Research Article - Imaging in Medicine (2017) Volume 9, Issue 6
The role of computed tomography as a prognostic tool in traumatic brain trauma
Cristian Rincon-Guio1, Ana M Gómez2 & José D Charry3*1Department of Engineering, Fundación Universitaria Navarra- Uninavarra, Colombia
2Department of Health Science, Fundación Universitaria Navarra- Uninavarra, Colombia
3Research Department, Fundación Universitaria Navarra- Uninavarra, Colombia
- Corresponding Author:
- José D
Charry
Research Department
Fundación Universitaria Navarra- Uninavarra, Colombia
E-mail: danielcharry06@gmail.com
Abstract
Traumatic brain injury (TBI) is a critical public health problem. It represents one of the most important medicalsurgical pathology worldwide. The occurrence indicate that this kind of injury cause a large number of deaths and impairments leading to permanent disabilities, that is why is necessary to triage the patient and identify the injury extension. Imaging has become a significant tool not only in the diagnosis of patients with TBI, but also as a patient outcome predictor. This review of literature provides evidence of the current state of TBI imaging as to what is considered to be the indications, benefits and limitations of computed tomography (CT).
Keywords
computed tomography ▪ traumatic brain injury ▪ prognosis
Introduction
Traumatic brain injury (TBI) is defined as any structural skull traumatic injury with alterations of cerebral physiology as a result of an external force either in the form of mechanical energy, chemical, electrical or thermal heating [1].
The annual incidence of TBI in the United States is frequently stated to be 200 cases per 100,000 people [2]. In low- and mediumincome countries, burden of disease is quite high and the few available data do not identify the extent of the problem [3].
Brain imaging is crucial in the attention of patients who suffer traumatic brain injury. Computed tomography (CT) is recommended for the initial assessment in the emergency services. It provides information and diagnosis to identify the need of surgery, but it also helps in the following of the patient and the evolution of pathology. Research suggests that CT can be used to predict patient outcomes. [4,5].
Many classifications have been proposed to predict clinical outcomes among patients with traumatic brain injury using computed tomography, however there are still limitations to find the best tool to perform an accurate prognosis [6].
Therefore, this review aims to assess the current literature on the role of Computed tomography and the classifications available to predict outcome in traumatic brain injury.
Methods
A literature search was performed in October 25 using PubMed and EMBASE databases. Search terms used included “traumatic brain injury” and “computed tomography” and “prognosis”. Search was filtered for articles in English, on human subjects and published in the last ten years. Articles were assessed based on information gleaned from the titles and abstracts initially. If they seemed appropriate for the purpose of this review, complete articles were obtained for further assessment. References from these selected papers were also examined to identify any article which could be included in this study.
The main inclusion criteria were the used of computed tomography in traumatic brain injury, clinical findings and outcome. Studies included must have clearly definition to determine diagnosis and prognosis in TBI by imaging. Abstracts, case reports, molecular studies, and neurologic or psychiatry comorbidities like dementia were excluded.
Results
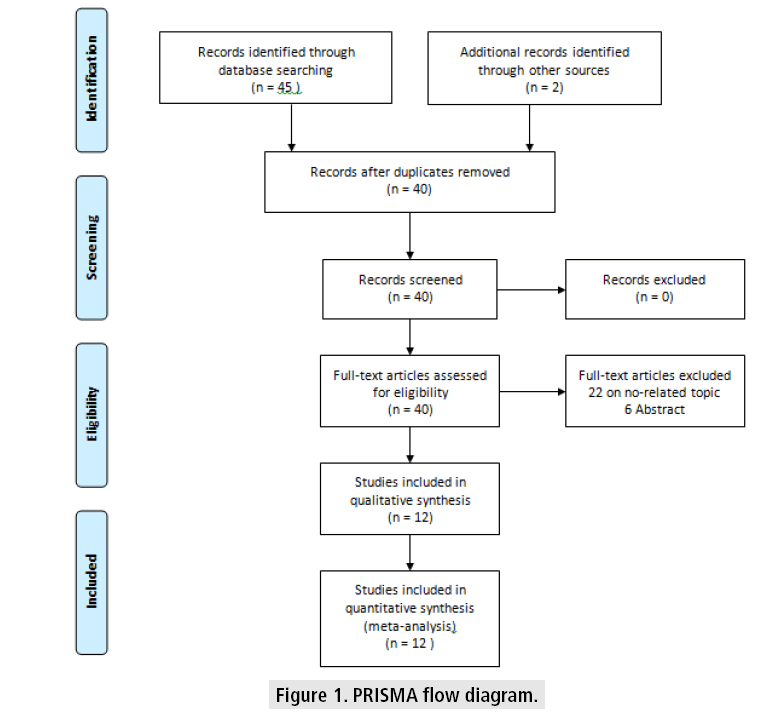
Based on the search terms mentioned before, 45 papers were initially identified. Twenty papers were selected for further scrutiny of their titles and abstracts. Upon further scrutiny, only 12 studies met the inclusion criteria of this study. (FIGURE 1) illustrated the flow of paper selection based on PRISMA guidelines.
