Review Article - Interventional Cardiology (2021) Volume 13, Issue 6
The promise and problems of metabolic-based therapies for heart failure
- Corresponding Author:
- Dianne M. Perez
The Lerner Research Institute,
The Cleveland Clinic Foundation,
9500 Euclid Ave, Cleveland,
OH 44195,
USA,
E-mail: Perezd@ccf.org
Received date: September 13, 2021; Accepted date: September 27, 2021; Published date: October 04, 2021
Abstract
Despite standard therapies, heart failure patients have high rates of morbidity highlighting the need to develop alternative therapeutic approaches. Heart failure has been described as an energy-starved condition that is hypothesized to drive the pathological remodeling of the heart. Numerous studies have described the metabolic defects that occur when the heart fails and adaptive changes that take place to maintain the energy needed for the heart to function properly. In this minireview we will summarize the metabolic requirements of a normal heart and what happens during failure. We will also summarize the various metabolic therapeutic strategies that have been developed over the years to treat heart failure and their results from clinical trials.
Keywords
Adrenergic receptor•Metabolism • Heart failure •Myocyte
Introduction
The heart requires a high metabolic rate to sustain needed Adenosine Trisphosphate (ATP) levels, more so than any other organ besides the kidney [1]. The heart also has tremendous flexibility to metabolize various types of substrates besides normal glucose and Fatty Acids (FA), such as lactate, amino acids, and ketone bodies though these are minor contributors to energy production in a normal heart [2]. During Heart Failure (HF), large energy deficits build up from alterations and defects in the ability to metabolize FAs properly, increasing oxidative stress and contractile dysfunction which ultimately contribute to progression of HF and poor clinical outcomes [3].
Literature Review
Fatty acid and glucose metabolism
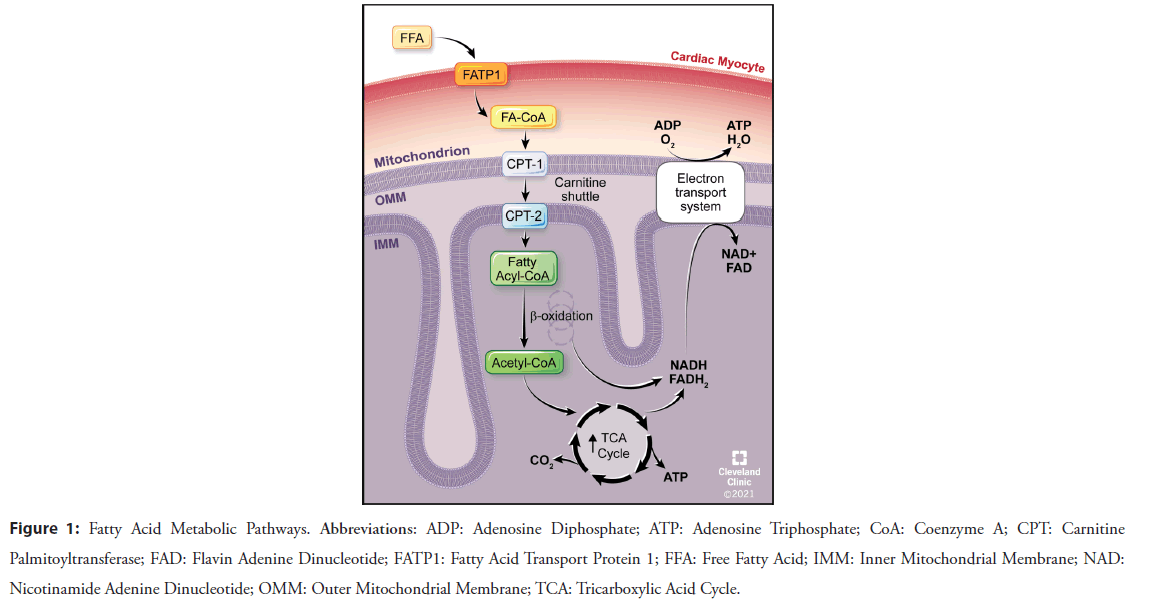
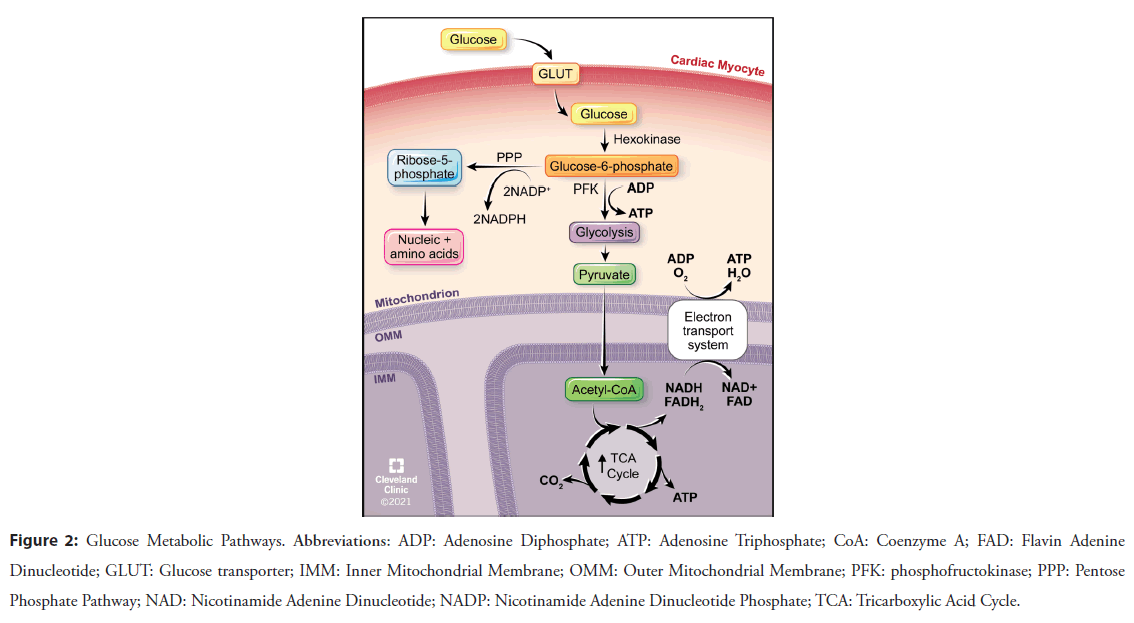
Fatty Acid Oxidation (FAO) (Figure 1) supplies the vast majority of the ATP requirements (60%-90%) in the normal heart, followed by glucose oxidation (Figure 2) [4]. FAO provides 105 ATP molecules for every 23 molecules of oxygen while glucose oxidation generates 31 ATP molecules per 6 molecules of oxygen, a more efficient use of oxygen even though less ATP is made [4].
