Review Article - Interventional Cardiology (2021) Volume 13, Issue 6
The repolarization during phase-1 defines Ca2+ transients and contractility in perfused mouse hearts
- Corresponding Author:
- Ariel L Escobar
Department of Bioengineering,
School of Engineering,
University of California,
Merced,
CA,
USA,
E-mail: aescobar4@ucmerced.edu
Received date: September 22, 2021; Accepted date: October 06, 2021; Published date: October 13, 2021
Abstract
In perfused hearts, Ca2+ influx through L-type Ca2+ channels triggers Ca2+ release from the sarcoplasmic reticulum. In nearly all mammals, the inflow which activates the sarcoplasmic reticulum Ca2+ release occurs during phase 2 of the action potential. Interestingly, in murine models, the triggering event occurs during phase 1 of the action potential. The objective of this review is to determine how much Ca2+ influx occurs during phase 1. Moreover, we want to determine how much Ca2+ gets into the myocytes when Kv4.3 is blocked with 4-aminopyridine (4-AP). Moreover, we will evaluate changes in the open probability of Kv 4.3 following blockage via 4-aminopyridine (4-AP). To test whether a decrease in a transient K+ current (Ito) will enhance Ca2+ influx across the plasma membrane and increase the amplitude of Ca2+ transients, pulsed local-field fluorescence microscopy, recordings using sharp microelectrodes, measurements of the developed pressure, and loose-patch photolysis were utilized. Furthermore, some experiments were performed using loose patch photolysis to evaluate the amplitude of the Ca2+ in intact beating hearts. Interestingly, 4-AP increased not only the time required for AP to reach 30% repolarization but also the amplitude of Ca2+ transients in the epicardium in comparison to the endocardium. Furthermore, the activation of Ito with N-[3,5-Bis(trifluoromethyl)phenyl]-N’-[2,4- dibromo-6-(2H-tetrazol-5-yl)phenyl]urea (NS5806) resulted in a reduction of Ca2+ current amplitude, which led to a reduction of the amplitude of Ca2+ transients.
Keywords
Heart rate • Electrocardiograms (ECGs) • Coronary circulation
Introduction
The time course of the cardiac ventricular Action Potential (AP) is the initial biological event controlling contraction during the cardiac cycle. Normally, the extended duration of the ventricular AP increases the open probability of the L-type Ca2+ channel, leading to a sustained increase in Ca2+ influx. In very small mammals, the duration of the AP needs to be short enough to handle the higher heart rate (600 beats/min for mice). This physiological property led previous researchers to incorrectly assume an absent plateau or phase 2 in the mouse ventricular AP [1,2]. However, experiments from our lab recording APs at the intact organ level suggest that mouse ventricular AP does indeed exhibit a phase-2 [3,4], which is dependent on the activation of the Na-Ca exchanger (NCX) [4]. Although, the membrane potential of this phase-2 is more hyperpolarized when compared with larger mammals [3,5,6]. Furthermore, our experiments also strongly suggest the influx of Ca2+ triggering Ca2+ transients occur during AP phase-1. This likely occurs due to the deactivation of the L-type Ca2+ current, which produces a tail current triggering Ca2+ release from the sarcoplasmic reticulum [4]. This has been proposed for dog [7-9], rat [10-12], and mouse [13-15]. The experiments performed in the ventricular epicardium under voltage-clamp conditions show the activation of the L-type Ca2+ current but also the activation of the NCX [4]. In the present review, we propose to gauge the role of a key K+ conductance activated during phase-1 repolarization in defining the transmural Ca2+ signaling. A combined and concurrent assessment of the mechanical activity, AP kinetics, electrocardiograms (ECGs), Ca2+ transients, and Ca2+ currents was performed on intact perfused hearts when Kv 4.3 channels that were inhibited by 4-aminopyridine (4-AP) or activated with 1-[2,4-dibromo-6-(1H-tetrazole-5-yl)-phenyl]-3- (3,5-bis-trifluoromethylphenyl)-urea (NS5806). Furthermore, the understanding obtained from the experiments presented here will help us to recognize pathophysiological mechanisms related to systolic heart failure and define novel pharmacological interferences to treat this set of clinical conditions.
Literature Review
Ethical approval
All animal experiments were performed on adult mice (Charles River Laboratories) following the Institutional Animal Care and Use Committee of the University of California, Merced. Animals were kept by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, Revised 1996). The Institutional Animal Care and Use Committee of the University of California Merced accepted the euthanasia method used (#2008-201).
Pulsed Local-Field Fluorescence Microscopy (PLFFM)
The PLFFM approach was developed in our laboratory to measure physiological data by exciting exogenous fluorescent indicators and sensing the light emitted by these molecules present in the tissue [4,7,16-18]. In PLFFM, the excitation and emitted light is conducted through a multimode optic fiber located either in the epicardium or in the endocardium. The measurement in these two regions allowed comparing the physiological properties of the epicardium and the endocardium where we measure membrane potential and Ca2+ transients. To study the time course and the physiological distribution of the signal, the emitted light from different sites must be recorded simultaneously from the epicardium and the endocardium.
The light source is a 532 nm, solid-state neodymium-doped yttrium-aluminum-garnet laser (Enlight Technologies), and we measure from the epicardium and the endocardium using optical fibers having a numerical aperture of 0.67. The beam is focused either with an aspheric lens or a microscope objective. The lens focused the light beam into an optical fiber that was in contact with the tissue. For endocardial measurements, we made a small incision on the surface of the left ventricular free wall, facilitating the placement of the fiber onto the epicardium and the endocardium.
Loose-Patch Photolysis (LPP)
The Loose-Patch Photolysis (LPP) permits the measurement of ionic currents in an intact heart during triggered physiological APs [5]. This includes PLFFM [4,7,16-18], photo-breaking compounds with Ultraviolet (UV) pulsing [18-22], microelectrode measurements, and loose-patch recordings [23,24].
The Loose-Patch Pipette (LPP) was made with a giant glass patch pipette and the tip was heated with a torch to decrease the diameter size of one end to ∼ 200 μm. This allows the optical fiber to be placed at the tip of the pipette. This fiber was used to record action potentials or Ca2+ transients using a potentiometric dye (di-8- Anepps) and the Ca2+ indicator Rhod-2. Small UV light pulses were used to break photosensitive compounds, allowing us to measure membrane currents during an action potential [5]. The giant patch pipette was filled with Tyrode’s solution. A micromanipulator was used to place the patch pipette on the epicardium of the ventricle, where the interior of the patch pipette was voltage-clamped with the same potential as the bath. A flash photolysis system let us fractionally change the L-type Ca2+ current ionic current (i.e., Ca2+ currents by photolyzing nifedipine) under the loose-patch pipette. Nifedipine was locally photo-inactivated by UV illumination generated by a diode-pumped solid-state UV laser (355 nm). UV light was applied through an external quartz multimode fiber-optic or by a fiber positioned inside the patch pipette. The distinction between the total current measured in the presence and the absence of the drug permits us to reveal the current that was pharmacologically blocked. As the area under the pipette is much smaller than the space constant, the neighboring tissue imposes an electrotonic coupling. This electrotonic coupling will act as an electric sink and impedes the activation of the photolitically activated current from producing any changes in the local AP. As the transmembrane current there does not produce a change of the membrane potential, mimics what happens under a voltage-clamp condition, but in this case, the ventricular syncytium is acting as a spatial clamp. Finally, hearts were paced between 4-6 Hz at 33°C.
