Review Article - Interventional Cardiology (2023)
The rationale behind adiponectin serum level as an independent predictor of mortality after TAVI
- Corresponding Author:
- Johan Bosmans
Department of Health Sciences and Medicine, University of Antwerp, Campus Drie Eiken Universiteitsplein 1, 2610 Wilrijk, Belgium,
E-mail: johan.bosmans@uza.be
Received date: 29-Sep-2023, Manuscript No. FMIC-23-115211; Editor assigned: 02-Oct-2023, PreQC No. FMIC-23-115211 (PQ); Reviewed date: 16-Oct-2023, QC No. FMIC-23-115211; Revised date: 23-Oct-2023, Manuscript No. FMIC-23-115211 (R); Published date: 31-Oct-2023, DOI: 10.37532/1755- 5310.2023.15(S19 ).472
Abstract
Transcatheter Aortic Valve Implantation (TAVI) is a suitable treatment for patients with severe symptomatic Aortic Stenosis (AS) ≥ 75 years or with high operative risk. Adiponectin, released by adipose tissue, has been associated with cardio protective properties: Anti-inflammatory and anti-atherosclerotic features, improved insulin sensitivity, and attenuated LV remodeling.
Nevertheless, several studies have reported the counter-intuitive finding that high adiponectin serum levels were associated with poor survival. Situations in which high adiponectin levels do not translate into beneficial insulin sensitivity and health benefits have been coined “the adiponectin paradox” in the medical literature.
A recent study reported that the adiponectin serum concentration before TAVI is an independent predictor of all-cause 5-year mortality after TAVI. Compared to patients with the lowest adiponectin level, patients in the third tertile had a hazards ratio of all−cause mortality after TAVI of 4.155 (95% CI: 1.364-12.655) (p=0.004), tested in a multivariable model, including classical clinical operative risk assessment, CT- measured psoas muscle density, and adiponectin serum level. In a supplemental multivariable model including LDL cholesterol serum levels, adiponectin plasma concentration remained an independent predictor of all-cause mortality after TAVI.
The European Prospective Investigation into Cancer and Nutrition (EPIC)-Heidelberg cohort study provided new insight into the adiponectin paradox and may explain these counter-intuitive study results in the TAVI population. A significant heterogeneity by N-Terminal pro B-type Natriuretic Peptide (NT-proBNP) in the association between total adiponectin and all-cause mortality was observed, with a significant increase in hazards of cardiovascular mortality restricted to participants in the highest N-Terminal pro B-type Natriuretic Peptide (NT-proBNP) levels. Among these latter participants, adiponectin showed a dose-response relationship with total mortality. Given the pathophysiological features of severe symptomatic AS and the clinical characteristics of the frail TAVI-candidates population, it is plausible that these latter patients represent a cohort with increased levels of NT-pro-BNP levels.
In this review, we briefly discuss the biological actions of adiponectin, the factors influencing adiponectin levels, the studies examining an association between adiponectin level and AS severity progression, the adiponectin paradox, and adiponectin as a biomarker to predict survival after TAVI.
Keywords
Transcatheter aortic valve implantation • Aortic stenosis • Adiponectin serum
Background
Physiological activity of adiponectin and shared pathophysiological processes by atherosclerosis and calcific AS severity progression
Physiological features of adiponectin: White adipocyte tissue secretes adiponectin. Adiponectin consists of 244 amino acids. The adiponectin molecule displays structural similarities to collagen and TNFalpha [1,2], and weights 30 kDa. Adiponectin is found in the blood in varying multimers, primarily as Low-Molecular-Weight (LMW) trimers, but also as Middle-Molecular Weight (MMW) hexamers, and High-Molecular-Weight (HMW) multimers [3,4]. The HMV multimers are most potent adiponectin multimers in activating AMP-activated Protein Kinase (AMPK), which is a stress-responsive kinase [5,6].
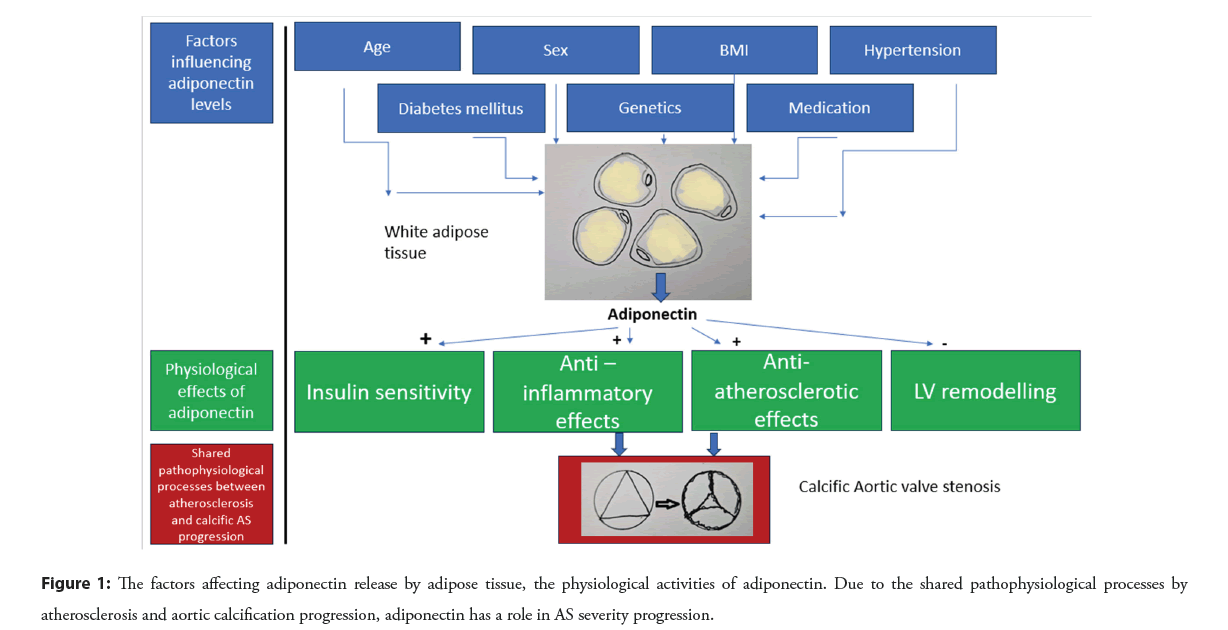
Several cardioprotective properties have been attributed to adiponectin [7]: (1) Increased insulin sensitivity, (2) Anti- inflammatory features, (3) Anti-atherosclerotic effects, (4) Protection against extreme LV remodeling.