During HF, the ability to metabolically utilize FA decreases [5,6], prompting a switch to greater carbohydrate metabolism. This switch in substrate utilization during HF is due to decreases in the number of mitochondria [7], mitochondrial dysfunction caused by Reactive Oxygen Species (ROS) [7-9], downregulation of FAO genes (i.e. FATP1, CPT-1) (Figure 1) [10] and enzymes involved in β-oxidation and oxidative phosphorylation (i.e. electron transport system), resulting in greater use of oxygensparing carbohydrate metabolism. HF also results in the upregulation of genes and activity associated with glucose oxidation or the flux of glucose into the heart, such as Glucose Transport proteins (GLUT), the enzyme Phosphofructokinase (PFK), Pyruvate Dehydrogenase (PDH) (Figures 2 and 3) [11-13]. Eventually, at end-stage HF, there is decreased ability to metabolize any type of substrate [14]. As such, HF is not merely shifting metabolically from FA to glucose utilization, but is also associated with decreased overall oxidation rates and increased oxidation of alternate substrates (i.e. amino acids, ketone bodies). While these metabolic changes occur during pressure overload and ischemic HF, an exception to this pathological metabolic remodeling occurs during diabetic-induced HF where there is an increase in FA uptake, shifting metabolism to even greater FAO which leads to damaging oxidative stress and dysfunction [15].
Randle effect
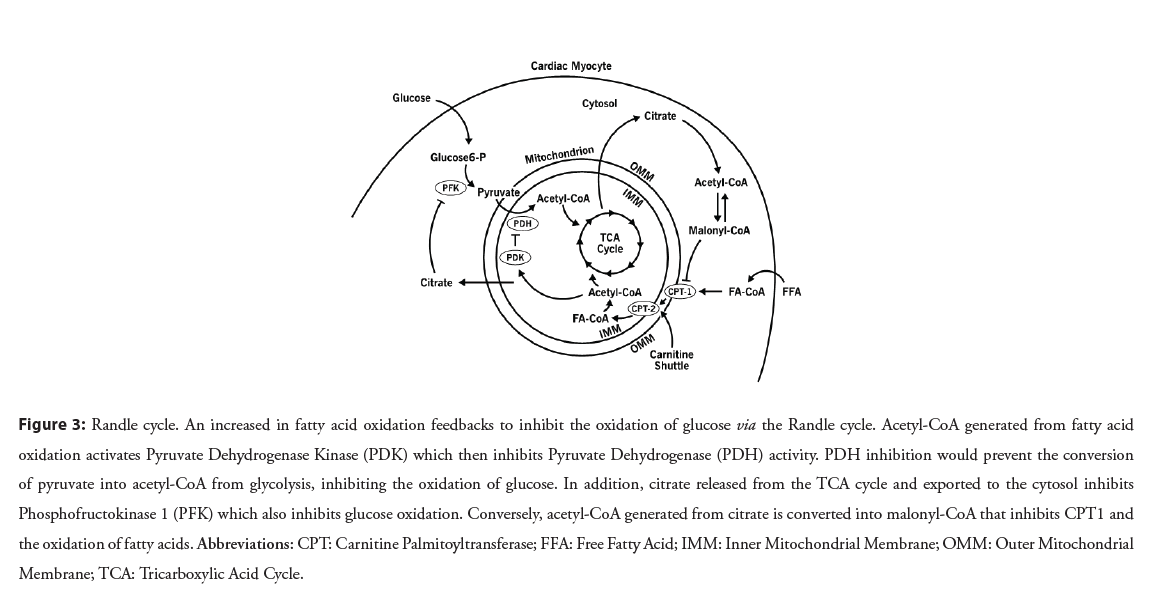
In cellular metabolism, there is a competition of substrates between the oxidation of FA and carbohydrates as described by the Randle cycle, also referred to as the glucose-fatty acid cycle (Figure 3) [16]. It’s role is thought to be a signaling mechanism to the cell that it has an excess of one type of fuel and allows for fine-tuning of metabolism without the intervention by hormonal signals. The Randle cycle describes the inverse relationship where the increase in utilization by one type of metabolic substrate will lead to the other’s inhibition. FAO increases acetyl-CoA which inhibits Pyruvate Dehydrogenase (PDH), which is essential to converting pyruvate to acetyl-CoA to feed the TCA cycle from glycolysis [17]. Increased levels of citrate from the TCA cycle inhibits a key glycolytic enzyme, PFK, the rate limiting enzyme for glycolysis and prevents pyruvate from accumulating [18]. The Randle cycle becomes important to understand why HF metabolic therapeutics are predicted to increase glucose oxidation when their main effect is to decrease FAO.
