diabetic group (CD) (Table 1). Lipid profile as serum total cholesterol, triacylglycerols and VLDL levels were significantly increased after duration four weeks in STZ- diabetic female rats (CD). Otherwise, diabetic MSCs group (ST) showed a significant reduction of lipid profile level as S. total cholesterol, TAG and VLDL-C concentration when compared with diabetic female group (CD). Duration of six weeks experiment displayed a significant reduction in lipid profile level as S. total cholesterol, triacylglycerols and VLDL level in STZ-diabetic female group (CD) as compared to normal group (CN). In contrast, MSCs group (ST) showed a non-significant change in S. total cholesterol in comparing with diabetic group. Otherwise, STZdiabetic rats that treated with MSCs group (ST) significantly increased both TAG and VLDL in comparing to STZ- diabetic group (CD) (Table 2). Kidney function test as serum urea presented in Table 3 showed an increament in STZ- diabetic female rats group (CD) all over the time of the experiment when compared to those of the control normal group (CN). Otherwise, diabetic MSCs groups (ST) were significantly decreased the elevated serum urea level after duration of four weeks but normal value has not achieved as compared with normal rats group (CN). Meanwhile, a non-significant change was observed in S. urea in diabetic MSCs group (ST) when compared with STZ- diabetic female group (CD). In addition, S. creatinine levels showed no- change in all experimental groups after duration four in comparison to normal group (CN). However, second duration of experiment showed an increament of S. creatinine in diabetic non treated group rats (CD) as compared to CN group. Otherwise, diabetic group that treated with MSCs group (ST) significantly reduced S. creatinine levels when compared with diabetic femlae group (CD) (Table 3). Serum L-MDA in STZ- diabetic female rats non treated group (CD) were increased in comparison to normal group (CN) all over the time of the experiment (Table 4). Conversely, diabetic MSCs group (ST) significantly reduced elevated serum MDA level after second duration when compared to the diabetic group (CD). Hepatic catalase had a non-significant change in diabetic group (CD) after duration of four weeks in comparison to normal group (CN). Conversely, diabetic MSCs group (ST) had significantly lowered catalase activity when compared with diabetic non treated group (CD). Meanwhile, duration of six, the activity of catalase of diabetic rats significantly reduced in comparing with the control normal (CN). Otherwise, diabetic MSCs group (ST) significantly increase the activity of catalase as compared to diabetic group but a no change was noticed in the activity of catalase in comparing with normal group (CN) (Table 4). Liver, kidney, heart and spleen tissues weight in the different treated groups indicated an increment in liver and kidney weights together all over the time of the experiment in diabetic group (CD). Otherwise, a no change in spleen weight has been noticed after duration four and on heart weight after six weeks, second duration. Moreover, a significant decreased in spleen weight noticed after duration six and in the heart weight of diabetic group (CD) after duration four in comparing with the control normal (CN). Diabetic MSCs (G. V) had a non-significant change in liver, kidney, heart and spleen weight after four weeks. Otherwise, a significant decrease was observed of liver, kidney and a no change of both heart and spleen weight after duration of six weeks as compared to the diabetic group (CD) (Table 5).
| Animal groups | Glucose (mg/dl) | a - amylase (U/L) | Hepatic Glycogen (µg/mg tissue) | |||
|---|---|---|---|---|---|---|
| 4 weeks | 6 weeks | 4 weeks | 6 weeks | 4 weeks | 6 weeks | |
| CN Range Mean ± SE |
67.3-75 70.16 ± 1.85b |
65-77 74.40 ± 2.14c |
204.26-348.84 280.01 ± 23.25a |
228.65-310.54 276.977 ± 16.238b |
0.066-0.151 0.088±0.03b |
0.079-0.11 0.095 ± 0.01b |
| CD Range Mean ± SE |
334-386 378.80 ± 9.68a |
329.5-465 399.90 ± 22.43a |
240.98-339.66 290.32 ± 16.47a |
429.17-688.5 550.50 ± 44.022a |
0.071-0.138 0.078 ± 0.02b |
0.064-0.103 0.088 ± 0.01b |
| ST Range Mean ± SE |
363.65-386 371.95 ± 4.16a |
275-355 300.00 ± 14.39b |
270.81-308.69 281.207 ± 6.712a |
201.96-262.47 230.14 ± 13.083b |
0.199-0.216 0.23 ± 0.005a |
0.20-0.252 0.24 ± 0.015a |
S.E=Standard error.