The studies characteristics are name in (TABLE 1), taking into account the title, the name of the author, year and study purpose.
| Title | Authors | Year | Study purpose |
|---|---|---|---|
| Imaging brain trauma | Josh et al. [7] | 2010 | Review recent advances in neuroimaging and discuss how they are Helping to address these fundamental gaps. |
| Structural imaging of mild traumatic brain injury May not be enough: overview of functional and metabolic imaging of mild traumatic brain injury |
Samuel et al. [8] | 2017 | The use of functional and metabolic imaging modalities can provide information on pathological changes in mild TBI patients that may not be detected by structural imaging. Each modality’s advantages and disadvantages are compared, and potential future applications of using combined modalities are explored. |
| Imaging evaluation of acute traumatic brain injury | Christopher et al. [9] | 2016 | Review the current state of TBI imaging including its indications, benefits and limitations of the modalities, imaging protocols, and imaging findings for each these pathoanatomic entities. |
| Current and future diagnostic tools for traumatic brain injury: ct, conventional mri, and diffusion tensor imaging | David et al. [10] | 2015 | The review is divided up into assessments based on specific goals, rather than based on scan modalities. It is not meant to supplant but rather to supplement the authoritative previous works on this topic. |
| Emerging imaging tools for use with traumatic brain injury research | Jill et al. [11] | 2012 | This article identifies emerging neuroimaging measures considered by the inter-agency Pediatric Traumatic Brain Injury (TBI) Neuroimaging Workgroup. This article attempts to address some of the potential uses of more advanced forms of imaging in TBI as well as highlight some of the current considerations and unresolved challenges of using them. |
| A systematic review of factors contributing to outcomes in patients with traumatic brain injury | Kim [12] | 2010 | Review systematically factors contributing to outcomes in patients with traumatic brain injury. |
| Imaging for the diagnosis and management of traumatic brain injury | Kim et al. [13] | 2011 | Both CT and MRI can be used to prognosticate clinical outcome, and there is particular interest in advanced applications of both techniques that may greatly improve the sensitivity of conventional CT and MRI for both the diagnosis and prognosis of TBI |
| Outcome of patients with severe head injury after decompressive craniectomy | Lemcke et al. [14] | 2010 | Decompressive craniectomy is an operative option for the neurosurgeon in cases of generalized traumatic brain edema. While the outcome of patients after decompressive craniectomy is often poor, we tried to identify predictors of a favorable course of the injury through GOS, CT and MRI. |
| Classification and prediction of outcome in traumatic brain injury based on computed tomographic imaging | Zhu et al. [15] | 2009 | Classifications based on the presence or absence of intracranial local lesions, diffuse injury, signs of subarachnoid or intraventricular haemorrhage and fractures or foreign bodies are considered, and their predictive value is discussed. Future studies should address the complicated issue of how optimally to combine CT characteristics for prognostic purposes and how to improve on currently used CT classifications to predict outcomes more accurately. |
| Prediction of outcome in severe traumatic brain injury | Menon and Zahed [16] | 2009 | Prognostic models for predicting outcome after severe traumatic brain injury (TBI) may be useful in several areas. However, established risk prediction models for general critical illness show significant limitations in neurotrauma. |
| Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients | MRC CRASH Trial Collaborators [17] | 2007 | To develop and validate practical prognostic models for death at 14 days and for death or severe disability six months after traumatic brain injury. |
| Imaging of traumatic brain injury | Uttam et al. [18] | 2015 | CT is the initial diagnostic test in TBI. Conventional MR imaging in the acute phase is used as a problem solver when CT does not explain the neurologic deficit. |
Table 1: Summary of study characteristics.