Statistical analysis
The physiological records of the APs, ECG, Ca2+ transients, Ca2+ currents, and ventricular pressure were evaluated. The AP trace for each set of experiments was evaluated at certain repolarization times (APD30 or APD90). The repolarization times between control and non-control experiments were then evaluated and normalized to the control values for each heart. After this normalization, values from five experiments (n=5 hearts, or otherwise noted) were compiled. Statistical analysis was performed with Origin 2020 using a Smirnoff-Kolmogorov approach. Specifically, we measured the fractional Ca2+ transient amplitude concerning a control trace; the rise time of Ca2+ transient, and the half relaxation time, measured as the time it takes for the Ca2+ transient to relax to half of its maximum amplitude. Each of these parameters recorded in control and non-control experiments was evaluated and normalized to the control values for each heart used. The data from all the Ca2+ transients obtained from five experiments (n=5 hearts unless stated otherwise) were compiled, and statistical analysis was performed with origin 2020 using a Smirnoff-Kolmogorov approach.
Results
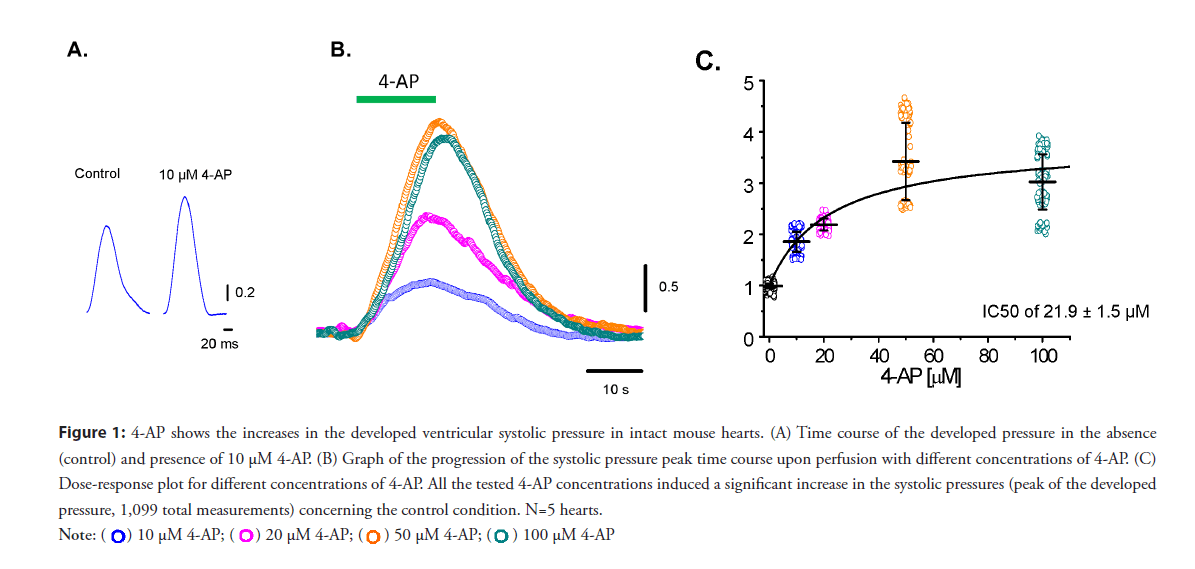
• 4-AP–sensitive Kv channels define the relaxation of phase-1 and define the mechanical properties on an intact beating mouse heart: To test if changes in phase 1 repolarization would impact cardiac contractility at the intact heart level. Moreover, experiments were performed measuring the developed pressure in the left ventricle when Ito was blocked with increasing concentrations of 4-AP. Figure 1 shows the effect of different concentrations of 4-AP and how an increase in the 4-AP concentration increases the developed pressure. Low concentrations of 4-AP (10 μM) produced a significant increase in the amplitude of the ventricular developed pressure (Figure 1A). Figure 1B shows the effect of increasing concentrations of 4-AP. At all concentrations, systolic pressure significantly decreased when 4-AP was washed out. A dose-response curve in Figure 1C shows significant increases in the measured pressure for each concentration of 4-AP (n=5 hearts). Increasing 4-AP concentration largely affected the developed pressure, suggesting that? Kv 4.3 and 4.2 channels can control contractility.
Figure 1: 4-AP shows the increases in the developed ventricular systolic pressure in intact mouse hearts. (A) Time course of the developed pressure in the absence
(control) and presence of 10 μM 4-AP. (B) Graph of the progression of the systolic pressure peak time course upon perfusion with different concentrations of 4-AP. (C)
Dose-response plot for different concentrations of 4-AP. All the tested 4-AP concentrations induced a significant increase in the systolic pressures (peak of the developed
pressure, 1,099 total measurements) concerning the control condition. N=5 hearts.
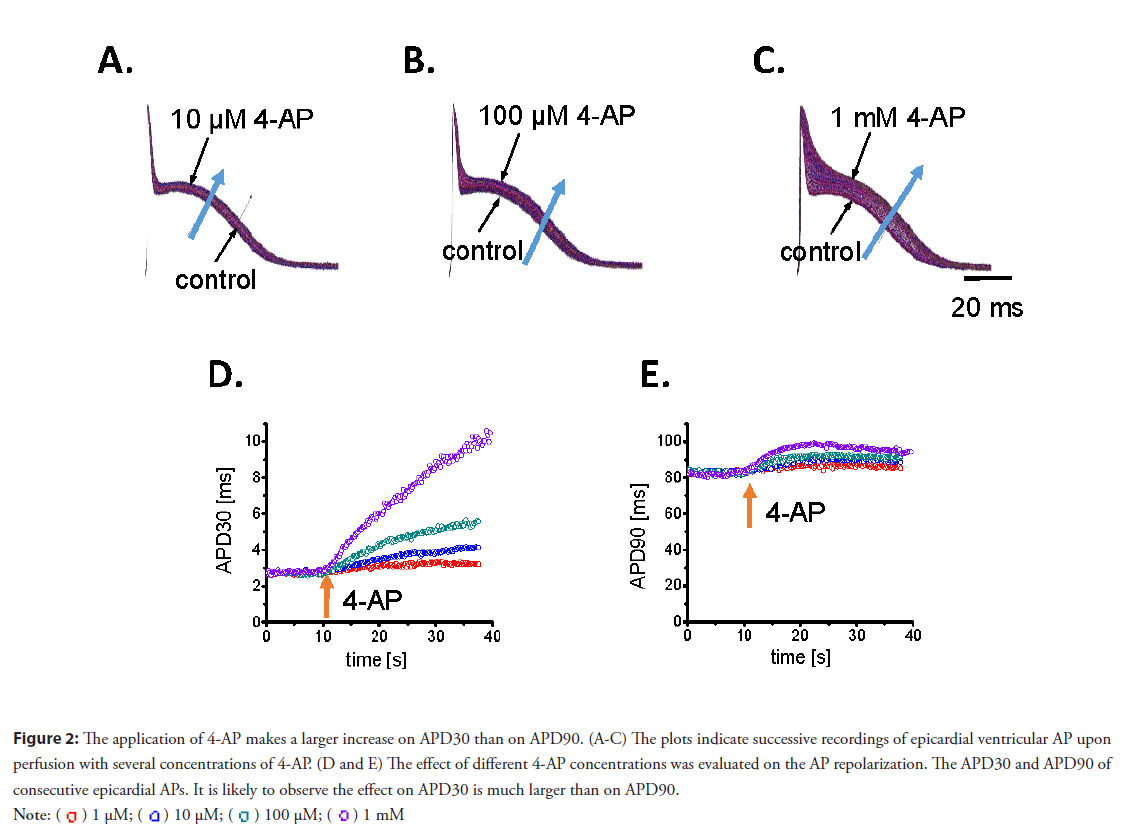
• 4-AP–sensitive Kv channels define the electrophysiological properties on an intact beating mouse heart: In addition, the impact of 4-AP on ventricular electrical activity was explored. Our lab previously revealed the mouse epicardial AP displays a fast phase-1. Figure 2 shows the effect of 4-AP on the phase-1 repolarization of the epicardial AP. Figures 2A-2C depicts the action of 4-AP when a heart was perfused with increasing concentrations. Additionally, perfusion with 4-AP slowed phase-1 repolarization and prolonged the duration of the AP. A prolongation in APD30 was observed in response to increasing concentrations of 4-AP (Figure 2D). Furthermore, a fractional increase of APD90 (Figure 2E), which was smaller than that of APD30, was also observed. Indeed, 10 μM of 4-AP increased APD30 and ADP 90 by 52% and 8.4%, respectively. This indicates channels blocked by 4-AP mostly define the early fast repolarization during phase-1. Although low concentrations of 4-AP have a profound effect on the mechanical response and AP repolarization, it is difficult to relate these two variables.