Metabolic effects of adiponectin
Adiponectin induces increased glucose tolerance: Increased insulin sensitivity is a feature attributed to adiponectin. Indeed, a prospective study demonstrated that the higher the blood adiponectin level, the lower the risk for diabetes in humans [8]. Lindsay, et al., found that blood adiponectin levels represent a better marker for the risk of onset of diabetes than serum glucose and insulin levels [9]. Another study demonstrated that the greater the HMW adiponectin level, the lower the incidence of diabetes [10].
Adiponectin and dyslipidemia: Clinical studies suggest a positive correlation between blood adiponectin levels and HDL-cholesterol levels and a negative correlation with triglyceride concentrations [11].
Anti-inflammatory effects of adiponectin
In a review, Hopkins, et al., concluded that in vitro studies indicate that adiponectin inhibits nuclear factor-κB (NF-κB) activation, effectively reducing [12]: (1) TNFα-stimulated monocyte adhesion to endothelial cells. (2) TNF-α-stimulated expression of adhesion molecules. (3) Expression of the pro-inflammatory cytokine IL-8 in endothelial cells [13,14].
Adiponectin reduces TNF-α production in human macrophages [15], increases the expression of the anti-inflammatory cytokine IL-10 [16], and inhibits the transformation of macrophages to foam cells [17].
The mechanisms mentioned above indicate that adiponectin has a key role in the genesis and progression of obesity-related vascular diseases via its anti-inflammatory features [12].
Anti-atherosclerotic effects of adiponectin
Adiponectin has anti-atherosclerotic properties, via its metabolic effects (See the Metabolic effects of adiponectin and Anti- inflammatory effects of adiponectin) and its anti-inflammatory features (See the Anti-inflammatory effects of adiponectin). Adiponectin also acts on vascular endothelial cells, thus directly inhibiting atherosclerosis in humans [18].
Iwabu, et al., summarized the key animal experiments [18]: (1) In adiponectin-deficient mice, vascular damage was associated with worsening of neointimal formation [2,19]. (2) Adiponectin overexpression leads to inhibition of atherosclerosis in ApoE- deficient mice, a well-known model of atherosclerosis [20].
There is also data involving humans. The beneficial association between adiponectin level and reduced risk of new onset of myocardial infarction remained after adjustment for other risk factors [1,21]. Kumada, et al., demonstrated that male patients with hypoadiponectinemia (<4.0 mcg/mL) had a significant 2-fold increase in CAD prevalence, independent of well-known CAD risk factors [22].
Adiponectin and LV remodeling
Experimental studies demonstrated that adiponectin influences cardiac remodeling and suppresses pathological cardiac growth. In APN-KO mice, pressure overload, created by aortic constriction, causes enhanced concentric ventricular hypertrophy and increased mortality [23]. However, adenovirus-mediated delivery of adiponectin attenuated cardiac hypertrophy in response to pressure overload in APN-KO mice [23].
Adiponectin promotes the phosphorylation of AMPK, resulting in the activation AMPK signaling cascade [12]. Experiments in rat cardiac myocytes show that adiponectin activates AMPK and inhibits the hypertrophic response to α-adrenergic receptor stimulation [23], while the inhibition of hypertrophic growth by adiponectin could be reversed by transduction with dominant- negative AMPK.
Literature Review
Factors affecting adiponectin concentration
Age [24], diabetes mellitus [25,26], obesity [27], sex [28], hypertension [29], Smoking [30-32], and medication [33,34], affect circulating adiponectin levels are shown in the Figure 1.
The anti-aging gene Sirtuin 1 has been identified as an essential determinant of adiponectin levels [35,36]. Authors have emphasized the role of Sirtuin 1 in proteomic-based approaches for biomarker investigation to interpret the severity of global disease progression [35,37]. Therefore, the measurements of adiponectin serum levels may need to be interpreted in conjunction with Sirtuin 1 levels to predict the survival of chronic cardiovascular disease.
Studies searching for an association between adiponectin serum concentration and AS severity progression
Studies examining the association between adiponectin serum concentration and AS severity progression, are relatively rare, and sample sizes are relatively small.
A study, including 58 patients with calcific aortic stenosis and 24 healthy controls, did not find an association between adiponectin levels and calcific aortic valve disease [38].
Another study (74 patients with AS and 74 control patients) showed that lower levels of adiponectin were associated with severe AS. According to the authors, these findings suggest that adipocytokines may be involved in the progression of AS, and adiponectin may play a protective role in this process [39].