■History of computed tomography
The development of CT is the result of the observations and theoretical correlations of many men throughout history, from Democritus reaching Comarck’s ideas, until Hounsfield’s advance of a practical device in the late 1960s [19].
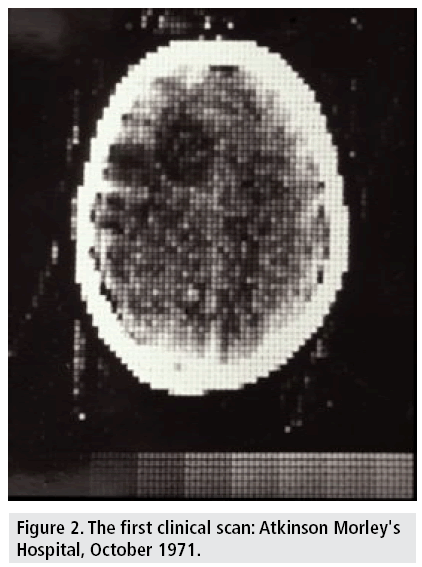
The first clinical CT on a patient took place on October 1971 at Atkinson Morley's Hospital. An English woman with a suspected frontal lobe tumor was scanned with a prototype scanner, which produced an image with an 80 × 80 matrix, taking about 5 min for each scan (FIGURE 2) with a similar time required to process the image data [20].
CT device consists of a tube that emits X-ray, which passes through the patient and impresses the detectors on the opposite side. The attenuated radiation that is received will be the attenuation coefficients or "densities" of the tissues, which will be interpreted by the CT device as Hounsfield units (UH). This range of attenuation varies between gray, from white (hyperdense) to black (hypodense); CT device can identify more than 2000 shades of gray, while the human eye only 30 shades [21,22].
Current CT scanners can produce images with a 1024 × 1024 matrix, acquiring data for a slice in less than 0.3 s and are an integral part of a modern hospital's imaging resources.
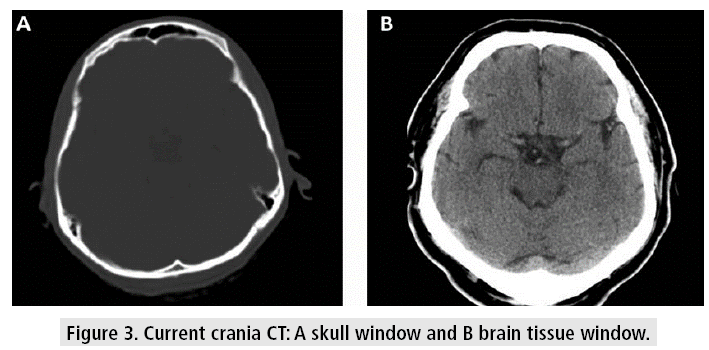
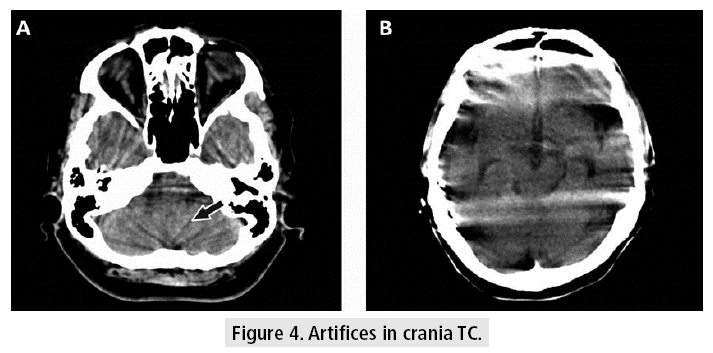
Crania CT allows to evaluate the tissues with very good resolution (FIGURE 3), even though there are some difficulties at the base of the skull, due to the artifacts generated by the bone tissue in the posterior fossa, which can be observed as hypodense bands, projected on the cerebellum. Other kinds of artifacts are due to patient movement, variations of volume or presence of metal, which can cause difficulties in diagnosis and lend themselves to misinterpretation, confusing these devices with images of hemorrhagic lesions (FIGURE 4).