Figure 2: The application of 4-AP makes a larger increase on APD30 than on APD90. (A-C) The plots indicate successive recordings of epicardial ventricular AP upon
perfusion with several concentrations of 4-AP. (D and E) The effect of different 4-AP concentrations was evaluated on the AP repolarization. The APD30 and APD90 of
consecutive epicardial APs. It is likely to observe the effect on APD30 is much larger than on APD90.
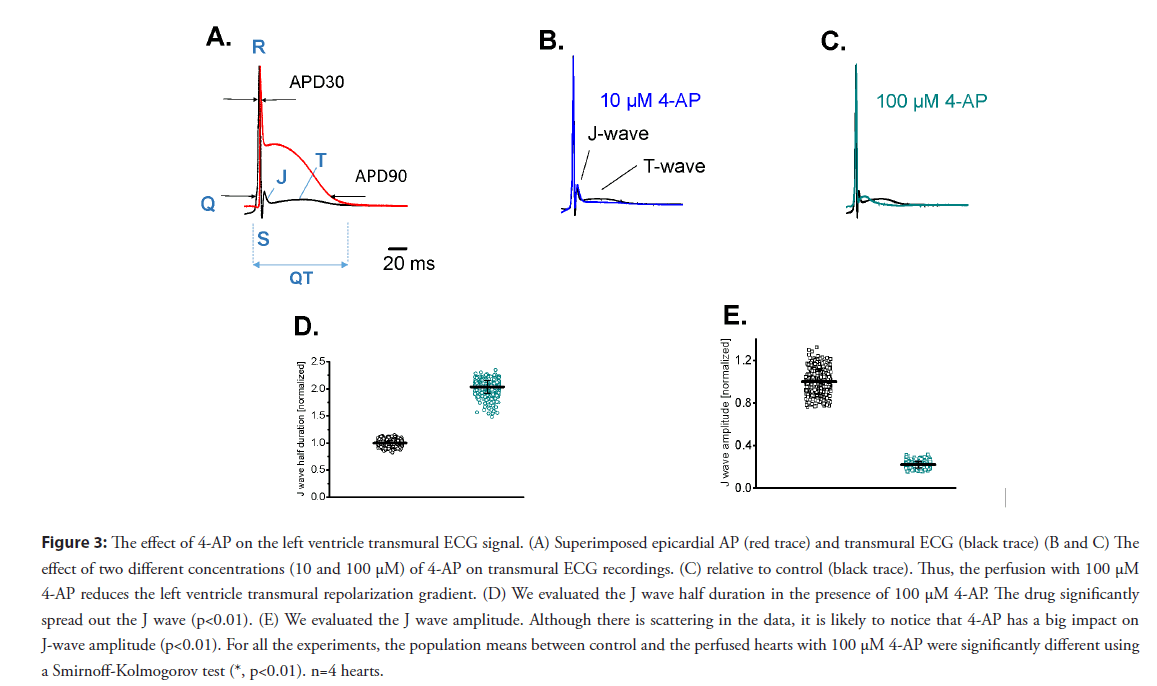
Therefore, it is very important to evaluate the waveform of the transmural ECG signals to have an idea of the relationship between the action potential and the EKG. In the mouse, the T-wave reflects the difference in timing between the repolarization of the apex and the repolarization of the base. However, when the ECG was transmurally recorded (with one electrode in the left ventricular chamber and a second one outside the left ventricular epicardial layer), the mouse T wave showed the temporal difference in the transmural repolarization, an event also observed in larger mammals. Furthermore, the transmural ECG is an ideal tool to explore the J wave produced because of the kinetic difference in phase-1 repolarization between epicardium and endocardium. Figure 3A illustrates a simultaneous recording of an epicardial AP and the corresponding transmural ECG. The 4-AP containing solutions were retro-perfused through the coronary circulation. The effect of different 4-AP concentrations on AP repolarization was evaluated by assessing the APD30 and APD90 of consecutive epicardial APs. Remarkably, since the J wave emerges during the fast phase-1 repolarization, evaluating this wave provides a precise way to assess the fast repolarization during phase 1 at different transmural levels.
Figure 3: The effect of 4-AP on the left ventricle transmural ECG signal. (A) Superimposed epicardial AP (red trace) and transmural ECG (black trace) (B and C) The effect of two different concentrations (10 and 100 μM) of 4-AP on transmural ECG recordings. (C) relative to control (black trace). Thus, the perfusion with 100 μM 4-AP reduces the left ventricle transmural repolarization gradient. (D) We evaluated the J wave half duration in the presence of 100 μM 4-AP. The drug significantly spread out the J wave (p<0.01). (E) We evaluated the J wave amplitude. Although there is scattering in the data, it is likely to notice that 4-AP has a big impact on J-wave amplitude (p<0.01). For all the experiments, the population means between control and the perfused hearts with 100 μM 4-AP were significantly different using a Smirnoff-Kolmogorov test (*, p<0.01). n=4 hearts.
Figures 3B and 3C shows the effect of 10 and 100 μM of 4-AP on the J wave amplitude and kinetics. As shown in the inset of Figure 3C, 4-AP increased the J wave duration and decreased the amplitude. Perfusion with 4-AP produced a significant increase (at the 0.01 level) in the J wave half duration of 100.3 ± 11% (Figure 3D). Additionally, 100 μM of 4-AP significantly decreased (at the 0.01 level) the amplitude of the J wave of 43 ± 32% in four different independent hearts (Figure 3E). Altogether, experiments presented in Figure 3 suggest 4-AP affects the rate of phase 1 repolarization (J wave half duration), and have a differential effect on epicardial and endocardial repolarization.