Ketone metabolism
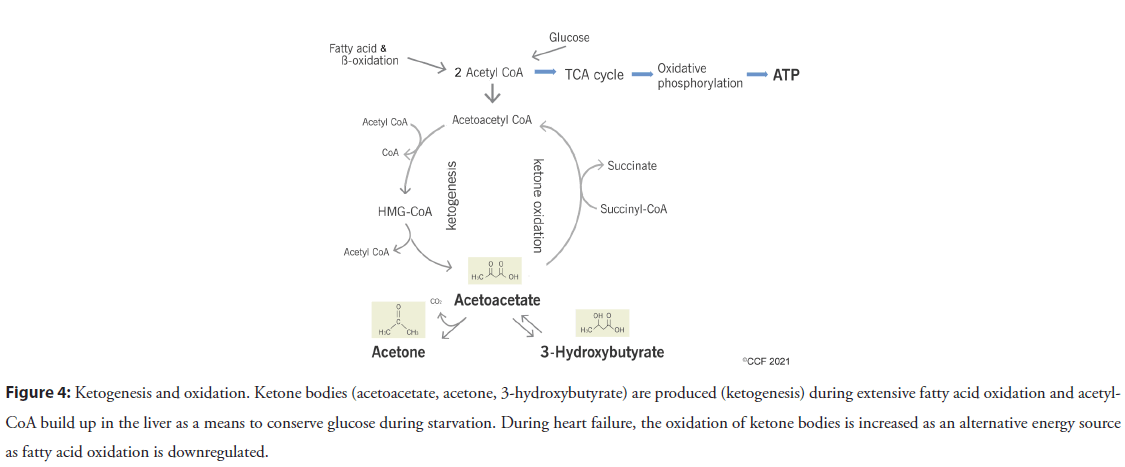
The heart is metabolically flexible due to its high metabolic rate requirements [1] and can adapt to utilizing various subtracts for fuel as they are available. Ketone bodies are generated as intermediate products of FAO in the liver and consist of acetoacetic acid, β-hydroxybutyric acid and acetone (Figure 4). During non-fasting conditions, ketone bodies are not utilized for energy but can be a critical part of maintaining energy homeostasis during stress and starvation by conserving glucose [19]. As HF is hypothesized to be in an “energy-starvation” state [20], the failing heart’s utilization of ketone bodies becomes more critical [21,22]. There is also an increase in the enzymes and metabolic intermediates associated with ketone metabolism during end-stage HF in addition to the expected downregulation of FAO associated proteins [21,22]. Ketones are very efficient in its energetic properties but circulating levels, while normally low, can increase quickly during starvation or HF. Ketone bodies can generate more ATP than glucose and with greater efficiency than FAO, but not as efficient as glucose. As HF progresses, later stages have deficits in both FA and glucose oxidation, causing the heart to utilize alternate metabolic substrates. Ketone oxidation increases during HF because it bypasses the normal oxidative pathways that are affected and downregulated during HF [19,21-23]. However, excessive ketone body oxidation could also lead to a depletion of TCA intermediates (Figure 4) [24] and will not provide the long-term energy needed for contractile function [25], so its usefulness as a therapeutic strategy for HF is limited.
Metabolic Therapeutics and Clinical Trial Outcomes
Our knowledge of cardiac metabolism and its alterations during failure as reviewed above has increased greatly during the years making metabolic therapeutic strategies an attractive target to treat HF. Traditional therapies to treat HF include renin-angiotensin inhibitors [26,27], β-blockers [28], mineralocorticoid antagonists [29], and vasodilators [30]. While these traditional therapeutics are the gold-standard and improve contractile function, long-term use has failed to improve outcomes.
β-Adrenergic receptor blockers
β-blockers are still a mainstay in HF treatment. Their primary action is to reduce heart rate which results in a decrease in work load and oxygen consumption. However, β-blockers are also being appreciated more for a secondary effect on cardiac metabolism. The best described β-AR blocker for its treatment for HF is carvedilol. Carvedilol is a β-AR antagonist and an β1/α1 antagonist [31]. While metaprolol and other α1-AR blockers have similar effects on cardiac metabolism, carvedilol has better effects on improving glucose metabolism [32], improve insulin sensitivity [33], and has greater antioxidative properties [34]. Carvedilol can achieve this by decreasing the pool of free fatty acids, which shifts energy substrate availability in the heart to increase glucose oxidation and energy efficiency [35,36]. In clinical trials, carvedilol reduces the mortality risk in HF patients better than metoprolol [37], but has not been assessed for its metabolic benefit in large scale studies. In a small clinical study of 9 patients with class III HF, carvedilol treatment for 3 months decreased myocardial free FA metabolism by 57% [38].
Heart Rate Control
The magnitude of heart rate reduction to the magnitude of survival benefit is strongly correlated in HF, more so than the type or dosage of β-AR blocker [39]. In a different class than β-AR blockers but with similar physiological effects, ivabradine lowers heart rate by inhibiting the inward Na+/K+ current that regulates sinus rhythm generation [40]. Ivabradine has an advantage over β-AR blockers in that it is a pure heart rate reducing drug, eliminating some of the potential side effects of β-AR blockers. During exercise studies, ivabradine caused a similar reduction in heart rate as β-AR blocker atenolol and improved cardiac oxygen consumption but without any negative lusitropic effect [41]. In several clinical studies, ivabradine reduced oxidative stress, improved cardiovascular endpoints, as well as morbidity and mortality in HF [42]. However, more clinical studies are needed directly comparing ivabradine vs. β-AR blockers to determine any significantly different long-term effects on efficacy or outcomes [42].
Sodium-Glucose Co-Transporter 2 inhibitors (SGLT2i)
Inhibitors of the type 2 Sodium Glucose Transporters (SGLT2) were originally approved for treatment of type 2 diabetes. Of the 12 family members, SGLT2 are expressed on the kidney proximal tubules and reabsorbs a large part (90%) of the glucose in the body [43]. Hence, SGLT2 inhibitors (SGLT2i) would increase the urinary excretion of glucose. They are also expressed on pancreatic alpha cells [44] and regulate glucagon release that exert beneficial effects on both glucose and lipid metabolism to improve cardiovascular outcomes [45].
A number of studies have shown that SGLT2i provide profound reductions in the hospitalizations for HF by at least 26% [46- 49]. It is postulated that the primary benefit of SGLT2i is to increase circulating ketone levels which increase ketogenesis, but this is not certain [50-52]. The SGLT2i, empagliflozin, improved remodeling and function in the heart by increasing ketogenesis which has better metabolic efficiency than glucose to produce ATP [53]. Empagliflozin also lowered levels of ROS and improved mitochondrial dysfunction in HF, resulting in a reduction of LV mass and fibrosis [54]. SGLT2i have been shown to also prevent and reduce NADPH-mediated oxidative stress and its resulting damage [55-57] that contributes to HF progression [58].