Figure 3: Current crania CT: A skull window and B brain tissue window.
■The use of CT in TBI
TBI represents a dynamic process with primary parenchymal damage from the moment of impact followed by a systemic response that involve local effects like hypoxia, hypotension, hypercarbia, brain swelling that combine lead to secondary brain damage [1].
Once determine the clinical state of the patient provides by the Glasgow Coma Scale (GCS) (TABLE 2), it is necessary to assess the type of injury and its extension using brain imaging [23]. The image recommended for the initial assessment of TBI in emergency services is crania CT. This must be requested in its two windows (bone window and tissue window). Currently, available technology allows TC scan in a few seconds, with cross-sectional, coronal, multiplanar and three-dimensional reconstructions. In patients with fronto-ethmoidal fractures, it is important to request coronal sections of the bone window if it is possible to position the patient for these. The crania CT provides a large amount of information and it has sensitivity for detection of acute hemorrhagic lesions for surgical intervention, as long as it is used in a rational way under the indicate circumstances [24-26].
| Eye opening response | Verbal response | Motor response |
|---|---|---|
| 4: Spontaneous | 5: Oriented | 6: Obeys commands |
| 3: To verbal stimuli | 4: Confused | 5: Localizes pain |
| 2: To pain | 3: Inappropriate words | 4: Withdraws from pain |
| 1: None | 2: Incoherent | 3: Flexion to pain or decorticate |
| 1: None | 2: Extension to pain or decerebrate | |
| 1: None |
Table 2: Glasgow coma scale
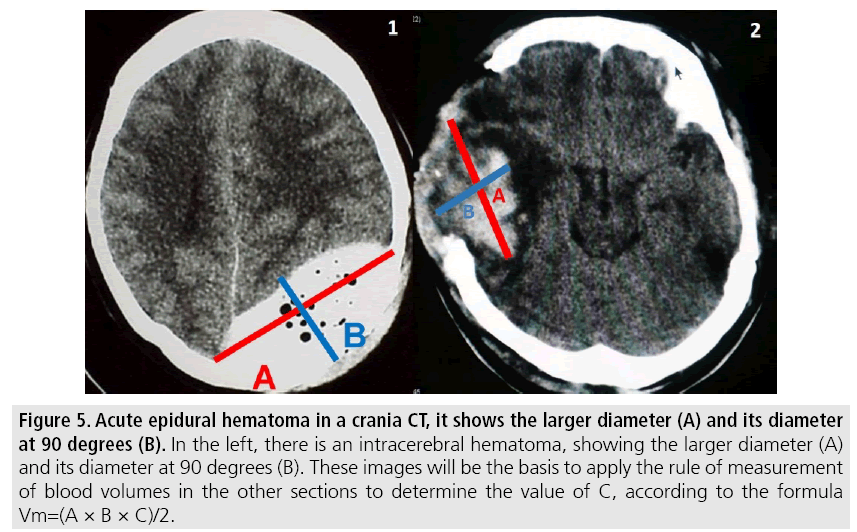
Volume measurement is a determining factor for the management of intracranial lesions [27]; because of emergency surgery decisions are made for each type of collections or for cranial decompression surgery to control cerebral edema.
Volumetric measurements are performed by the CT software and for its application use the following points (FIGURE 5).
Figure 5: Acute epidural hematoma in a crania CT, it shows the larger diameter (A) and its diameter at 90 degrees (B). In the left, there is an intracerebral hematoma, showing the larger diameter (A) and its diameter at 90 degrees (B). These images will be the basis to apply the rule of measurement of blood volumes in the other sections to determine the value of C, according to the formula Vm=(A × B × C)/2.
1. Identify slice where the lesion with the largest diameter is evident.
- A=Larger diameter of the lesion.
- B=Larger diameter measured at 90° from the previous diameter in the same section.
- C=Number of sections of 1 cm where the lesion is evident. (Cuts larger than 1cm are usually made by technical requirement in diagnostic images above the posterior fossa).
Each section should be compared with the base one where the measurements were taken, to find the rule:
- Hemorrhage greater than 75% of the initial section volume is counted as 1.