Shetty, et al., including a cohort of 220 patients with aortic bioprosthetic valves, demonstrated that bioprosthetic valve dysfunction was significantly associated with an increased proportion of small, dense LDL. Moreover, patients with an elevated level of %LDL along with a decreased plasma adiponectin level were at a very high risk of developing early bioprosthetic hemodynamic dysfunction (OR=2.54, p=0.04) [40].
Another study, including 122 patients undergoing Aortic Valve Replacement (AVR), found that patients with lower plasma levels of adiponectin had a faster progression rate of the mean transvalvular gradient before surgery than those with higher levels (p=0.008) [41].
Mizia-Stec, et al., demonstrated that there were no differences in the adipokines concentrations between patients with AS (n=65) and a control group (n=24). Compared to patients without CAD, patients with AS and coexisting CAD had decreased serum adiponectin (p=0.040) levels. Multivariate regression analysis demonstrated that age (p=0.015) and E/E’ index (p=0.032) were independent predictors of the adiponectin level in the AS group [42]. The authors concluded that AS with preserved EF does not change the adipokine serum profile. Co-existing atherosclerosis modified the adipokines levels. Degeneration of inconsistent findings, there seems to be a trend suggesting that low adiponectin serum levels promote a faster AS severity progression or aortic valve bioprosthetic degeneration.
NT-pro-BNP and the adiponectin paradox: New insights EPIC-Heidelberg cohort study
Based on the biological activity of adiponectin, hypoadiponectinemia is expected in patients with insulin resistance, metabolic syndrome, atherosclerotic disease, vascular depression, cancer, and dementia [43-46]. Nevertheless, studies reported counterintuitive results. A review by Menzaghi and Trischitta demonstrated that several studies found that all-cause mortality correlated positively with hyperadiponectinemia instead of hypoadiponectinemia in many clinical conditions such as type 2 diabetes mellitus [47]. These authors stated that the underlying biology of this paradox is unknown, but several hypotheses have been proposed. They emphasized that none of these hypotheses are based on robust data. The following mechanisms have been proposed [47]:
a) Increasing adiponectin levels may fail to protect individuals with a high risk of mortality due to adiponectin resistance in metabolically active organs [48].
b) Based on the observation that Natriuretic Peptides (NPs) enhance adiponectin production in human adipocytes [49], the adiponectin paradox may be driven by the direct and strong correlation between adiponectin and NPs [49,50]. This hypothesis postulates that NPs are the real mortality risk factors, with adiponectin being only a marker of increased NPs. However, studies searching for the association between serum adiponectin and mortality rate have generated conflicting data [47]. After adjustment for the NPs, the adiponectin paradox was no longer consistently present among these reports [51- 55].