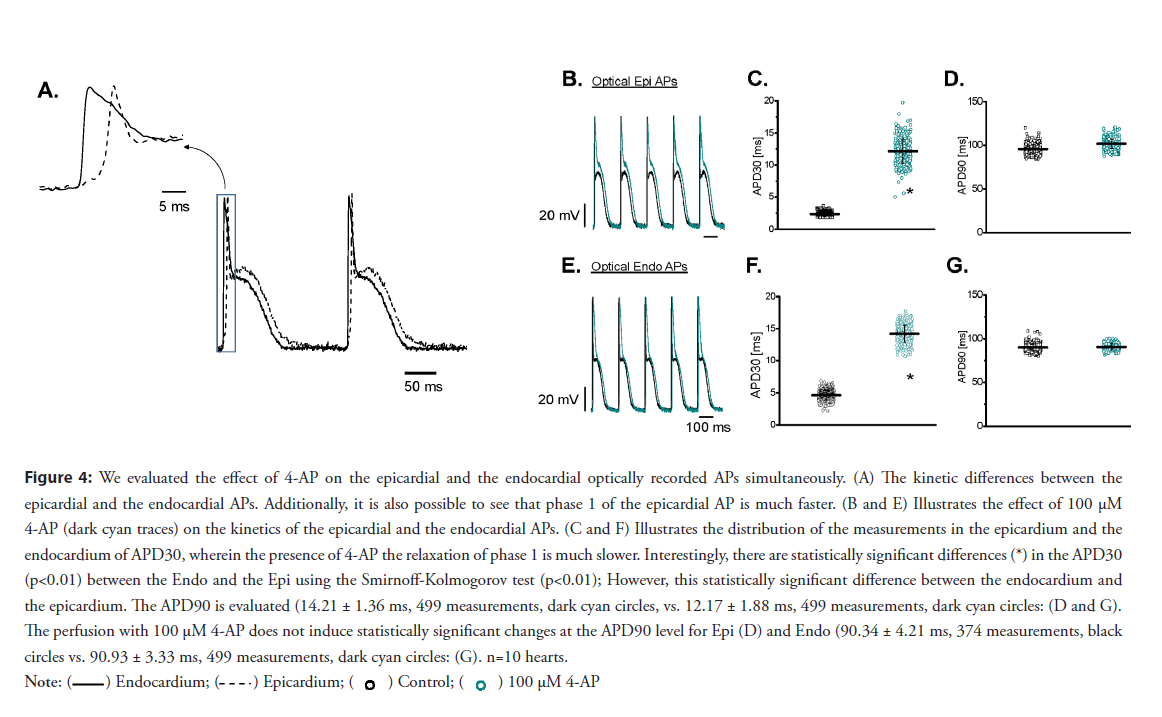
• The block of 4-AP–sensitive Kv channels have a large effect in controlling the phase 1 electrophysiological behavior of the epicardium to the endocardium: Recently, our group reported for the first time the assessment of epicardial and endocardial APs using potentiometric dyes and a dual PLFFM. The idea is to use retroperfusion a beating heart through the coronary bed with the potentiometric dye Di-8-ANNEPS. After the heart was retroperfused with the dye, two independent high-numerical-aperture optical fibers attached to PLFFM record changes in membrane potential in the epicardium and the endocardium. PLFFM allows the assessment of electrophysiological parameters across the ventricular wall. A typical experiment in which epicardial and endocardial AP were simultaneously recorded is presented in Figure 4A, where three main features can be observed. First, the endocardium depolarizes before the epicardial layer. For all the experiments, the population means between the control and the perfused hearts with 100 μM 4-AP were significantly different (*, p<0.01), n=4 hearts. Second, the repolarization during phase-1 was slower in the endocardium than in the epicardium (4.66 ± 0.76 ms vs. 2.35 ± 0.37 ms, n=10 hearts). Finally, the epicardial layer presented a prominent spike-and-dome morphology, an experimental observation presented in several mammalian models. As the epicardium and the endocardium display significant differences in the early repolarization kinetics, we decided to evaluate the effect of blocking Kv channels with 4-AP on each layer (Figures 4A-4G). Interestingly, 4-AP perfusion had a larger effect on the APD30 of the epicardial AP (Figure 4C) than on the APD90 (Figure 4D). Although 4-AP also slowed down the rate of repolarization in the endocardium (Figures 4E and 4F), the effect on the phase 1 repolarization rate was smaller in the endocardium than on the epicardial AP. Indeed, the ratios of the APD30 in the absence and presence of 100 μM 4-AP were 5.3 times in epicardium versus 3 times in endocardium. This lower sensitivity in the endocardium can reflect the lower expression of Kv 4. x. A differential effect of 4-AP on simultaneous epicardial and endocardial optically recorded APs can be observed in Figure 5A. In the picture, we can see the differences between the relaxations of phase 1 and the difference in timing for the activation between the endocardium and the epicardium. The kinetic differences between epicardial and endocardial Ca2+ transients recorded simultaneously on an intact perfused heart (Figures 5A-5H). It is possible to observe the differences in the kinetics of these transmurally recorded Ca2+ transients. The effect of 100 μM 4-AP (dark cyan traces) on the kinetics of epicardial and endocardial Ca2+ transients, respectively. (Figures 5C and 5F) The distribution of the APD30 measurements of epicardial and endocardial APs before and after the application of 100 μM 4-AP. 4-AP induced a significant decrease in the rate of repolarization. Interestingly, there were statistically significant differences (*) in the Ca2+ transients (P<0.01); F) and epicardium (2.34 ± 0.37 ms, 376 measurements, black circles; C) before the application of the drug. However, this statistically significant difference between endocardium and epicardium disappeared Ca2+ transients level (5D and 5G).; D) and endocardium (90.34 ± 4.21 ms, 374 measurements, black circles vs. 90.93 ± 3.33 ms, 499 measurements, dark cyan circles; G, n=10 hearts.
Figure 4: We evaluated the effect of 4-AP on the epicardial and the endocardial optically recorded APs simultaneously. (A) The kinetic differences between the
epicardial and the endocardial APs. Additionally, it is also possible to see that phase 1 of the epicardial AP is much faster. (B and E) Illustrates the effect of 100 μM
4-AP (dark cyan traces) on the kinetics of the epicardial and the endocardial APs. (C and F) Illustrates the distribution of the measurements in the epicardium and the
endocardium of APD30, wherein the presence of 4-AP the relaxation of phase 1 is much slower. Interestingly, there are statistically significant differences (*) in the APD30
(p<0.01) between the Endo and the Epi using the Smirnoff-Kolmogorov test (p<0.01); However, this statistically significant difference between the endocardium and
the epicardium. The APD90 is evaluated (14.21 ± 1.36 ms, 499 measurements, dark cyan circles, vs. 12.17 ± 1.88 ms, 499 measurements, dark cyan circles: (D and G).
The perfusion with 100 μM 4-AP does not induce statistically significant changes at the APD90 level for Epi (D) and Endo (90.34 ± 4.21 ms, 374 measurements, black
circles vs. 90.93 ± 3.33 ms, 499 measurements, dark cyan circles: (G). n=10 hearts.
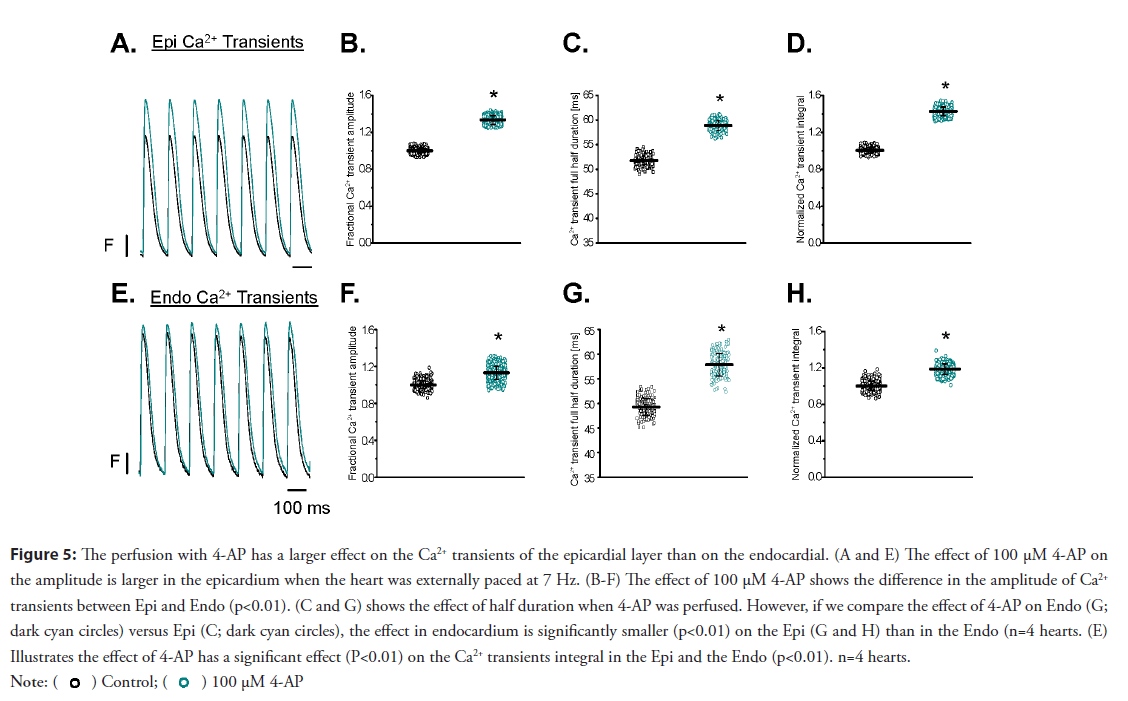
Figure 5: The perfusion with 4-AP has a larger effect on the Ca2+ transients of the epicardial layer than on the endocardial. (A and E) The effect of 100 μM 4-AP on
the amplitude is larger in the epicardium when the heart was externally paced at 7 Hz. (B-F) The effect of 100 μM 4-AP shows the difference in the amplitude of Ca2+
transients between Epi and Endo (p<0.01). (C and G) shows the effect of half duration when 4-AP was perfused. However, if we compare the effect of 4-AP on Endo (G;
dark cyan circles) versus Epi (C; dark cyan circles), the effect in endocardium is significantly smaller (p<0.01) on the Epi (G and H) than in the Endo (n=4 hearts. (E)
Illustrates the effect of 4-AP has a significant effect (P<0.01) on the Ca2+ transients integral in the Epi and the Endo (p<0.01). n=4 hearts.