Mean values with different superscript letters in the same column are significantly different at (P = 0.05).
Control normal group (CN), diabetic group (CD), and diabetic MSCs (ST).
Table 1. Serum glucose, a-amylase and hepatic Glycogen parameters between the different groups under study
| Animal groups | Cholesterol (mg/dl) | Triacylglycerol (mg/dl) | VLDL (mg/dl) | |||
|---|---|---|---|---|---|---|
| 4 weeks | 6 weeks | 4 weeks | 6 weeks | 4 weeks | 6 weeks | |
| CN Range Mean ± SE |
35.4-50 46.18 ± 2.55c |
68-75.4 71.11 ± 1.67a |
50.14-64.5 52.09 ± 2.54c |
56.21-62.5 54.81 ± 1.06a |
10.03-12.9 10.55 ± 0.509c |
11.242-12.5 10.86 ± 0.205a |
| CD Range Mean ± SE |
109-120 109.85 ± 1.91a |
36-50 44.39 ± 2.42b |
230-339.3 290.89 ± 18.90a |
20-37 32.11 ± 3.59b |
46-67.86 60.23 ± 3.780a |
4-7.4 4.49 ± 0.718b |
| ST Range Mean ± SE |
64.03-84.9 70.00 ± 3.86b |
37-52 40.23 ± 2.89b |
148-230 185.10 ± 15.74b |
38-71.1 55.43 ± 5.79a |
29.6-46 33.23 ± 3.147b |
7.6-14.22 11.35 ± 1.158a |
S.E=Standard error.
Mean values with different superscript letters in the same column are significantly different at (P = 0.05).
Control normal group (CN), diabetic group (CD), and diabetic MSCs (ST).
Table 2.Serum lipid profile between the different groups under study
| Animal groups | Urea (mg/dl) | Creatinine (mg/dl) | ||
|---|---|---|---|---|
| 4 weeks | 6 weeks | 4 weeks | 6 weeks | |
| CN Range Mean ± SE |
39.5-64.53 44.11 ± 4.343c |
38.21-49.4 40.11 ± 2.103b |
0.676-1.27 0.89 ± 0.103a |
0.798-1.18 0.89 ± 0.564b |
| CD Range Mean ± SE |
107.4-125.2 109.26 ± 2.999a |
65.4-87 70.04 ± 3.924a |
0.985-1.278 1.01 ± 0.056a |
1.43-1.454 1.41 ± 0.004a |
| ST Range Mean ± SE |
86.6-124.61 98.07 ± 6.425b |
56.3-92.82 71.04 ± 7.066a |
0.864-1.18 1.00 ± 0.055a |
0.929-1.27 1.05 ± 0.065b |
S.E=Standard error.
Mean values with different superscript letters in the same column are significantly different at (P = 0.05).
Control normal group (CN), diabetic group (CD), and diabetic MSCs (ST).
Table 3. Serum kidney function test between the different groups under study
| Animal groups | SerumMDA (µmol /L) | Hepatic catalase (mmol/min/mg protein) | ||
|---|---|---|---|---|
| 4 weeks | 6 weeks | 4 weeks | 6 weeks | |
| CN Range Mean ± SE |
0.0057-0.0.012 0.0085 ± 0.002b |
0.0178-0.01889 0.010 ± 0.0004b |
1.98-2.27 2.02 ± 0.08a |
2.0-2.14 2.10 ± 0.04a |
| CD Range Mean ± SE |
0.021-0.024 0.025 ± 0.001a |
0.033-0.041 0.039 ± 0.003a |
2.02-3.7 2.81 ± 0.50a |
0.6-1.34 0.89 ± 0.22b |
| ST Range Mean ± SE |
0.0189-0.019 0.016 ± 3.33a |
0.0045-0.0159 0.0084 ± 0.004c |
0.75-0.87 0.92 ± 0.04b |
1.18-1.83 1.46 ± 0.19ab |
S.E=Standard error.