c) Genetic evidence suggests a direct role of adiponectin in increasing the risk of death [56,57]. In a prospective study, including 356 patients with diabetes mellitus and proven coronary artery disease, a single nucleotide polymorphism in the ADIPOQ locus was strongly associated with adiponectin levels and cardiovascular mortality. According to the investigators, these findings suggest a direct deleterious role of adiponectin in increasing the mortality risk [56].
d) Unfavourable effects of adiponectin on inflammatory processes under specific conditions have been reported and may contribute to the adiponectin paradox. In colonic epithelial cells, adiponectin exerted proinflammatory effects by inducing chemokine production [58]. In rheumatoid arthritis, recombinant adiponectin induced synthesis of IL-6 [59]. These data seem to suggest that under some chronic inflammatory conditions, adiponectin exacerbates inflammation, instead of being an anti-inflammatory factor. Interestingly, Menzaghi and Trischitta speculated whether those unexpected unfavourable effects of adiponectin on some low-grade chronic inflammatory conditions, such as CVD and in many frail individuals, may explain/contribute to the adiponectin paradox [47].
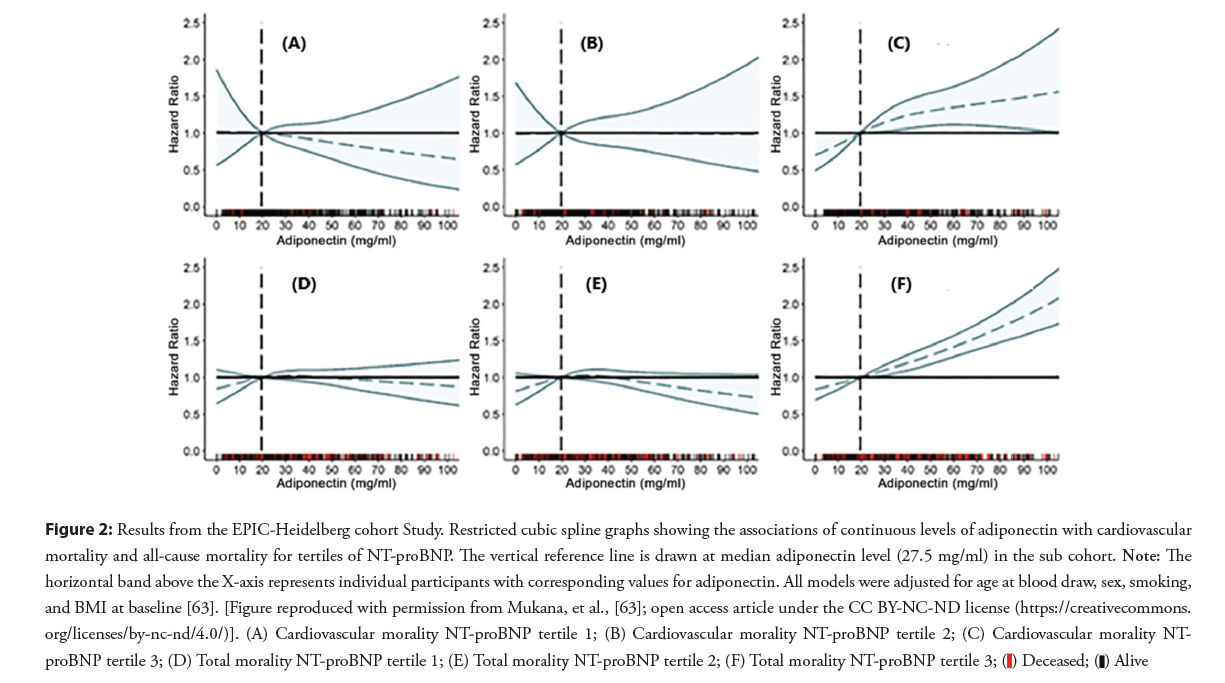
e) The conditions in which high adiponectin levels do not translate into beneficial insulin sensitivity and health benefits are often referred to as “the adiponectin paradox” [46,47,60-62]. The case-cohort design nested within the EPIC-Heidelberg cohort included 25,540 participants with a median follow-up of 15.6 years [63]. Adiponectin was statistically significantly associated with all-cause mortality, but not with cardiovascular mortality. Significant heterogeneity by NT-proBNP in the association between total adiponectin and all-cause mortality was observed. A significant increase in hazards of mortality was restricted to participants in the highest tertile of NT-pro-BNP. Among these participants, adiponectin showed a dose-response relationship with total mortality (Figure 2). Compared to participants in the lowest quintile, participants in the third, fourth, and fifth were at 1.22, 1.50, and 1.59 higher hazards of mortality, respectively. This study supports the hypothesis that NT-proBNP may explain the adiponectin paradox [63].
Figure 2: Results from the EPIC-Heidelberg cohort Study. Restricted cubic spline graphs showing the associations of continuous levels of adiponectin with cardiovascular
mortality and all-cause mortality for tertiles of NT-proBNP. The vertical reference line is drawn at median adiponectin level (27.5 mg/ml) in the sub cohort. Note: The
horizontal band above the X-axis represents individual participants with corresponding values for adiponectin. All models were adjusted for age at blood draw, sex, smoking,
and BMI at baseline [63]. [Figure reproduced with permission from Mukana, et al., [63]; open access article under the CC BY-NC-ND license (https://creativecommons.
org/licenses/by-nc-nd/4.0/)]. (A) Cardiovascular morality NT-proBNP tertile 1; (B) Cardiovascular morality NT-proBNP tertile 2; (C) Cardiovascular morality NT-
proBNP tertile 3; (D) Total morality NT-proBNP tertile 1; (E) Total morality NT-proBNP tertile 2; (F) Total morality NT-proBNP tertile 3; 
Adiponectin as a predictor of 5-year mortality after TAVI treatment for AS
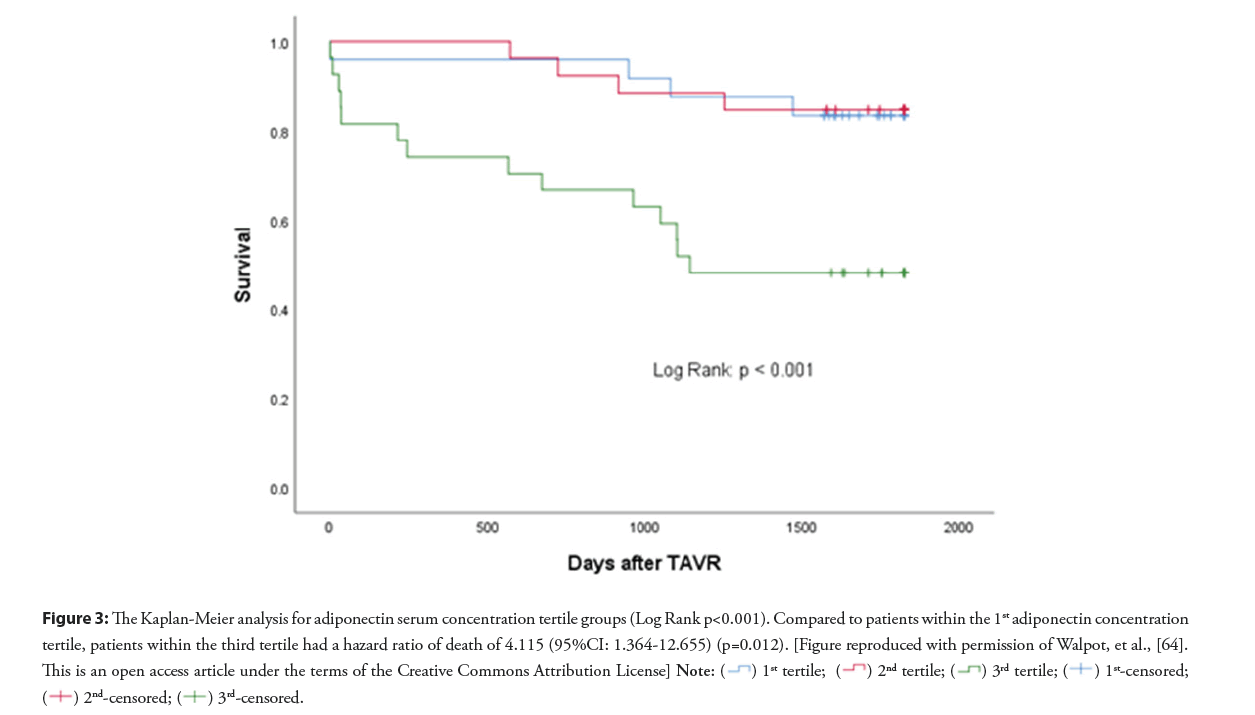
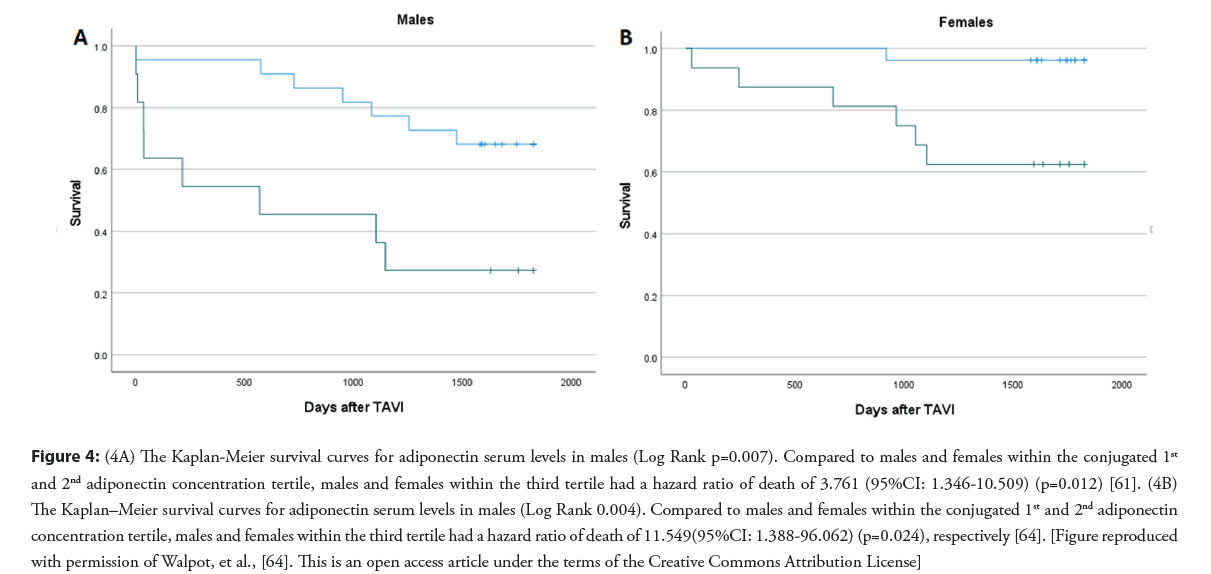