The cardioprotective benefits of SGLT2i have also been ascribed to the activation of sirtuin-1 (SIRT1: Silent Information Regulator 1) signaling pathways [59]. SIRT1 is a NAD-dependent deacetylase of the histone deacetylase family [60] and transcriptionallyregulates many of the major genes involved in aging, metabolism, oxidative stress, and mitochondrial biogenesis and function in the heart through its downstream signals [61,62]. During starvation or energy-starved states such as HF, SIRT1 stimulation promotes FAO and gluconeogenesis which can drive ketone body production and ketogenesis [63], thus, increasing metabolic efficiency. These results may explain the cardioprotective effects of the red wine chemical, resveratrol and its derivatives, which has a SIRT1 activating effect [64,65]. Clinical trials with synthetic SIRT1 activators have shown cardiovascular benefit [66,67] but have not been tested in HF. Future clinical studies may reveal a potential for the consumption of SIRT1 activators as an attractive target to treat HF.
However, not all cardioprotective effects of SGLT2i may be metabolic [68]. The heart contains some of the highest levels of the enzymes required for ketone oxidation in the body [69] but the receptors for SGLT2 are not expressed in the myocardium [70]. One hypothesis is that HF causes an increase in SGLT1 receptor expression in the heart which undergo non-specific inhibition by SGLT2i [71] or that ketosis is indirect [70]. Other benefits from SGLT2i are 45% reduction in the progression of kidney failure [72]. A large scale meta-analysis of four different SGLT2is also revealed significantly increased risk of diabetic ketoacidosis and genital infection [73].
Carnitine Shuttle
l-Carnitine is important in FAO, being utilized as a cofactor in the carnitine shuttle that transports fatty acids into the mitochondria where FA undergo oxidative phosphorylation (Figure 1). l-propionylcarnitine has been previously studied for metabolic enhancement in the heart as carnitine supplementation may improve FAO through substrate availability. However, the actions of l-propionylcarnitine cannot be explained by stimulation of FAO but by increasing glucose oxidation via relief of PDH inhibition. The metabolic premise is that inhibition of FAO shifts cardiac metabolism towards utilizing more of the energy-efficient glucose metabolism via the Randle cycle. Although l-propionylcarnitine showed increased exercise tolerance and improved symptoms in HF patients compared with a placebo control group [74], a large randomized and double-blind clinical trial failed to reach sufficient efficacy [75]. Similar results have been found with meldonium, a FAO inhibitor used illegally by Soviet and Latvian professional athletes to increase performance. Meldonium (i.e. Mildronate®) is biochemically related to l-carnitine and partially inhibits the last enzymatic step in the body’s synthesis of l-carnitine. There are reports of positive outcomes in HF when meldonium is used in combination therapy [76-78]; however, there are no large-scaled controlled clinical trials as the drug is not available in the United States.
Figure 1: Fatty Acid Metabolic Pathways. Abbreviations: ADP: Adenosine Diphosphate; ATP: Adenosine Triphosphate; CoA: Coenzyme A; CPT: Carnitine Palmitoyltransferase; FAD: Flavin Adenine Dinucleotide; FATP1: Fatty Acid Transport Protein 1; FFA: Free Fatty Acid; IMM: Inner Mitochondrial Membrane; NAD: Nicotinamide Adenine Dinucleotide; OMM: Outer Mitochondrial Membrane; TCA: Tricarboxylic Acid Cycle.
An alternate way to modulate the carnitine pathway and shuttle is through the enzymes, Carnitine Palmitoyl Transferase (CPT), which has two variants: CPT1 and CPT2 (Figures 1 and 4). Long-chain fatty acyl-CoA is converted to acylcarnitine by CPT1 on the outer membrane of mitochondria. Acylcarnitine is then transported to the inner membrane by a transposase and CPT2 is used to transfer the acyl group on acylcarnitine to long-chain fatty acyl-CoA. Once inside the mitochondria, FAs are oxidatively metabolized. The CPT1 inhibitors, etomoxir or perhexiline, have been shown to improve cardiac energetics, exercise capacity, and diastolic function in hypertrophic cardiomyopathy patients after 4-5 months of dosing [79-82]. However, it has a narrow therapeutic window with possible neurotoxic and hepatoxic side effects [83,84]. A clinical trial is now underway that will test whether prolonged treatment with perhexiline for 12 months will improve left ventricular hypertrophy which is the main driver of systolic dysfunction and HF [85].
General FAO inhibition
Trimetazidine or ranolazine are anti-anginal drugs that inhibit FAO to indirectly increase glucose oxidation via the Randle cycle. Once thought to act by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase [86], a subsequent study have kinetically shown that both trimetazidine and ranolazine are not substrates for the thiolase but do inhibit FAO [87]. In general, smaller clinical trials and meta-analysis with either trimetazidine or ranolazine demonstrated efficacy against HF, particularly when the dosing was long-term [88-94] but other studies did not improve exercise capacity or mortality in HF but this was attributed to them being weaker FAO inhibitors than the CPT1 inhibitor perhexiline [95- 98]. Trimetazidine is currently approved for coronary artery disease [94] but not HF due to the lack of large-scale clinical trials. A meta-analysis is currently being undertaken to assess trimetazidine in combination therapy with the β-AR blocker, bisoprolol [99] after a smaller combination therapy clinical study demonstrated efficacy to treat HF [100].