Mean values with different superscript letters in the same column are significantly different at (P = 0.05).
Control normal group (CN), diabetic group (CD), and diabetic MSCs (ST).
Table 4. Serum L-MDA level and liver catalase activity between the different groups under study
| Animal groups | Liver weight (g) | Spleen weight (g) | Kidney weight (g) | Heart weight (g) | ||||
|---|---|---|---|---|---|---|---|---|
| 4 weeks | 6 weeks | 4 weeks | 6 weeks | 4 weeks | 6 weeks | 4 weeks | 6 weeks | |
| CN Range Mean ± SE |
5.04-5.7 5.47 ± 0.12b |
5.84-6.23 6.02 ± 0.07c |
0.45-0.60 0.53 ± 0.04a |
0.599-0.687 0.64 ± 0.03a |
1.32-1.45 1.39 ± 0.03b |
1.48-1.64 1.57 ± 0.03c |
0.567-0.663 0.60 ± 0.03a |
0.665-0.70 0.68 ± 0.01a |
| CD Range Mean ± SE |
6.33-6.63 6.48 ± 0.07a |
7.98-10 9.33 ± 0.36a |
0.275-0.33 0.31 ± 0.02a |
0.32-0.41 0.37 ± 0.03b |
1.598-1.796 1.70 ± 0.04a |
2.2-2.5 2.36 ± 0.07a |
0.441-0.48 0.46 ± 0.01b |
0.57-0.98 0.73 ± 0.13a |
| ST Range Mean ± SE |
6.44-6.55 6.49 ± 0.03a |
6.55-7.85 7.45 ± 0.24b |
0.19-0.51 0.32 ± 0.07a |
0.39-0.5 0.46 ± 0.03b |
1.54-1.6 1.57 ± 0.02a |
1.89-2.13 2.01 ± 0.05b |
0.449-0.469 0.46 ± 0.01b |
0.397-0.685 0.53 ± 0.06a |
S.E=Standard error.
Mean values with different superscript letters in the same column are significantly different at (P = 0.05).
Control normal group (CN), diabetic group (CD), and diabetic MSCs (ST).
Table 5.Organs weight between the different groups under study
Histopathological findings
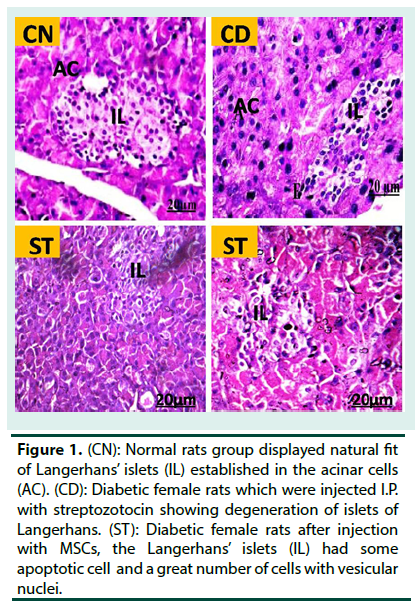
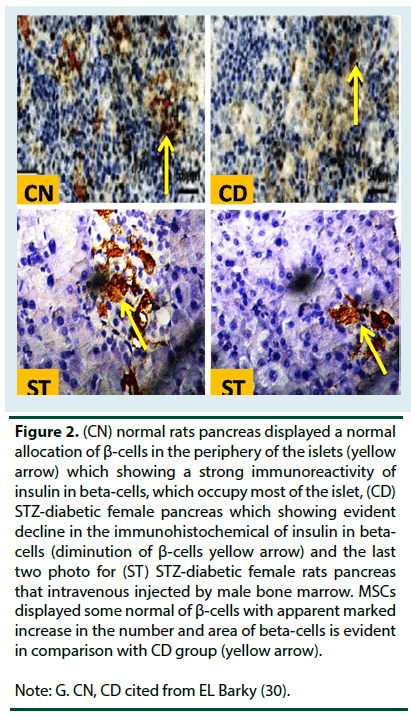
Control rats (CN) group displayed intact of the langerhans’ islets with well-defined edges and plump islets cells. The portion that stained by insulin antisera showed that β-cells were clear enough to be recognized in islets particularly in peripheral parts. On the other hand, the pancreatic of diabetic female rats displayed a large destruction of the pancreatic islet langerhans. The islets have ill-defined boundary and several necrotic areas were predominatingly seen. Severe inflammatory cells infiltrations were observed. Some endocrine cells had vacuolization in their cytoplasm and others had no cytoplasmic secretion. Diabetic pancreas which was stained with insulin antisera displayed a profound reduction in the β-cells. The pancreas section of diabetic rats that treated with MSCs (ST) repaired comparatively the normal appearance of the beta cell Langerhans’ islets. Moreover, the pancreas portion which were stained with insulin antisera displayed normal detection with β-cells though their numbers were still minimal as compared to normal control islets (Figure 1 and 2).