Previously, we reported study results that adiponectin serum concentration is an independent predictor of al-cause 5-year mortality after TAVI (Figures 3 and 4) [64]. In this study, the value of adiponectin serum level before TAVI to predict the 5-year survival after TAVI was tested in a multivariable model, including classical clinical operative risk assessment, CT-measured low muscle density fraction of the psoas muscle (LDM%), and adiponectin serum level. Compared to patients with the lowest adiponectin level, patients in the third tertile had a hazards ratio of all−cause mortality after TAVI of 4.155 (95% CI: 1.364-12.655) (p=0.004) [64]. Adiponectin level was a predictor of all−cause mortality in females and males (p=0.012 and 0.024, respectively) [64].
Figure 3: The Kaplan-Meier analysis for adiponectin serum concentration tertile groups (Log Rank p<0.001). Compared to patients within the 1st adiponectin concentration
tertile, patients within the third tertile had a hazard ratio of death of 4.115 (95%CI: 1.364-12.655) (p=0.012). [Figure reproduced with permission of Walpot, et al., [64].
This is an open access article under the terms of the Creative Commons Attribution License] 
Figure 4: (4A) The Kaplan-Meier survival curves for adiponectin serum levels in males (Log Rank p=0.007). Compared to males and females within the conjugated 1st and 2nd adiponectin concentration tertile, males and females within the third tertile had a hazard ratio of death of 3.761 (95%CI: 1.346-10.509) (p=0.012) [61]. (4B) The Kaplan–Meier survival curves for adiponectin serum levels in males (Log Rank 0.004). Compared to males and females within the conjugated 1st and 2nd adiponectin concentration tertile, males and females within the third tertile had a hazard ratio of death of 11.549(95%CI: 1.388-96.062) (p=0.024), respectively [64]. [Figure reproduced with permission of Walpot, et al., [64]. This is an open access article under the terms of the Creative Commons Attribution License]
It should be emphasized that the Society of Thoracic Surgeons’ risk score (STS score) includes most known factors influencing adiponectin levels, such as age, sex, hypertension, diabetes mellitus, and tobacco use. In a supplemental multivariable model also including LDL cholesterol serum level, adiponectin serum level remained an independent predictor of all-cause mortality after TAVI. Compared to patients in the conjugated first and second tertiles, patients in the third adiponectin tertile had an HR of 4.382 (95%CI 1.317-14,575; p=0.016).
Discussion
Given the cardioprotective features of adiponectin, our study results with excessive 5-year mortality after TAVI in patients within the highest adiponectin serum concentration tertile, are counter-intuitive.
The ´NT-pro-BNP and adiponectin paradox´ hypothesis from the EPIC-Heidelberg cohort study and insights from frailty studies provide important clues to explain our study results. The arguments below sound sensible:
Homogeneity of our study population
It can be argued that a new insight, learned from the ´NT-pro- BNP and adiponectin paradox´ hypothesis from the EPIC- Heidelberg cohort study, is that studies, examining the clinical utility of adiponectin as a predictor of clinical outcomes, should be performed in relatively homogeneous study populations.