Directly increasing glucose oxidation
While several metabolic inhibitors of FOA indirectly increase glucose oxidation via the Randle cycle (Figure 3), potential therapeutics that can directly increase glucose uptake and/ or its oxidation is an alternative approach to metabolically treat HF. During glycolysis, pyruvate is transported into the mitochondria for subsequent oxidation (Figures 2 and 4). Pyruvate Dehydrogenase (PDH) coverts pyruvate into acetyl CoA which enters the TCA cycle and fuels oxidative phosphorylation. PDH is tightly regulated by Pyruvate Dehydrogenase Kinases (PDK) and pyruvate dehydrogenase phosphatases [101]. PDKs phosphorylate PDH, which reduces its activity and phosphate is removed by the phosphatase, increasing PDH activity. This highly-regulated control makes PDH a potential therapeutic target. Dichloroacetate (DCA) is an inhibitor of PDKs, increasing PDH activity by promoting the conversion of pyruvate into acetyl- CoA, thereby increasing oxidation through the TCA cycle [102]. In early animal studies, DCA showed promising results [103,104] but human studies are limited and conflicting. A small clinical trial tested DCA (50 mg/kg) in HF patients [105] resulted in increased left ventricular mechanical efficiency and stimulated lactate consumption. However, another study showed that DCA administration did not improve LV function [106].
Figure 2: Glucose Metabolic Pathways. Abbreviations: ADP: Adenosine Diphosphate; ATP: Adenosine Triphosphate; CoA: Coenzyme A; FAD: Flavin Adenine Dinucleotide; GLUT: Glucose transporter; IMM: Inner Mitochondrial Membrane; OMM: Outer Mitochondrial Membrane; PFK: phosphofructokinase; PPP: Pentose Phosphate Pathway; NAD: Nicotinamide Adenine Dinucleotide; NADP: Nicotinamide Adenine Dinucleotide Phosphate; TCA: Tricarboxylic Acid Cycle.
Figure 3: Randle cycle. An increased in fatty acid oxidation feedbacks to inhibit the oxidation of glucose via the Randle cycle. Acetyl-CoA generated from fatty acid oxidation activates Pyruvate Dehydrogenase Kinase (PDK) which then inhibits Pyruvate Dehydrogenase (PDH) activity. PDH inhibition would prevent the conversion of pyruvate into acetyl-CoA from glycolysis, inhibiting the oxidation of glucose. In addition, citrate released from the TCA cycle and exported to the cytosol inhibits Phosphofructokinase 1 (PFK) which also inhibits glucose oxidation. Conversely, acetyl-CoA generated from citrate is converted into malonyl-CoA that inhibits CPT1 and the oxidation of fatty acids. Abbreviations: CPT: Carnitine Palmitoyltransferase; FFA: Free Fatty Acid; IMM: Inner Mitochondrial Membrane; OMM: Outer Mitochondrial Membrane; TCA: Tricarboxylic Acid Cycle.
Figure 4: Ketogenesis and oxidation. Ketone bodies (acetoacetate, acetone, 3-hydroxybutyrate) are produced (ketogenesis) during extensive fatty acid oxidation and acetyl- CoA build up in the liver as a means to conserve glucose during starvation. During heart failure, the oxidation of ketone bodies is increased as an alternative energy source as fatty acid oxidation is downregulated.
DCA has side effects of neurotoxicity and carcinogenicity that restricts clinical use and further development [107,108]. However, a more recent clinical study using lower dose of DCA (3-6 mg/ kg b.i.d) for 4 months to treat pulmonary arterial hypertension improved lung capacity and was well tolerated [109]. In this study, an increase in right ventricular ejection fraction was detected even with this lower dose which might mitigate potential side effects. DCA treatment may also induce epigenetic remodeling during HF through histone acetylation that may help reverse the cardiac pathophysiology [110].
Discussion
Various modulators of the metabolic pathways have been discussed here with their results from clinical trials. Early clinical trials that used FAO inhibitors to improve oxygen efficiency and increase glucose oxidation did produce some favorable results but could not reach efficacy milestones. While our understanding of the metabolic changes that occur during the progression of HF has increased, targeting these changes with metabolic small molecules have not done particularly well in clinical trials. SGLT2is have shown the most consistent and efficacious results in clinical trials [111]. Several metabolic therapeutics developed undesirable side effects or were variable in outcomes. One explanation for the variable outcomes of metabolic-based clinical trials in HF may be due to comorbidities which also vary upon gender and type of HF [112]. During the SGLT2i clinical trial using empagliflozin which reduced adverse cardiovascular events in HF patients with type 2 diabetes [113], a subsequent post-hoc analysis [46] indicated that the beneficial outcome of SGLT2i did not depend upon a HF diagnosis and variable differences were noted depending upon the type of cardiovascular disease [72] or type of SGLT2i used [114]. The vast majority of clinical trials in HF are performed in patients under the age of 65 [115]. A common comorbidity and potential clinical trial outcome variability that is often overlooked is a sedentary lifestyle brought on by the chronic illness of HF, particularly in the aged population. A randomized and controlled clinical trial on the effects of exercise training in elderly HF patients significantly improved both cardiac and pulmonary function after only 4 weeks of training [116]. Since HF patients often develop multiple and variable comorbidities, a holistic and multidisciplinary approach is needed to manage HF and to include these populations in future clinical trials.
Conclusion
Metabolic therapies have promise as a sole or add-on to currently used treatments, such as β-blockers to treat HF. SGLT2is have the current best potential to become the new standard in HF care but their mechanistic actions are not known if they are truly metabolic. As HF is very complex with variable comorbidities, a patient-specific approach may be more viable and conjoined with metabolomics, could provide information for the better design of future clinical trials and which metabolic therapy may provide the best treatment options.
Funding
This work was supported by a grant from The Edward N. & Della L. Thome Memorial Foundation Award Programs in Alzheimer’s Disease Drug Discovery Research and an RO1 from the National Institute of Aging (AG066627).
References
- Elia M. Organ and tissue contribution to metabolic rate. In Kinney JM, Tucker HN, Energy metabolism: Tissue determinants and cellular corollaries. New York, NY: Raven Press. 61-80 (1992).
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 85 (3): 1093-1129 (2005).
- Cheng ML, Wang CH, Shiao MS, et al. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: Diagnostic and prognostic value of metabolomics. J Am Coll. Cardiol. 65 (15): 1509-20 (2015).
- De Jong KA, Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol. 33: 860-871 (2017).
- Sack MN, Rader TA, Park S, et al. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 94(11): 2837-2842 (1996).