• Evaluation of the Ca2+ transients at the epicardial and endocardial layer: Undoubtedly, the perfusion of the hearts with 4-AP had a dramatic effect on both the mechanical and electrical properties of the mouse heart. However, none of the experiments presented above defined the relationship between excitability and contractility. The most likely scenario is that 4-AP will enhance Ca2+ release from the SR by changing the amplitude and the kinetics of the L-type Ca2+ currents. The Ca2+ transient recordings were obtained by perfusing the heart with the Ca2+ indicator Rhod-2 and simultaneously recording fluorescence signals with two independent optical fibers. Experiments presented in Figure 5 illustrate the effect produced by the perfusion of the heart with 100 μM 4-AP on the amplitude is larger in the epicardial than in the endocardial Ca2+ transients (n=4 hearts). Perfusion with 4-AP induced an increase in the amplitude and half relaxation time of the epicardial Ca2+ transients (Figures 5A-5D). This is consistent with the larger epicardial effects of 4-AP due to the heterogeneous expression of Kv 4.x channels across the ventricular wall. The results presented in Figure 5 demonstrate the increase in the systolic pressure with 4-AP observed in Figure 1 is due to an increase in the amplitude and the kinetics of the Ca2+ transient.
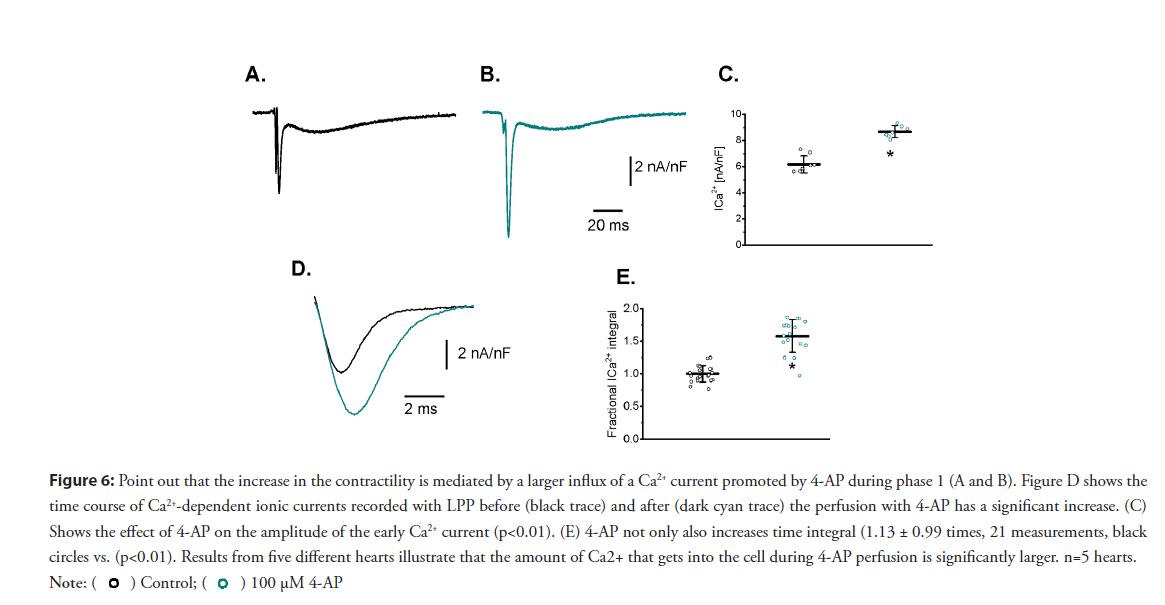
• Effects on the L-type Ca2+ current: Changes in the AP phase 1 repolarization will likely induce an increase in the amplitude of the L-type Ca2+ current. Recently, our laboratory was able to measure for the first time Ca2+-dependent currents during a triggered AP at the intact heart level using the novel LPP technique [4]. To assess if changes during the repolarization of phase 1 defined the kinetics of Ca2+ influx into the cell, we performed LPP experiments in hearts exposed to 4-AP. A typical experiment is shown in Figures 6A and 6B, which illustrates epicardial currents recorded in the absence and presence of 100 μM 4-AP, respectively. The LPP recording shows two components, one fast early current, and a late slower current. As demonstrated in a previous article [4], the current during the early phase is carried by Ca2+ ions permeating through L-type Ca2+ channels, and the late component is mediated by the influx of Na+ through the NCX. Furthermore, in [4], this Ca2+ influx was terminated by voltage-dependent deactivation and not by Ca2+ dependent inactivation. This late Na+ current through the NCX is activated by Ca2+ released from the SR. Remarkably, the perfusion of the heart with 4-AP not only induced an increase in the amplitude of the fast inward component of the current but also increased its mean duration. Figures 6C and 6D reinforces this observation: Currents recorded from five different animals show the increase in the current amplitude and the total charge carried through the L-type Ca2+ currents during phase-1 was significantly larger in hearts perfused with 4-AP (57% for the total charge) than in control hearts (Figure 6E).
Figure 6: Point out that the increase in the contractility is mediated by a larger influx of a Ca2+ current promoted by 4-AP during phase 1 (A and B). Figure D shows the
time course of Ca2+-dependent ionic currents recorded with LPP before (black trace) and after (dark cyan trace) the perfusion with 4-AP has a significant increase. (C)
Shows the effect of 4-AP on the amplitude of the early Ca2+ current (p<0.01). (E) 4-AP not only also increases time integral (1.13 ± 0.99 times, 21 measurements, black
circles vs. (p<0.01). Results from five different hearts illustrate that the amount of Ca2+ that gets into the cell during 4-AP perfusion is significantly larger. n=5 hearts.