We think our study population meets this criterion. All patients suffered from severe symptomatic AS and were elderly, with a mean age of 80.8 ± 7.4 years [64].
Furthermore, the enrolment of patients in our study happened between January 2014 and June 2016, according to the ESC guidelines for TAVI implantation in charge at that time. At the time of enrolment in our study, high operative risk was mandatory to be a TAVI candidate, further narrowing the study population to a very frail study cohort.
NT-pro-BNP and adiponectin
The EPIC-Heidelberg cohort study only demonstrated an association between adiponectin serum level and cardiovascular mortality in the subgroup with the highest NT-pro-BNP levels.
Given the pathophysiology of AS, it is reasonable to assume that study populations of patients undergoing TAVI are most likely to correspond best with patients in the third NT-pro-BNP tertile of the EPIC-Heidelberg cohort study. The high-pressure gradient across the stenotic aortic valve implicates high systolic LV pressure during LV emptying, increasing LV wall stress, and triggering NT- pro-BNP release. Cardiac Magnetic Resonance (CMR) studies have documented that the presence of late gadolinium enhancement, a marker for scar, in the LV myocardium in patients with AS is associated with all-cause and cardiovascular mortality [65,66]. The adaptive LV concentric remodeling in AS may contribute to LV diastolic dysfunction, which has been associated with mortality in patients with AS [67]. AS has become a disease that predominantly affects the elderly, with a prevalence of 1.7% in the population>65 years old [68]. The effect of age on diastolic dysfunction has been well documented [69], and LV diastolic dysfunction has been associated with NT-proBNP levels in patients with AS [70].
NT pro-BNP is linked to the severity of AS and New York Heart Association class and is an indication for AVR [71]. Weber, et al., demonstrated NT-proBNP is elevated in patients with aortic valve disease, and linked to disease severity.
Many studies reported that NT-pro-BNP level before AVR or TAVI is an independent predictor of adverse outcomes after TAVI [72-74]. A meta-analysis, pooling the data of 21 studies found that all-cause mortality after intervention for AS was significantly associated with elevated baseline levels of NT-pro-BNP (HR 1.73; 95% CI 1.45-2.06; p=0.00001) compared to lower baseline biomarker levels [75].
Conclusion
In this review, we aimed to explain the rationale behind adiponectin serum concentration as a predictor of adverse clinical outcomes after TAVI. The following messages can be retrieved from the medical literature findings:
1. Adiponectin has cardioprotective properties.
2. Despite inconsistent study results, there is a trend toward a correlation between adiponectin serum concentration and AS severity progression, with low adiponectin levels favoring AS severity progression.
3. Adiponectin is an independent predictor of 5-year survival after TAVI. Patients with the highest adiponectin have the worst survival.
4. The literature findings in (1) and (3) seem contradictory. Previous frailty studies also found that higher adiponectin levels were correlated with frailty and cognitive disorders. Authors have coined these seemingly contradictory findings as ´the adiponectin paradox´.
5. Recent findings from the EPIC- Heidelberg study cohort have provided valuable new insights into the adiponectin paradox. It was demonstrated that in patients with the highest NT-pro-BNP levels, there was a dose response curve between adiponectin levels and cardiovascular death, while this was not found in the patients in the lower NT-pro-BNP level tertiles. These results may clarify why adiponectin serum level functions well as a predictor of mortality after TAVI. It is sensible to assume that the population of TAVI candidates represents a population with increased NT-pro-BNP levels, given the nature of the underlying severe symptomatic AS as well as the age of this population.
Disclosure
HH: Dr. Heidbuchel did not receive any personal honoraria. He received unconditional research grants through the University of Antwerp and/or the University of Hasselt from Bayer, Boehringer- Ingelheim, Bracco Imaging Europe, Abbott, Medtronic, Biotronik, Daicchi-Sankyo, Pfizer-BMS, and Boston-Scientific, all outside the scope of this work. JB: Dr. Bosmans is a proctor for Medtronic CoreValve.
References
- Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules. Circulation. 100(25):2473-2476 (1999).
- Matsuda M, Shimomura I, Sata M, et al. Role of adiponectin in preventing vascular stenosis: The missing link of adipo-vascular axis. J Biol Chem. 277(40):37487-37491 (2002).
- Pajvani UB, Du X, Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. J Biol Chem. 278(11):9073-9085 (2003).
- Waki H, Yamauchi T, Kamon J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. J Biol Chem. 278(41):40352-40363 (2003).
- Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 8(11):1288-1295 (2002).
- Kobayashi H, Ouchi N, Kihara S, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 94(4) (2004).
- Ouchi N, Shibata R, Walsh K, et al. Cardioprotection by adiponectin. Trends Cardiovasc Med. 16(5):141-146 (2006).
- Li S, Shin HJ, Ding EL, et al. Adiponectin levels and risk of type 2 diabetes. JAMA. 302(2):179 (2009).
- Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 360(9326):57-58 (2002).
- Nakashima R, Kamei N, Yamane K, et al. Decreased total and high molecular weight adiponectin are independent risk factors for the development of type 2 diabetes in Japanese-Americans. J Clin Endocrinol Metab. 91(10):3873-3877 (2006).
- Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, Metabolic risk factors, and Cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 13(1):134-141 (2002).
- Hopkins T, Ouchi N, Shibata R, et al. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 74(1):11-18 (2007).
- Kobashi C, Urakaze M, Kishida M, et al. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 97(12):1245-1252 (2005).
- Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signalling through a camp-dependent pathway. Circulation. 102(11):1296-1301 (2000).
- Yokota T, Oritani K, Takahashi I, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 96(5):1723-1732 (2000).
- Kumada M, Kihara S, Ouchi N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 109(17):2046-2049 (2004).
- Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class a scavenger receptor expression in human monocyte-derived macrophages. Circulation. 103(8):1057-1063 (2001).
- Iwabu M, Okada-Iwabu M, Yamauchi T, et al. Adiponectin/adipor research and its implications for lifestyle-related diseases. Front Cardiovasc Med. (2019).
- Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 277(29):25863-25866 (2002).
- Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes and apoe-deficient mice from atherosclerosis. J Biol Chem. 278(4):2461-2468 (2003).
- Hara K, Yamauchi T, Imai Y, et al. Reduced adiponectin level is associated with severity of coronary artery disease. Int Heart J. 48(2):149-153 (2007).
- Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 23(1):85-89 (2003).
- Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 10(12):1384-1389 (2004).
- Obata Y, Yamada Y, Takahi Y, et al. Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clin Endocrinol (Oxf). 79(2):204-210 (2013).
- Zusi C, Csermely A, Rinaldi E, et al. Crosstalk between genetic variability of adiponectin and leptin, glucose−insulin system and subclinical atherosclerosis in patients with newly diagnosed type 2 diabetes (2023).
- Gao S, Su S, Zhang E, et al. The effect of circulating adiponectin levels on incident gestational diabetes mellitus: Systematic review and meta analysis. Ann Med. 55(1):2224046 (2023).
- Tadiotto MC, Corazza PRP, Menezes Junior FJ, et al. Lower adiponectin is associated with higher anthropometry and insulin resistance but not with low cardiorespiratory fitness in adolescents. J Endocrinol Invest (2023).
- Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia. 46(4):459-469 (2003).
- Cunha WR, Gaspar IC, de Souza BC, et al. High molecular weight adiponectin as a biomarker of hypertension in children and adolescents with obesity. Eur J Pediatr. 182(6):2925-2931 (2023).
- Abbasi F, Farin HMF, Lamendola C, et al. The Relationship between plasma adiponectin concentration and insulin resistance is altered in smokers. J Clin Endocrinol Metab. 91(12):5002-5007 (2006).
- Iwashima Y, Katsuya T, Ishikawa K, et al. Association of hypoadiponectinemia with smoking habit in men. Hypertension. 45(6):1094-1100 (2005).
- Kawamoto R, Tabara Y, Kohara K, et al. Smoking status is associated with serum high molecular adiponectin levels in community-dwelling Japanese men. J Atheroscler Thromb. 2010;17(4):423-430 (2010).
- Youssef ME, Yahya G, Popoviciu MS, et al. Unlocking the full potential of SGLT2 inhibitors: expanding applications beyond glycemic control. Int J Mol Sci. 24(7):6039 (2023).
- Shevchuk S V, Seheda YS, Kuvikova IP, et al. The effect of atorvastatinum in the treatment of patients with rheumatoid arthritis. Wiad Lek. 73(11):2427-2430 (2020).
- Martins IJ. Sirtuin 1. A diagnostic protein marker and its relevance to chronic disease and therapeutic drug interventions. EC Pharmacol Toxicol. 6(4):209-215 (2018).
- Martins I. The role of clinical proteomics, lipidomics, and genomics in the diagnosis of alzheimer’s disease. Proteomes. 4(2):14 (2016).
- Martins IJ. Anti-Aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Adv Aging Res. 05(01):9-26 (2016).
- Gucuk Ipek E, Guray U, Guray Y, et al. Relationship between serum adiponectin levels and calcific aortic valve disease. Kardiol Pol. 71(3):241-246 (2013).
- Kolasa-Trela R, Miszalski-Jamka T, Grudzień G, et al. Adiponectin, leptin, and resistin in patients with aortic stenosis without concomitant atherosclerotic vascular disease. Pol Arch Med Wewn. 121(10):352-359 (2011).
- Shetty R, Girerd N, Côté N, et al. Elevated proportion of small, dense low-density lipoprotein particles and lower adiponectin blood levels predict early structural valve degeneration of bioprostheses. Cardiology. 121(1):20-26 (2012).
- Mohty D, Pibarot P, Côté N, et al. Hypoadiponectinemia is associated with valvular inflammation and faster disease progression in patients with aortic stenosis. Cardiology. 118(2):140-146 (2011).
- Mizia-Stec K, Bochenek T, Kusz B, et al. Severe degenerative aortic stenosis with preserved ejection fraction does not change adipokines serum levels. Cardiol J. 26(5):483-492 (2019).
- Wang Z V, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 28(2):93-100 (2016).
- Shehzad A, Iqbal W, Shehzad O, et al. Adiponectin: Regulation of its production and its role in human diseases. Hormones. 11(1):8-20 (2012).
- Liu Z, Liang S, Que S, et al. Meta-analysis of adiponectin as a biomarker for the detection of metabolic syndrome. Front Physiol. 9 (2018).
- Kalkman HO. An Explanation for the adiponectin paradox. Pharmaceuticals (Basel). 14(12) (2021).
- Menzaghi C, Trischitta V. The adiponectin paradox for all-cause and cardiovascular mortality. Diabetes. 67(1):12-22 (2018).
- Wang Y, Ma XL, Lau WB, et al. Cardiovascular adiponectin resistance: The critical role of adiponectin receptor modification. Trends Endocrinol Metab. 28(7):519-530 (2017).