- Aubert G, Vega RB, Kelly DP. Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart. Biochim Biophys Acta. 1833(4): 840-847 (2013).
- Mentesana G, Baez A, Cordoba R, et al. Role of mitochondria and reactive oxygen species in the progression of heart failure. Rev Fac Cien Med Univ Nac Cordoba. 67 (4): 150-8 (2010).
- Battogtokh G, Choi YS, Kang DS, et al. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: Current strategies and future perspectives. Acta Pharm Sin B. 8 (6): 862-880 (2018).
- Maack C, Bohm M. Targeting mitochondrial oxidative stress in heart failure throttling the afterburner. J Am Coll Cardiol. 58 (1): 83-6 (2011).
- Sorokina N, O'Donnell JM, McKinney RD, et al. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 115 (15): 2033-41 (2007).
- Burke MA, Chang S, Wakimoto H, et al. Molecular profiling of dilated cardiomyopathy that progress to heart failure. JCI Insight. 1 (6): e86898 (2016).
- Diakos NA, Navankasattusas S, Abel ED, et al. Evidence of glycolysis up-regulation and pyruvate mitochondrial oxidation mismatch during mechanical unloading of the failing human heart: Implications for cardiac reloading and conditioning. JACC Basic Transl Sci. 1 (6): 432-444 (2016).
- Sheeran FL, Angerosa J, Liaw NY, et al. Adaptations in protein expression and regulated activity of pyruvate dehydrogenase multienzyme complex in human systolic heart failure. Oxid Med Cell Longev. 2019: 4532592 (2019).
- Gupte AA, Hamilton DJ, Cordero-Reyes AM, et al. Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ Cardiovasc Genet. 7 (3): 266-76 (2014).
- Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 11 (1): 31-39 (2010).
- Randle PJ, Garland PB, Hales CN, et al. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1 (7285): 785-9 (1963).
- Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 284 (5): E855-E862 (2003).
- Randle PJ, England PJ, Denton RM. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 117 (4): 677-695 (1970).
- Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25 (2): 262-284 (2017).
- Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 356 (11): 1140-1151 (2007).
- Bedi KC Jr., Snyder NW, Brandimarto J, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 133 (8): 706-716 (2016).
- Aubert G, Martin OJ, Horton JL, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 133 (8): 698-705 (2016).
- Horton JL, Davidson MT, Kurishima C, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 4 (4): e124079 (2019).
- Russell RR, Taegtmeyer H. Changes in citric acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J Clin Invest. 87 (2): 384-90 (1991).
- Taegtmeyer H. On the ability of ketone bodies to serve as the only energy providing substrate for rat heart at physiological work load. Basic Res. Cardiol. 78: 435-450 (1983).
- Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. New Eng J Med. 325 (5): 293-302 (1991).
- Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: The optimaal randomised trial. Optimal trial in myocardial infarction with angiotensin II antagonist losartan. Lancet. 360 (9335): 752-760 (2002).
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US carvedilol heart failure study group. New Eng J Med. 334 (21): 1349-1355 (1996).
- Bolam H, Morton G, Kalra PR. Drug therapies in chronic heart failure: A focus on reduced ejection fraction. Clin Med (Lond). 18 (2): 138-145 (2018).
- Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. New Eng J Med. 351 (20): 2049-2057 (2004).
- Yoshikawa T, Port JD, Asano K, et al. Cardiac adrenergic receptor effects of carvedilol. Eur Heart J. 17 (SB): 8-16 (1996).
- Kveiborg B, Christiansen B, Major-Petersen A, et al. Metabolic effects of β-adrenoceptor antagonists with special emphasis on carvedilol. Am J Cardiovasc Drugs. 6 (4): 209-217 (2006).
- Jacob S, Rett K, Wicklmayr M, et al. Differential effect of chronic treatment with two β‐blocking agents on insulin sensitivity: The carvedilol‐metoprolol study. J Hypertens. 14 (4): 489-494 (1996).
- Arumanayagam M, Chan S, Tong S, et al. Antioxidant properties of carvedilol and metoprolol in heart failure: A double-blind randomized controlled trial. J Cardiovasc Pharmacol. 37 (1): 48-54 (2001).
- Eichhorn EJ, Bedotto JB, Malloy CR, et al. Effect of β-adrenergic blockade on myocardial function and energetics in congestive heart failure: Improvements in hemodynamic, contractile, and diastolic performance with bucindolol. Circulation. 82 (2): 473-483 (1990).
- Heesch C, Marcoux L, Hatfield B, et al. Hemodynamic and energetic comparison of carvedilol and metoprolol for the treatment of congestive heart failure. Am J Cardiol. 75: 360-364 (1995).
- Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): Randomised controlled trial. Lancet. 362 (9377): 7-13 (2003).
- Wallhaus TR, Taylor M, DeGrado TR, et al. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 103 (20): 2441-6 (2001).
- McAlister FA, Wiebe N, Ezekowitz JA, et al. Meta-analysis: β-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 150 (11): 784-94 (2009).
- Thollon C, Vilaine JP. I(f) inhibition in cardiovascular diseases. Adv Pharmacol. 59: 53-92 (2010).
- Colin P, Ghaleh B, Hittinger L, et al. Differential effects of heart rate reduction and beta-blockade on left ventricular relaxation during exercise. Am J Physiol Heart Circ Physiol. 282 (2): H672-9 (2002).
- Scicchitano P, Cortese F, Ricci G, et al. Ivabradine, coronary artery disease, and heart failure: Beyond rhythm control. Drug Des Devel Ther. 8: 689-700 (2014).
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 91 (2): 733-94 (2011).
- Bonner C, Kerr-Conte J, Gmyr V, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 21 (5): 512-517 (2015).
- Ceriello A, Genovese S, Mannucci E, et al. Glucagon and heart in type 2 diabetes: New perspectives. Cardiovasc Diabetol. 15 (1): 123 (2016).
- Fitchett D, Butler J, van de Borne P, et al. EMPA-REG OUTCOME® trial investigators. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 39 (5): 363-370 (2018).