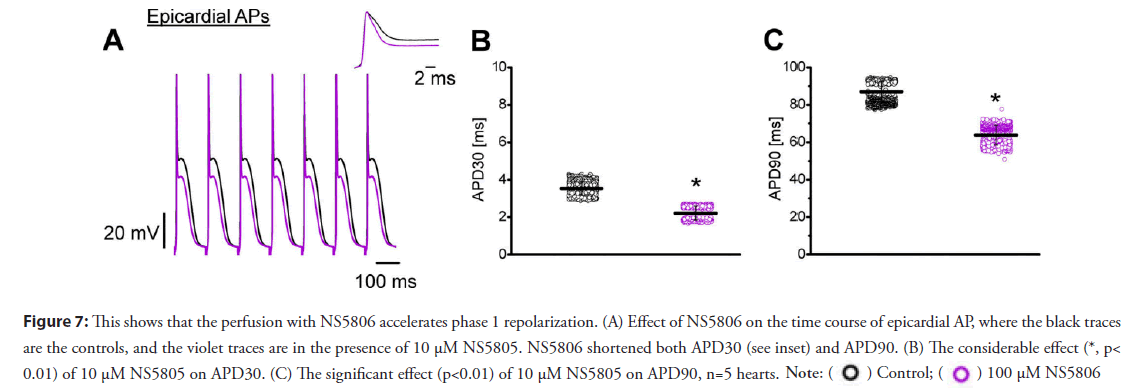
• Activation of Kv 4.x channels speed the relaxation of the action potential during phase-1, reduces the mechanical response by increasing the rate of repolarization during phase-1; this finally leads to an attenuation in the L-type Ca2+ and the Ca2+ transient: Results presented in this review indicate the blockage of Kv channels increases the systolic force because a slower AP repolarization phase-1 increases the Ca2+ influx through L-type channels, activating a larger Ca2+ transient with the concomitant increase in contractility. A final proof of concept supporting this idea is the assessment of contractility (i.e., Ca2+) when the Kv channels are activated instead of inhibited. Kv channels are tetramers in which each α subunit has six transmembrane segments. Although this is the main structure for K+ permeation, other regulatory subunits can modify the level of expression and also can regulate the kinetics of the channels. Moreover, a central regulatory subunit, K+ channel-interacting protein (KChIP), has a central role in regulating the Kv 4. x channels. Furthermore, some drugs can interact with Kv 4.x channels when KChIP is also expressed in the plasma membrane. Interestingly, NS5806 can slow the rate of inactivation of Kv 4.x when the α subunit interacts with KChIP [25]. Thus, we expect that NS5806 will accelerate the rate of repolarization during phase 1. Figure 7 illustrates experiments on epicardium that were designed to test this idea. Figure 7A shows 10 μM NS5806 dramatically accelerated phase-1 repolarization (see inset) and decreased both the amplitude of phase-2 and the APD90. Statistical data obtained from five different hearts (Figures 7B and 7C) show significant changes in the APD30 and APD90 of epicardial AP. If the prolongation of phase-1 induced an increase in contractility, we expect an increase in the rate of repolarization will attenuate the amplitude of myoplasmic Ca2+ transients. Indeed, when the hearts were perfused with NS5806, there was a strong decrease in the heart mechanical activity.
Figure 7: This shows that the perfusion with NS5806 accelerates phase 1 repolarization. (A) Effect of NS5806 on the time course of epicardial AP, where the black traces
are the controls, and the violet traces are in the presence of 10 μM NS5805. NS5806 shortened both APD30 (see inset) and APD90. (B) The considerable effect (*, p<
0.01) of 10 μM NS5805 on APD30. (C) The significant effect (p<0.01) of 10 μM NS5805 on APD90, n=5 hearts. 
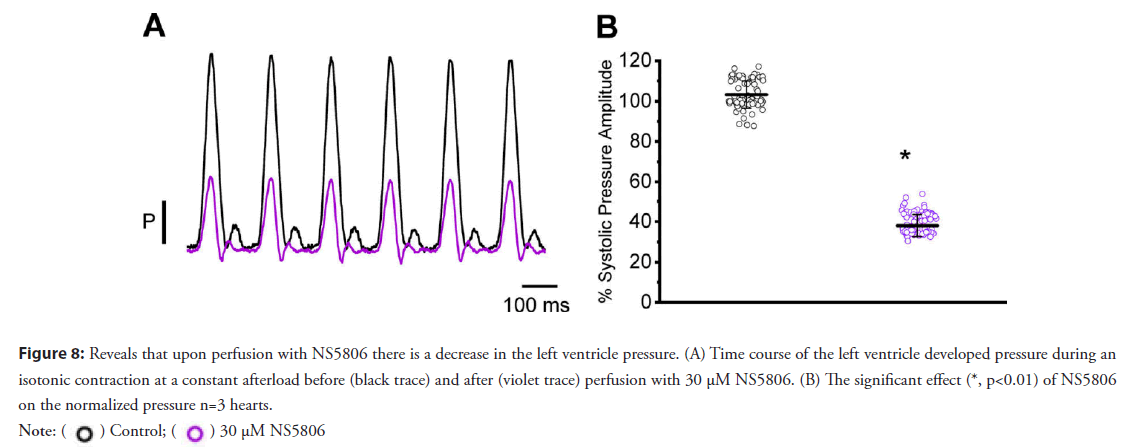
• Activation of Kv 4.3 reduces the mechanical response by increasing the rate of repolarization during phase 1; this finally leads to an attenuation in the L-type Ca2+ and the Ca2+ transient: Figure 8A shows the time course of the normalized developed pressure in a heart that was perfused with 30 μM NS5806. A statistical analysis of the effect of NS5806 on the developed pressure is shown in Figure 8B (103.2 ± 6.7% for the control and 38.0 ± 5.5% for NS5806; n=3 hearts).
Figure 8: Reveals that upon perfusion with NS5806 there is a decrease in the left ventricle pressure. (A) Time course of the left ventricle developed pressure during an
isotonic contraction at a constant afterload before (black trace) and after (violet trace) perfusion with 30 μM NS5806. (B) The significant effect (*, p<0.01) of NS5806
on the normalized pressure n=3 hearts. 
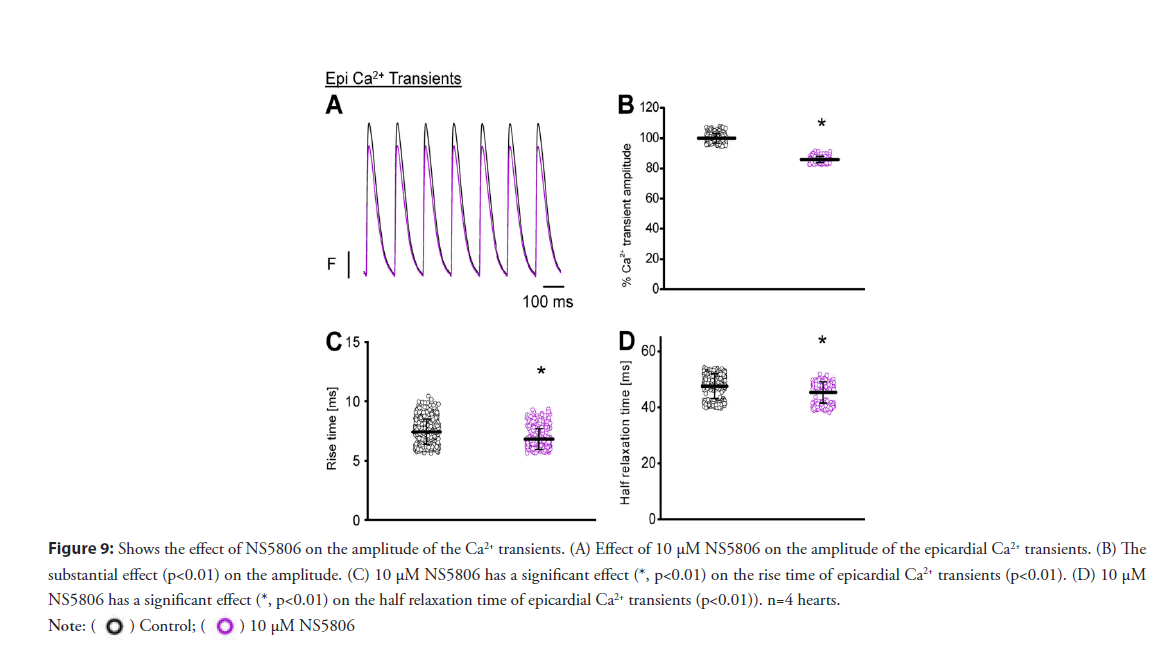
• Activation of Kv 4.3 reduces the amplitude of Ca2+ transient: Moreover, Figures 9A-9D reveals 10 μM NS5806 perfusion induced a reduction in the amplitude, rise time, and half relaxation time, and the duration of epicardial Ca2+ transients. Furthermore, to unmask the molecular mechanism by which NS5806 reduces the amplitude of Ca2+ transient, we performed experiments to record the Ca2+ influx through L-type Ca2+ channels in the presence of 10 μM NS5806.