Figure 1. (CN): Normal rats group displayed natural fit of Langerhans’ islets (IL) established in the acinar cells (AC). (CD): Diabetic female rats which were injected I.P. with streptozotocin showing degeneration of islets of Langerhans. (ST): Diabetic female rats after injection with MSCs, the Langerhans’ islets (IL) had some apoptotic cell and a great number of cells with vesicular nuclei.
Figure 2. (CN) normal rats pancreas displayed a normal
allocation of ß-cells in the periphery of the islets (yellow
arrow) which showing a strong immunoreactivity of
insulin in beta-cells, which occupy most of the islet, (CD)
STZ-diabetic female pancreas which showing evident
decline in the immunohistochemical of insulin in betacells
(diminution of ß-cells yellow arrow) and the last
two photo for (ST) STZ-diabetic female rats pancreas
that intravenous injected by male bone marrow. MSCs
displayed some normal of ß-cells with apparent marked
increase in the number and area of beta-cells is evident
in comparison with CD group (yellow arrow).
Note: G. CN, CD cited from EL Barky (30).
Discussion
Mesenchymal stem cells injection resulted in decrease the concentration of blood glucose in diabetic group that treated with 1 x 105 cell/ rat after duration of 6 weeks. These results are almost identical to El Said [25] they demonstrated that STZ-diabetic rats which were received a subcutaneous injection of BMMSCs significantly reduced blood glucose level, butpancreatic beta cells including high expression of pancreatic and duodenal homeobox 1, insulin, and glucagon and have the ability to secrete insulin that led to amendment the diabetes [28]. MSCs group significantly diminish S. alphaamylase activity as compared to diabetic group (CD). This suggests that homing ability of MSCs due to the severity of injury. Hepatic glycogen content had an incredible increase in MSCs group (ST) after duration of four and six weeks by comparing with both normal rats group (CN) and diabetes female non treated group (CD). Glycogen and glucose are the two forms of sugar utilized by the body as a means of storing and providing energy at the cellular level. The liver excrete glucose into the bloodstream as a major mechanism to hold blood glucose levels steady. Glycogen is the main intracellular storable shape of glucose in different tissues and its level in such tissues especially the liver is a direct reflection of insulin activity [29]. The ability of MSCs to increase liver glycogen content may be due it raising the synthesis of glycogen through modulatory effect on the activities of glycogen synthase and glycogen phosphorylase or increase the insulin level that encourage glycogenesis. Decrease glycolysis, and expanded gluconeogenesis are a progressive part of glucose synthesis in diabetic liver [30].The increment of hepatic glycogen in MSCs treated group supposedly refer to the more utilization of glucose in the hepatic rather than insulin excretion. This study showed an incredible increase in lipid profile as S. total cholesterol, TAG, and VLDL concentrations after duration four in the diabetic non-treated groups (CD). Otherwise, all of lipid profile which analyses had a significant decrease in the serum of diabetic non-treated group (CD) after duration six weeks by comparing with normal group (CN). The diminution of serum lipid profile possibly due to rats that suffering from diabetes requirement need more food so, it search of other sources as lipid, so it break lipids to get their need of energy which in matching with EL Barky [31,32]. Meanwhile, MSCs diabetic group (ST) treatment had a significant increases of all lipid profile parameter which analyzed which resemble the results of Jung [33]. Remediation rats suffering from diabetes by MSCs had significantly diminish the increment of lipid profile as serum total cholesterol, triacylglycerols and VLDL after duration four. While, duration of six weeks had a significant increment of serum lipid profile by comparing with the diabetic group (CD), the obtained data were in agreement with Pan [34]. The decrease of lipid profile perhaps is related with mesenchymal stem cells which mend the hyperglycemia and so have the ability to increase serum insulin that is able of activate lipoprotein lipase [34]. Otherwise, the noticed increase of lipid profile second duration of the experiment might be due to