- Tsukamoto O, Fujita M, Kato M, et al. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 53(22):2070-2077 (2009).
- Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 350(7):655-663 (2004).
- Witberg G, Ayers CR, Turer AT, et al. Relation of adiponectin to all-cause mortality, cardiovascular mortality, and major adverse cardiovascular events (from the Dallas heart study). Am J Cardiol. 117(4):574-579 (2016).
- Wilson SR, Sabatine MS, Wiviott SD, et al. Assessment of adiponectin and the risk of recurrent cardiovascular events in patients presenting with an acute coronary syndrome: Observations from the pravastatin or atorvastatin evaluation and infection trial-thrombolysis in myocardial infarction. Am Heart J.161(6):1147-1155 (2011).
- Wannamethee SG, Welsh P, Whincup PH, et al. High adiponectin and increased risk of cardiovascular disease and mortality in asymptomatic older men: Does NT-proBNP help to explain this association? Eur J Cardiovasc Prev Rehabil. 18(1):65-71 (2011).
- Drechsler C, Krane V, Winkler K, et al. Changes in adiponectin and the risk of sudden death, stroke, myocardial infarction, and mortality in hemodialysis patients. Kidney Int. 76(5):567-575 (2009).
- Pilz S, Mangge H, Wellnitz B, et al. Adiponectin and mortality in patients undergoing coronary angiography. J Clin Endocrinol Metab. 91(11):4277-4286 (2006).
- Ortega Moreno L, Copetti M, Fontana A, et al. Evidence of a causal relationship between high serum adiponectin levels and increased cardiovascular mortality rate in patients with type 2 diabetes. Cardiovasc Diabetol. 15(1):17 (2016).
- Uetani E, Tabara Y, Kawamoto R, et al. CDH13 genotype-dependent association of high–molecular weight adiponectin with all-cause mortality: The J-SHIPP study. Diabetes Care. 37(2):396-401 (2014).
- Ogunwobi OO, Beales ILP. Adiponectin stimulates proliferation and cytokine secretion in colonic epithelial cells. Regul Pept. 134(2-3):105-113 (2006).
- Liu D, Luo S, Li Z, et al. Multifaceted roles of adiponectin in rheumatoid arthritis. Int Immunopharmacol. 28(2):1084-1090 (2015).
- Semple RK, Halberg NH, Burling K, et al. Paradoxical elevation of high-molecular weight adiponectin in acquired extreme insulin resistance due to insulin receptor antibodies. Diabetes. 2007;56(6):1712-1717 (2007).
- Ramsay JE, Jamieson N, Greer IA, et al. Paradoxical elevation in adiponectin concentrations in women with preeclampsia. Hypertens. 42(5):891-894 (2003).
- Woodward L, Akoumianakis I, Antoniades C, et al. Unravelling the adiponectin paradox: novel roles of adiponectin in the regulation of cardiovascular disease. Br J Pharmacol. 174(22):4007-4020 (2017).
- Mukama T, Johnson T, Kaaks R, et al. A case-cohort study of the association between adiponectin and mortality in EPIC–Heidelberg: NT-proBNP may explain the adiponectin paradox. Nutr Metab Cardiovasc Dis. 33(4):853-863 (2023).
- Walpot J, van Herck P, Collas V, et al. Adiponectin serum level is an independent and incremental predictor of all−cause mortality after transcatheter aortic valve replacement. Clin Cardiol. 45(10):1060-1069 (2022).
- Kwak S, Everett RJ, Treibel TA, et al. Markers of myocardial damage predict mortality in patients with aortic stenosis. J Am Coll Cardiol. 78(6):545-558 (2021).
- Balciunaite G, Skorniakov V, Rimkus A, et al. Prevalence and prognostic value of late gadolinium enhancement on CMR in aortic stenosis: Meta-analysis. Eur Radiol. 30(1):640-651 (2020).
- Thellier N, Altes A, Layec J, et al. Impact of left atrial and diastolic ventricular dysfunction on mortality in patients with aortic stenosis. Arch Cardiovasc Dis. 116(3):126-135 (2023).
- Lindman BR, Clavel M-A, Mathieu P, et al. Calcific aortic stenosis. Nat Rev Dis Prim. 2(1):16006 (2016).
- Chai J, Mohiaddin H, Mandal AKJ, et al. Role of open access echocardiography in detection of cardiac structural and functional abnormalities. Postgrad Med J. 99(1170):308-312 (2023).
- Anantha−Narayanan M, Malik U, Mbai M, et al. Impact of diastolic dysfunction on long−term mortality and quality of life after transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 95(5):1034-1041 (2020).
- Weber M, Arnold R, Rau M, et al. Relation of n-terminal pro–b-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol. 94(6):740-745 (2004).
- Vale NC, Campante TR, Madeira S, et al. Post-procedural N-terminal pro-brain natriuretic peptide predicts one-year mortality after transcatheter aortic valve implantation. Rev Port Cardiol. 37(1):67-73 (2018).
- Koskinas KC, O’Sullivan CJ, Heg D, et al. Effect of B-type natriuretic peptides on long-term outcomes after transcatheter aortic valve implantation. Am J Cardiol. 116(10):1560-1565 (2015).
- Katz M, Tarasoutchi F, Pesaro AEP, et al. Natriuretic peptides and long-term mortality in patients with severe aortic stenosis. J Heart Valve Dis. 21(3):331-336 (2012).
[Google Scholar] [PubMed]
- White M, Baral R, Ryding A, et al. Biomarkers associated with mortality in aortic stenosis: A systematic review and meta-analysis. Med Sci. 9(2):29 (2021).