Figure 9: Shows the effect of NS5806 on the amplitude of the Ca2+ transients. (A) Effect of 10 μM NS5806 on the amplitude of the epicardial Ca2+ transients. (B) The
substantial effect (p<0.01) on the amplitude. (C) 10 μM NS5806 has a significant effect (*, p<0.01) on the rise time of epicardial Ca2+ transients (p<0.01). (D) 10 μM
NS5806 has a significant effect (*, p<0.01) on the half relaxation time of epicardial Ca2+ transients (p<0.01)). n=4 hearts. 
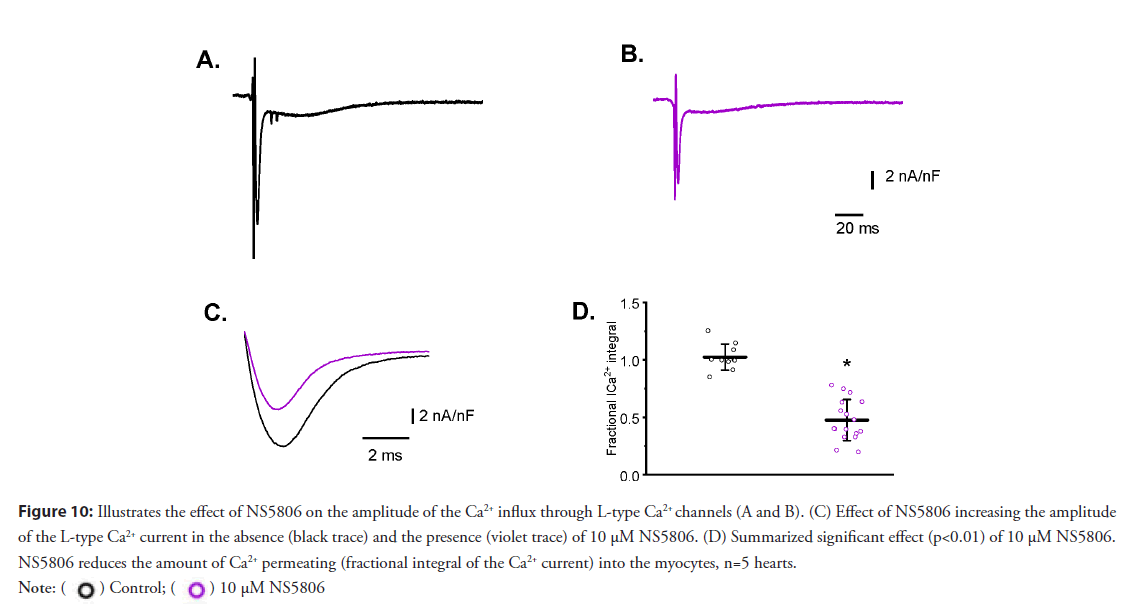
• Activation of Kv 4.3 induces attenuation in the L-type Ca2+: Figures 10A and 10B show an increase in the magnitude of Ito decreases the amplitude of Ca2+ currents measured with LPP. Moreover, increasing the rate of repolarization not only affected the amplitude of the Ca2+ current but also decreased the duration of the current (Figures 10C and 10D). This effect seems to be a consequence of faster deactivation of the L-type Ca2+ current due to an increased rate of AP repolarization during phase-1.
Figure 10: Illustrates the effect of NS5806 on the amplitude of the Ca2+ influx through L-type Ca2+ channels (A and B). (C) Effect of NS5806 increasing the amplitude
of the L-type Ca2+ current in the absence (black trace) and the presence (violet trace) of 10 μM NS5806. (D) Summarized significant effect (p<0.01) of 10 μM NS5806.
NS5806 reduces the amount of Ca2+ permeating (fractional integral of the Ca2+ current) into the myocytes, n=5 hearts. 
Discussion
We have provided evidence that strongly suggests the Ca2+ influx which led to an activation of Ca2+ transients occurs during phase-1 of the action potential. Furthermore, the kinetics of this fast repolarization can control contractility in mice hearts. The main property is the presence of a strong phase-1 during the early repolarization of the action potential. This event permits the heart of a mouse to beat at an extremely high frequency. The concept that the phase-1 rate of repolarization could be involved in regulating the amplitude of Ca2+ transients has been historically debated by several authors [11,12,25]. Moreover, in this review, we integrated, at the intact heart level the relationship between the rate of phase-1 repolarization, Ca2+ influx, and contractility throughout the free ventricular wall. The Ito current is encoded by two genes, Kv 4.2 and Kv 4.3 [26-30]. Figure 1 shows increasing concentrations of 4-AP resulted in increased systolic pressures, an effect previously observed in isolated myocytes described by [14] was increasing the concentration of 4-AP to 100 μM increased the mechanical shortening of mouse myocytes. Moreover, our pressure recordings were made under isotonic conditions at a constant load, suggesting an increase in the pressure during 4-AP was due to the blocking effect of this drug on the phase-1 repolarization of the AP.
Data presented in Figure 2 show perfusion with 4-AP slowed down the AP repolarization during phase-1. Furthermore, the effect on APD30 was much larger than on APD90. Moreover, as we previously proved [4,6,7,31-33], APs recorded from different ventricular regions on perfused heart shows an important phase [4,7,26,31-33]. The transmural ECG recordings in mouse hearts displayed a notable J wave [31]. This J wave occurs because of the different rates of repolarization during the AP phase-1 [34].
Experiments described in Figure 3 show 4-AP perfusion in intact mouse hearts reduced the amplitude of the J wave (Figures 3C-3E) and significantly widened its half duration (Figure 3D). One likely effect for the reduction in the amplitude of the J wave could be that 4-AP reduces the changes among the epicardial and endocardial rate of phase-1 repolarization.
Results presented in Figure 4 show the difference between epicardial and endocardial AP repolarization during phase-1. This variation can be described by a diminished expression of Kv 4.3 at the endocardium ventricular layer [32,33,35]. Remarkably, the differences between endocardial and epicardial APD30 were highly reduced when the heart was coronary perfused with 100 μM 4-AP (Figures 4C and 4F). Even more, no significant changes were observed at the APD90 level in both regions during the 4-AP treatment (Figures 4D and 4G). 4-AP had a bigger effect on the epicardium due to the higher expression of Kv. 4.3 in this ventricular layer. The fact that 4-AP had a larger effect on the epicardial than on the endocardial, is because there is a larger expression of Kv. 4.3 in the epicardium [36].
Figure 5 showed that 100 μM 4-AP had a smaller effect on the amplitude of the endocardial Ca2+ transient than on the epicardial Ca2+ transient. Our experiments were performed at 33°C and 7 Hz. 4-AP had a significant effect on both phase-1 repolarization and the amplitude of Ca2+ transient during systole.
We have established the LPP technique allows the recording of Ca2+ currents at the whole heart level. Moreover, Figures 6 illustrates 4-AP perfusion increased the amplitude of the L-type Ca2+ current. Likewise, if the same method were applied when Ito had been partially blocked with 100 μM 4-AP, the effect would become dramatically larger.
Interestingly, the APD30 measurements shown in Figures 7B and 7C become much faster when following 10 μM NS5806 perfusion. Phase-1 repolarization had a profound effect on epicardial and endocardial contractility.
Figures 8A and 8B reveals that 10 μM NS5806 accelerated phase-1 thus, reducing the ventricular developed pressure (Figures 8A and 8B).
Furthermore, a significant change was observed in the amplitude, the rise time, and the half duration of the Ca2+ transients in the epicardial layer (Figures 9A-9D).
This effect correlates very well with the effect of NS5806 on the L-type Ca2+ currents (Figures 10A-10D). This data further solidifies the notion that Kv.4.3 channels regulate contractility. Moreover, NS5806 only interacts with KChiP, a regulatory subunit that interacts with Kv. 4.3 channels [37].
Conclusion
Taken together, the results presented in this review demonstrate, for the first time, the role of Ito in explaining how cardiac contractility is reduced when the heart is perfused with NS5806. Furthermore, ventricular myocytes are electrically coupled through connexins 43. Finally, this step repolarization gradient can produce a graded contractility response across the ventricular wall that is defined by each layer. This suggests the transmural differences in Ca2+ signaling are imperative in defining the contractile properties of the ventricular wall.