- Wojcik C, Warden BA. Mechanisms and evidence for heart failure benefits from SGLT2 Inhibitors. Curr Cardiol Rep. 21 (10): 130 (2019).
- Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 396 (10254): 819-829 (2020).
- Gager GM, Gelbenegger G, Jilma B, et al. Cardiovascular outcome in patients treated with SGLT2 inhibitors for heart failure: A meta-analysis. Front Cardiovasc Med. 8: 691907 (2021).
- Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA-REG OUTCOME trial: A “thrifty substrate” hypothesis. Diabetes Care. 39: 1108-1114 (2016).
- Al Jobori H, Daniele G, Adams J, et al. Determinants of the increase in ketone concentration during SGLT2 inhibition in NGT, IFG and T2DM patients. Diabetes Obes Metab. 19 (6): 809-813 (2017).
- Lopaschuk GD, Verma S. Empagliflozin's fuel hypothesis: Not so soon. Cell Metabolism. 24: 200-2 (2016).
- Santos-Gallego CG, Requena-Ibanez JA, San Antonio R et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 73 (15): 1931-1944 (2019).
- Oh CM, Cho S, Jang JY, et al. Cardioprotective potential of an SGLT2 inhibitor against doxorubicin-induced heart failure. Korean Circ J. 49: 1183-1195 (2019).
- Uthman L, Baartscheer A, Schumacher CA, et al. Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front Physiol. 9: 1575 (2018).
- Li C, Zhang J, Xue M, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 18 (1): 15 (2019).
- Xing YJ, Liu BH, Wan SJ, et al. A SGLT2 Inhibitor dapagliflozin alleviates diabetic cardiomyopathy by suppressing high glucose-induced oxidative stress in vivo and in vitro. Front Pharmacol. 12: 708177 (2021).
- Van der Pol A, van Gilst WH, Voors AA, et al. Treating oxidative stress in heart failure: Past, present and future. Eur J Heart Fail. 21: 425-435 (2019).
- Packer M. Cardioprotective effects of Sirtuin-1 and its downstream effectors: Potential role in mediating the heart failure benefits of SGLT2 (Sodium-Glucose Cotransporter 2) inhibitors. Circ Heart Fail. 13 (9): e007197 (2020).
- Haigis MC, Sinclair DA. Mammalian sirtuins: Biological insights and disease relevance. Annu Rev Pathol. 5: 253-295 (2010).
- Alcendor RR, Gao S, Zhai P, et al. SIRT1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 100 (10): 1512-1521 (2007).
- Planavila A, Redondo-Angulo I, Ribas F, et al. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res. 106 (1): 19-31 (2015).
- Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 434 (7029): 113-118 (2005).
- Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 425 (6954): 191-6 (2003).
- Guo Y, Zhang L, Li F, et al. Restoration of SIRT1 function by pterostilbene attenuates hypoxia-reoxygenation injury in cardiomyocytes. Eur J Pharmacol. 776: 26-33 (2016).
- Libri V, Brown AP, Gambarota G, et al. A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS One. 7 (12): e51395 (2012).
- Venkatasubramanian S, Noh RM, Daga S, et al. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J Am Heart Assoc. 2 (3): e000042 (2013).
- Nikolic M, Zivkovic V, Jovic JJ, et al. SGLT2 inhibitors: A focus on cardiac benefits and potential mechanisms. Heart Fail Rev. (2021).
- Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 60 (1): 143-187 (1980).
- Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium glucose cotransporter-2 inhibition in heart failure: Potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 136: 1643-1658 (2017).
- Sayour AA, Oláh A, Ruppert M, et al. Characterization of left ventricular myocardial sodium-glucose cotransporter 1 expression in patients with end-stage heart failure. Cardiovasc Diabetol. 19 (1): 159 (2020).
- Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 393 (10166): 31-39 (2019).
- Qiu M, Ding LL, Zhang M, et al. Safety of four SGLT2 inhibitors in three chronic diseases: A meta-analysis of large randomized trials of SGLT2 inhibitors. Diab Vasc Dis Res. 18 (2): 14791641211011016 (2021).
- Caponnetto S, Canale C, Masperone MA, et al. Efficacy of l-propionylcarnitine treatment in patients with left ventricular dysfunction. Eur Heart J. 15: 1267-1273 (1994).
- Russian Study on propionyl-L-carnitine in chronic heart failure. Eur Heart J. 20 (1): 70-6 (1999).
- Dzerve V, Matisone D, Kukulis I, et al. Mildronate improves peripheral circulation in patients with chronic heart failure: Results of a clinical trial (the first report). Cardiology. 11: 56-64 (2005).
- Tsverava MD. Influence of mildronat on left ventricular systolic, diastolic functional parameters, pulmonary arterial flow and systolic dyssynchrony in patients with congestive heart failure. Georgian Med News. 218: 34-40 (2013).
- Statsenko ME, Shilina NN, Turkina SV. Use of meldonium in the combination treatment of patients with heart failure in the early postinfarction period. Ter Arkh. 86 (4): 30-5 (2014).
- Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci. 99 (1): 27-35 (2000).
- Holubarsch CJ, Rohrbach M, Karrasch M, et al. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: The ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci. 113 (4): 205-212 (2007).
- Abozguia K, Elliott P, McKenna W, et al. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation. 122 (16): 1562-1569 (2010).
- Lee L, Campbell R, Scheuermann-Freestone M, et al. Metabolic modulation with perhexiline in chronic heart failure: A randomized, controlled trial of short-term use of a novel treatment. Circulation. 112: 3280-3288 (2005).
- Horowitz JD, Sia ST, Macdonald PS, et al. Perhexiline maleate treatment for severe angina pectoris: Correlations with pharmacokinetics. Int J Cardiol. 13 (2): 219-229 (1986).
- Cole PL, Beamer AD, McGowan N, et al. Efficacy and safety of perhexiline maleate in refractory angina: A double-blind placebo-controlled clinical trial of a novel antianginal agent. Circulation. 81: 1260-1270 (1990).