the diminish of glucose level and increase level of insulin and thus prevent rats need any sources of carbohydrate from other sources like lipid source and this not lead to increase cholesterol and TAG from other sources. The obtained data showed a significant increase in both sera urea and creatinine levels in STZ- induced diabetes. The high concentration levels of urea in STZ-diabetic female rats is related to much protein catabolism. Mesenchymal cells can improve kidney function test for instance Castiglionea [35]. Mesenchymal cells have the ability to stop renal damage and beta cell pancreas islet degenerative because of the normalization of glucose in the body as a result of restoration pancreatic beta cell islets [36]. The present study displayed an increment of serum MDA and displayed a diminish in the activity of liver catalase in the STZ-diabetic female rat group (CD). On contrast, a significant reduction in the serum MDA of STZ-diabetic female rats that injected intravenous with male bone marrow mesenchymal stem cells after six weeks. The obtained data were in agreement with [37] they reported that treatment diabetic rats with mesenchymal stem cells significantly inhibited the increment of MDA and carbonyl protein in comparison to the diabetic animals. Treating STZ-diabetic female rats with male bone marrow mesenchymal stem cells significantly decrease the activity of catalase in hepatic tissue after duration four weeks. The diminish of the activity might be due to protein glycation and consumption by an excess demand [38]. The data obtained in our results indicated the elevation in the weight of liver and kidney in STZ-diabetic female rats (CD). Otherwise, there was a considerable diminution in spleen and heart weight was observed. The increment of the liver and kidney organs were in harmony with Al-Enazi [39]. The enlargement of liver could be impute to the increment triacylglycerol which accumulate in hepatic tissue causing enlargement of the liver. This increment is due to the increase outflow of fatty acids into the hepatic tissue which induced due to decrease level of insulin and the minimum strength of secretion of lipoprotein excretion of liver which give rise to a lack of apolipoprotein B structure [40]. Renal hypertrophy may be an increment in average of protein structure and lowering in the in the degradation of kidney matrix compounds that happen because of experimental diabetes [41] The hypertrophy of diabetic organs in general may be due to oxidative stress which resulted from hyperglycemia [42]. Cellular damages can be showed by both histopathology and immunohistochemistry. The diabetic non treated group (CD) showed a significant decreased in the number of insulin immunoreactive cells in the pancreatic islets, with a disrupted ultrastructure of beta-cells which is similar to [43,44].The present results show that MSCs at a dose of 1 x 105 cell /rat can cause a significant improvements in the functioning of beta-cells. The results could be slightly clarified by considering the mode of action of STZ, which induces an increased release of reactive oxygen species, thereafter causing DNA damage [45]. This damage leads to activation of DNA repair enzyme poly ADPribose polymerase-1. The result, a reduction in intracellular NAD is followed by ATP depletion, that cause a pancreatic beta-cell death [45]. It has been proposed that, MSCs protects pancreatic beta-cells from the damage caused by the free radicals induced by oxidative stress, and prevents membrane perturbation. The amelioration of histopathology tissue in diabetic rats that injected intravenous with MSCs (ST) in comparing to the STZ-diabetic female non-treated group (CD) was assured by the histological results and also by the immunohistochemical feedback. The result is because of the hypoglycemic act of mesenchymal stem cell. MSCs have the ability to decrease S. glucose concentration and ameliorate insulin secretion. This was related with repressed inflammation in the liver and pancreas and wellpreserved pancreatic β -cell mass [46].
Conclusion
Treatment diabetic rats with MSC may be more effective in repeated doses and long period. This was confirmed by lowering blood glucose level as compared to other diabetic non treated group in experimental six weeks. Moreover, immunehisto chemistry showed more insulin released from β- cell.