Acknowledgements
We want to thank Dr. Alicia Mattiazzi for reviewing this review. Supported by NIH (R01 R01GM132753 and NIH 1R01HL152296 to ALE).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
ALE designed the research; ALE performed the research; ALE analyzed data; and EM, MB, ALE wrote the review.
References
- Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 85(4): 1205-1253 (2005).
- Dilly KW, Rossow CF, Votaw VS, et al. Mechanisms underlying variations in excitation-contraction coupling across the mouse left ventricular free wall. J Physiol. 572(Pt1): 227-241 (2006).
- Ferreiro M, Petrosky AD, Escobar AL. Intracellular Ca2+ release underlies the development of phase 2 in mouse ventricular action potentials. Am J Physiol Heart Circ Physiol. 302(5): H1160-H1172 (2012).
- Ramos-Franco J, Aguilar-Sanchez Y, Escobar AL. Intact heart loose patch photolysis reveals ionic current kinetics during ventricular action potentials. Circ Res. 118(2): 203-215 (2016).
- Kornyeyev DM. Reyes M, Escobar AL. Luminal Ca2+ content regulates intracellular Ca2+ release in subepicardial myocytes of intact beating mouse hearts: Effect of exogenous buffers. Am J Physiol. Heart Circ Physiol. 298(6): H2138-H2153 (2010).
- Valverde CA, Kornyeyev D, Ferreiro M, et al. Transient Ca2+ depletion of the sarcoplasmic reticulum at the onset of reperfusion. Cardiovasc. Res. 85(4): 671-680 (2010).
- Zygmunt AC, Robitelle DC, Eddlestone GT. Ito1 dictates the behavior of ICl(Ca) during the early repolarization of the canine ventricle. Am J Physiol. 273(3 Pt 2): H1096-H1106 (1997).
- Banyasz T, Fülop L, Magyar J, et al. Endocardial versus epicardial differences in L-type calcium current in canine ventricular myocytes studied by action potential voltage clamp. Cardiovasc Res. 58(1): 66-75 (2003).
- Cordeiro JM, Greene L, Heilmann C, et al. Transmural heterogeneity of calcium activity and mechanical function in the canine left ventricle. Am J Physiol Heart Circ Physiol. 286(4): H1471-H1479 (2004).
- Bouchard RA, Clark RB, Giles WR. Effects of action potential duration on excitation-contraction coupling in rat ventricular myocytes. Action potential voltage-clamp measurements. Circ Res. 76(5): 790-801 (1995).
- Sah R, Ramirez RJ, Backx PH. Modulation of Ca2+ release in cardiac myocytes by changes in repolarization rate: Role of phase 1 action potential repolarization in excitation-contraction coupling. Circ Res. 90(2): 165-173 (2002).
- Cooper PJ, Soeller C, Cannell MB. Excitation-contraction coupling in human heart failure examined by action potential clamp in rat cardiac myocytes. J Mol Cell Cardiol. 49(6): 911-917 (2010).
- Dilly KW, Rossow CF, Votaw VS, et al. Mechanisms underlying variations in excitation-contraction coupling across the mouse left ventricular free wall. J Physiol. 572: 227-241 (2006).
- Kondo RP, Dederko DA, Teutsch C, et al. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: A role for repolarization waveform. J Physiol. 571(Pt1): 131-146 (2006).
- Mejıa-Alvarez R, Manno C, Villalba-Galea CA, et al. Pulsed local-field fluorescence microscopy: A new approach for measuring cellular signals in the beating heart. Pflugers Arch. 445(6): 747-58 (2003).
- Kornyeyev D, Petrosky AD, Zepeda B, et al. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J Mol Cell Cardiol. 52: 21-31 (2003).
- Morad M, Goldman YE, Trentham DR. Rapid photochemical inactivation of Ca2+-antagonists shows that Ca2+ entry directly activates contraction in the frog heart. Nature. 304(5927): 635-638 (1983).
- Gurney AM, JM Nerbonne, Lester HA. Photoinduced removal of nifedipine reveals mechanisms of calcium antagonist action on single heart cells. J Gen Physiol. 86(3): 353-379 (1985).
- Sanchez JA, Vergara J. Modulation of Ca2+ transients by photorelease of caged nucleotides in frog skeletal muscle fibers. Am J Physiol. 266(5 Pt1): C1291-C1300 (1994).
- Escobar AL, Velez P, Kim AM, et al. Kinetic properties of DM-nitrophen and calcium indicators: Rapid transient response to flash photolysis. Pflugers Arch. 434: 615-631 (1997).
- Escobar AL, Perez CG, Reyes ME, et al. Role of inositol 1,4,5-trisphosphate in the regulation of ventricular Ca2+ signaling in intact mouse heart. J Mol Cell Cardiol. 53(6): 768-779 (2012).
- Almers W, Roberts WM, Ruff RL. Voltage clamp of rat and human skeletal muscle: Measurements with an improved loose-patch technique. J Physiol. 347: 751-768 (1984).
- Roberts WM, Almers W. An improved loose patch voltage-clamp method using concentric pipettes. Pflugers Arch. 402: 190-196 (1984).
- Gonzalez WG, Pham K, Miksovska J. Modulation of the voltage-gated potassium channel (Kv4.3) and the auxiliary protein (KChIP3) interactions by the current activator NS5806. J Biol Chem. 289: 32201-32213 (2014).
- Volk T, Nguyen TH, Schultz JH, et al. Relationship between transient outward K+ current and Ca2+ influx in rat cardiac myocytes of endo- and epicardial origin. J Physiol. 519(Pt 3): 841-850 (1999).
- Guo W, Xu H, London B, et al. Molecular basis of transient outward K+ current diversity in mouse ventricular myocytes. J Physiol. 521(Pt 3): 587-599 (1999).
- Rossow CF, Dilly KW, Santana LF. Differential calcineurin/NFATc3 activity contributes to the Ito transmural gradient in the mouse heart. Circ Res. 98(10): 1306-1313 (2006).
- Rossow CF, Dilly KW, Yuan C, et al. NFATc3-dependent loss of I(to) gradient across the left ventricular wall during chronic β adrenergic stimulation. J Mol Cell Cardiol. 46(2): 249-256 (2009).
- Teutsch C, Kondo RP, Dederko DA, et al. Spatial distributions of Kv4 channels and KChip2 isoforms in the murine heart based on laser capture microdissection. Cardiovasc Res. 73(4): 739-749 (2007).
- Huo R, Sheng Y, Guo WT, et al. The potential role of the Kv4.3 K+ channel in heart hypertrophy. Channels (Austin). 8(3): 203-209 (2014).
- Kornyeyev D, Petrosky AD, Zepeda B, et al. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J Mol Cell Cardiol. 52(1): 21-31 (2012).
- Mattiazzi A, Argenziano M, Aguilar-Sanchez Y, et al. Ca2+ sparks and Ca2+ waves are the subcellular events underlying Ca2+ overload during ischemia and reperfusion in perfused intact hearts. J Mol Cell Cardiol. 79: 69-78 (2015).
- Aguilar-Sanchez Y, Fainstein D, Mejia-Alvarez R. Local field fluorescence microscopy: Imaging cellular signals in intact hearts. J Vis Exp. 121: 55202 (2017).
- Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 93(2): 372-379 (1996).
- Brunet SF. Aimond F, Li H. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol. 559(Pt 1): 103-120 (2004).
- Aguilar-Sanchez Y, de Yurre AR, Argenziano M, et al. Transmural autonomic regulation of cardiac contractility at the intact heart level. Front Physiol. 10: 773 (2019).
- Li H, Guo W, Mellor RL. KChIP2 modulates the cell surface expression of Kv 1.5-encoded K+ channels. J Mol Cell Cardiol. 39(1): 121-132 (2005).