- Ananthakrishna R, Lee SL, Foote J, et al. Randomized controlled trial of perhexiline on regression of left ventricular hypertrophy in patients with symptomatic hypertrophic cardiomyopathy (RESOLVE-HCM trial). Am Heart J. 240: 101-113 (2021).
- Kantor PF, Lucien A, Kozak R, et al. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 86 (5): 580-8 (2000).
- MacInnes A, Fairman DA, Binding P, et al. The antianginal agent trimetazidine does not exert its functional benefit via inhibition of mitochondrial long-chain 3-ketoacyl coenzyme a thiolase. Circ Res. 93 (3): e26-32 (2003).
- Hayashida W, van Eyll C, Rousseau MF, et al. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther. 8 (5): 741-7 (1994).
- Fragasso G, Palloshi A, Puccetti P, et al. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol. 48 (5): 992-998 (2006).
- Fragasso G, Rosano G, Baek SH, et al. Effect of partial fatty acid oxidation inhibition with trimetazidine on mortality and morbidity in heart failure: Results from an international multicentre retrospective cohort study. Int J Cardiol. 163 (3): 320-325 (2013).
- Di Napoli P, Di Giovanni P, Gaeta MA, et al. Beneficial effects of trimetazidine treatment on exercise tolerance and B-type natriuretic peptide and troponin T plasma levels in patients with stable ischemic cardiomyopathy. Am Heart J. 154: 602.e1-602e5 (2007).
- Tuunanen H, Engblom E, Naum A, et al. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation. 118 (12): 1250-1258 (2008).
- Gao D, Ning N, Niu X, et al. Trimetazidine: A meta-analysis of randomised controlled trials in heart failure. Heart. 97 (4): 278-86 (2011).
- Milinković I, Rosano G, Lopatin Y, et al. The role of ivabradine and trimetazidine in the New ESC HF guidelines. Card Fail Rev. 2 (2): 123-129 (2016).
- Coats CJ, Pavlou M, Watkinson OT, et al. Effect of trimetazidine dihydrochloride therapy on exercise capacity in patients with nonobstructive hypertrophic cardiomyopathy: A randomized clinical trial. JAMA Cardiol. 4 (3): 230-235 (2019).
- Pepine CJ, Wolff AA. A controlled trial with a novel anti-ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional antianginal agents. Ranolazine study group. Am J Cardiol. 84 (1): 46-50 (1999).
- Maier LS, Layug B, Karwatowska-Prokopczuk E, et al. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: The RALI-DHF proof-of-concept study. JACC Heart Fail. 1 (2): 115-122 (2013).
- Olivotto I, Camici PG, Merlini PA, et al. Efficacy of ranolazine in patients with symptomatic hypertrophic cardiomyopathy: The RESTYLE-HCM randomized, double-blind, placebo-controlled study. Circ Heart Fail. 11 (1): e004124 (2018).
- Zhang X, Ma S, Fu B. Effects of trimetazidine in combination with bisoprolol in patients with chronic heart failure and concomitant chronic obstructive pulmonary disease: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 100 (15): e25491 (2021).
- Ke Y, Xu D, Li M, et al. Effects of bisoprolol in combination with trimetazidine on the treatment of heart failure and concomitant chronic obstructive pulmonary disease. Pak J Med Sci. 32 (5): 1208-1212 (2016).
- Ke Y, Xu D, Li M, et al. Effects of bisoprolol in combination with trimetazidine on the treatment of heart failure and concomitant chronic obstructive pulmonary disease. Pak J Med Sci. 32 (5): 1208-1212 (2016).
- Chambers KT, Leone TC, Sambandam N, et al. Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response. J Biol Chem. 286 (13): 11155-1162 (2011).
- Atherton HJ, Dodd MS, Heather LC, et al. Role of pyruvate dehydrogenase inhibition in the development of hypertrophy in the hyperthyroid rat heart: A combined magnetic resonance imaging and hyperpolarized magnetic resonance spectroscopy study. Circulation. 123 (2): 2552- 2561 (2011).
- Wang P, Lloyd SG, Chatham JC. Impact of high glucose/high insulin and dichloroacetate treatment on carbohydrate oxidation and functional recovery after low-flow ischemia and reperfusion in the isolated perfused rat heart. Circulation. 111 (16): 2066-2072 (2005).
- Bøgh N, Hansen ESS, Omann C, et al. Increasing carbohydrate oxidation improves contractile reserves and prevents hypertrophy in porcine right heart failure. Sci Rep. 10 (1): 8158 (2020).
- Bersin RM, Wolfe C, Kwasman M, et al. Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. J Am Coll Cardiol. 23 (7): 1617-1624 (1994).
- Lewis JF, DaCosta M, Wargowich T, et al. Effects of dichloroacetate in patients with congestive heart failure. Clin Cardiol. 21 (12): 888-892 (1998).
- Abdelmalak M, Lew A, Ramezani R, et al. Long-term safety of dichloroacetate in congenital lactic acidosis. Mol Genet Metab. 109 (2): 139- 143 (2013).
- Tataranni T, Piccoli C. Dichloroacetate (DCA) and cancer: An overview towards clinical applications. Oxid Med Cell Longev. (2019).
- Michelakis ED, Gurtu V, Webster L, et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med. 9 (413): eaao4583 (2017).
- Matsuhashi T, Hishiki T, Zhou H, et al. Activation of pyruvate dehydrogenase by dichloroacetate has the potential to induce epigenetic remodeling in the heart. J Mol Cell Cardiol. 82: 116-24 (2015).
- Halimi JM. SGLT2 inhibitors: A new era for our patients. Nephrol Ther. 17 (3): 143-148 (2021).
- Correale M, Paolillo S, Mercurio V, et al. Comorbidities in chronic heart failure: An update from Italian Society of Cardiology (SIC) working group on heart failure. Eur J Intern Med. 71: 23-31 (2020).
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 373 (22): 2117-28 (2015).
- Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 139 (22): 2516-2527 (2019).
- Orimo H, Ito H, Suzuki T, et al. Reviewing the definition of “elderly”. Geriat Geront Int. 6 (3): 149-158 (2006).