References
- El Barky A, Hussein S, Alm-Eldeen A et al. Saponins and their potential role in diabetes mellitus. Diabetes. Manag. 7(1), 148–158(2017).
- Ghosh S, Bhattacharyya S, Rashid K et al. Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways, Toxicol. Rep. 2, 365–376(2015).
- Kayama Y, Raaz U, Jagger A et al. Diabetic cardiovascular disease induced by oxidative stress. Int. J. Mol. Sci. 16(10), 25234–25263(2015).
- Deavall D, Elizabeth A, Judith M et al. Drug-induced oxidative stress and toxicity. J. Toxicol. 1–13(2012).
- Villeneuve L, Natarajan R. The role of epigenetics in the pathology of diabetic complications. Am. J. Physiol. Renal. Physiol. 299(1),14–25(2010).
- Guo T, Hebrok M. Stem cells to pancreatic β-cells: New sources for diabetes cell therapy. Endocrine. Reviews. 30(3), 214–227 (2009).
- Matsumoto S. Clinical allogeneic and autologous islet cell transplantation: Update. J. Diabetes. Metab. 35(3), 199–206(2011).
- Kaur H, Bhaskar N, Ishaq S et al. Stem cells: Source for diabetes cell therapy. Jour. Diabe. 3(3)9 pages(2012).
- El Barky A, Ezz A, Alm-Eldeen A et al. Can stem cells ameliorate the pancreatic damage induced by streptozotocin in rats? Can. J. Diabetes. 42(1), 61–70(2018).
- Luo L, Badiavas E, Luo J et al. Allogeneic bone marrow supports human islet beta cell survival and function over six months. Biochem. Biophys. Res. Commun. 361(4),859–864(2007).
- Kim J, Luo J, Luo L et al. Islet transplantation: Potential role of stem cells. Insights. In. Stem. Cells. 2(1), 1–11(2016).
- Tariq M, Masoud M, Mehmood A et al. Stromal cell derived factor-1alpha protects stem cell derived insulin-producing cells from glucotoxicity under high glucose conditions in-vitro and ameliorates drug induced diabetes in rats. J. Transl. Med. 11,115(2013).
- Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: Characteristics and clinical applications. Folia. Histochem.Cytobiol. 44(4), 215–30 (2006).
- Tietz N, ed., Clinical guide to laboratory tests. 3rd ed. Philadeiphia. WB Saunders,268-273,(1995).
- Winn-Deen E, David H, Sigler G et al. Development of a direct assay for alpha-amylase. Clin. Chem. 34(10), 2005-2008(1988).
- Ellefson R, Caraway W. Fundamentals of clinical chemistry. Ed Tietz NW, 506(1976).
- Stein E. lipids, lipoproteins, and apolipoproteins. In NW Tietz, ed. Fundamentals of clinical chemistry, 3rd ed. Philadelphia: WB Saunders, 448(1987).
- Bauer J. "Clinical laboratory methods" 9th Ed, the C.V. Company Waistline Industrial Missouri 63116 Chapter 33, p.555(1982).
- Tietz N, ED. Clinical guide to laboratory tests. 2 ND ED.philadelphia: WB Saunders, 566(1990).
- Tietz N, Textbook of clinical chemistery. WB saunders, Philadelphia, 1271-1281(1986).
- Mesbah L, Soraya B, Narimane S et al. protective effect of flavonides against the toxicity of vinblastine cyclophosphamide and paracetamol by inhibition of lipid-peroxydation and increase of liver glutathione. Haematol. 759-67(2004).
- Xu J, Yuan X, Lang P. Determination of catalase activity and catalase inhibition by ultraviolet spectrophtometry. Chinese Environ. Chem. 16(1), 73–76(1997).
- Togenu M, Austin U, Joyce A et al. Anti diabetic effect of aqueous leaf extract of Heinsia crinata on key glycolytic enzymes and glycogen in streptozotocin induced diabetic rats. Academia Journal of Scientific Research 1, 109-114(2013).
- Abou-Zaid F, Salem S, Madkour G et al. Histological and immunohistochemical studies on the pigeon endocrine pancreas at different ages. Egypt. J. Exp. Biol. (Zool.), 6(2) 385–394 (2010).
- El Said H, Gabrand H, Ammar R. The effect of human bone marrow mesenchymal stem cells on diabetic heart failure rats. Lif. Sci. Jour. 10(1), 3413– 3425 (2013).
- Ehsan A, Solmaz M, Ghaznavi H et al. A comparative study of mesenchymal stem cell transplantation with its paracrine effect on control of hyperglycemia in type 1 diabetic rats. J. Diabetes. Metab. Disord. 13(1), 76 (2014).
- Bell G, Broughton H, Levac K et al. Transplanted human bone marrow progenitor subtypes stimulate endogenous islet regeneration and revascularization. Stem. Cells. Dev. 21(1), 97–109 (2011).
- Zanini C, Bruno S, Mandili G et al. Differentiation of mesenchymal stem cells derived from pancreatic islets and bone marrow into islet-like cell phenotype. PLoS. One. 6(12), e28175 (2011).
- Nidhi S, Veena G, Arpita P. Antihyperglycemic, antihyperlipi-demic and antioxidative potential of Prosopis cineraria bark. Indian. J. Clin. Biochem. 25(2), 193–200 (2010).
- Baquer N, Gupta D, Raju J. Regulation of metabolic pathways in liver and kidney during experimental diabetes: effects of antidiabetic compounds, Ind. J. Clin. Biochem. 13(2), 63–80 (1998).
- El Barky A. Biochemical influence of alpha-lipoic acid on lipid peroxidation and antioxidant enzymes in blood and tissues of streptozotocin induced diabetes in rats. Ms. thesis, faculty of Veterinary Medicine, Moshthor, Benha University (2012).
- El Barky A, Hussein S, Alm-Eldeen A et al. Anti-diabetic activity of Holothuria thomasi saponin. Biomed. Pharmacother. 84, 1472–1487 (2016).
- Jung U, Cho Y, Choi M. Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients. 8(5), 305 (2016).
- Intrahepatic transplantation of adipose derived stem cells attenuates the progression of non alcoholic fatty liver disease in rats. Mol. Med. Rep. 12(3), 3725–3733 (2015).
- Castiglione R, Maron-Gutierrez T, Barbosa C et al. Bone marrow-derived mononuclear cells promote improvement in glomerular function in rats with early diabetic nephropathy. Cell. Physiol. Biochem. 32, 699–718 (2013).
- Lee R, Seo M, Reger R et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Natl. Acad. Sci. USA 103(46), 17438–17443 (2006).
- Fang Y, Tian X, Bai S et al. Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int. J. Mol. Med. 30 (1), 85–92 (2012),.
- DavI G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid. Redox. Signal. 7(1-2),256–268 (2005).
- Al-Enazi M. Combined therapy of rutin and silymarin has more protective effects on streptozotocin-induced oxidative stress in rats. J.A.P.S. 4 (1), 21–28 (2014).
- Zafar M, Naqvi S. Effects of STZ-induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: A comparative study. Int. J. Morphol. 28(1), 135–142 (2010).
- Wu K, Setty S, Mauer S et al. Altered kidney matrix gene expression in early stages of experimental diabetes. Acta. Anat. (Basel), 158(3), 155–165 (1997).
- Arozal W, Watanabe K, Veeraveedu P et al. Effects of angiotensin receptor blocker on oxidative stress and cardio-renal function in streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 32(8), 1411–1416 (2009).
- Ahmadi S, Karimian S, Sotoudeh M. Pancreatic islet beta cell protective effect of oral vanadyl sulphate in streptozotocin-induced diabetic rats, an ultrastructure study. Pak. J. Biol. Sci. 13(23), 1135–1140 (2010).
- Abunasef K, Amin H, Abdel-Hamid A. histological and immunohistochemical study of beta cells in streptozotocin diabetic rats treated with caffeine. Folia. Histochem. Cytobiol. 52(1), 42–50 (2014).
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 50(6), 537– 546 (2001).
- Cao M, Pan Q, Dong H et al. Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice. Stem. Cell. Res. Ther. 208, (2015).