- Hemorrhage between 25 and 75% of the volume of the initial section is counted as half (1/2 section).
- Hemorrhage less than 25% with respect to the base section is not taken into account.
In addition to measuring the volume, the presence of lesions that determine prognosis and an urgent intervention in diffuse pathologies with cerebral edema should be reviewed:
- Midline shift.
- Presence of traumatic subarachnoid hemorrhage in the basal cisterns.
- Obliteration of cisterns of the base.
The classification of the severity of the TBI according to the Glasgow coma scale and the correlation with the CT images have allowed to associate prognosis and surgical indications in a better-standardized way. One of the most used is the Marshall CT classification (TABLE 3), which allows to correlate tomographic patterns of injury that produce an important morbidity and mortality, including diffuse and focal lesions. The Marshall CT classification is a valid instrument, which is based on the CT findings on the status of the mesencephalic cisterns, the degree of midline shift and the presence or absence of local mass lesions [27,28].
| Category | Definition | Mortality* |
|---|---|---|
| Diffuse injury I (no visible pathology) | No visible intracranial pathology on CT scan. | 10% |
| Diffuse injury II | Cisterns present with midline shift </=5 mm and/or lesion densities present, no high- or mixed-density lesion >25 cc, may include bone fragments and foreign bodies. | 14% |
| Diffuse injury III | Cisterns compressed or absent with midline shift </=5 mm, no high- or mixed-density lesion >25 cc | 34% |
| Diffuse injury VI | Midline shift >5 mm, no high- or mixed-density lesion >25 cc | 56% |
| Evacuated mass lesion | Any lesion surgically evacuated | 39% |
| No evacuated mass lesion | High- or mixed-density lesion >25 cc, not surgically evacuated | 53% |
| *Mortality at discharge | ||
Table 3: Marshal classification of head injury (Trauma coma data bank)
Marshal classification has limitation in patients with multiple injuries, and its applications is not linear, which difficult its use for severity. In the other hand Rotterdam score (TABLE 4) is based only in CT findings such as the presence of subarachnoid or intraventricular hemorrhage, effacement of the basilar cisterns, midline shift >5 mm, presence of subdural hemorrhage, and absence of epidural hemorrhage, in an effort to predict 6-month mortality [29].
| Predictor | Score |
|---|---|
| Basal Cistern | |
| Normal | 0 |
| Compressed | 1 |
| Absent | 2 |
| Midline Shift | |
| No shift or shift </=5 mm | 0 |
| Shift >5 mm | 1 |
| Epidural mass lesion | |
| Present | 0 |
| Absent | 1 |
| Intraventricular blood or subarachnoid hemorrhage | |
| Absent | 0 |
| Present | 1 |
| Sum score | +1 |
| In the Rotterdam scoring system, 1 point is added as a sum score to make the Rotterdam grade numerically total 6 points, consistent with the motor core of the Glasgow Coma Scale and the Marshal classification. From Maas et al. [37] | |
Table 4: Rotterdam score
Even other prognostic tools exist, the Marshall and Rotterdam score are the well standardized and validated with CT findings.
Discussion
■Role of CT in prognosis
CT is the brain imaging of choice for the initial assessment of TBI because it is fast, widely available, and highly accurate in the detection of skull fractures and intracranial lesions like hemorrhages, herniation and hydrocephalus, which may necessitate immediate surgical evacuation. However, in case of concussion, the role of CT is less well defined [30]. CT is recommended in patients with neurological deterioration following TBI; studies have shown little benefit for routine follow up imaging. In rare cases where the clinical presentation is far worse than can be explained based on the CT scan, MRI may be helpful [15].
To evaluate prognosis Glasgow Outcome Scale (GOS) can be used. It categorizes the outcomes of patients after traumatic brain injury, as follows [31]: 1. Death, 2. Persistent vegetative state: Minimal responsiveness, 3. Severe disability: Conscious but disabled; dependent on others for daily support, 4. Moderate disability: Disabled but independent; can work in sheltered setting, 5. Good recovery: Resumption of normal life despite minor deficits.
However, there are other elements valuable in the prognosis of TBI patients. Kim [12] described in his paper that older age, severe injury measured by GCS or injury severity score (ISS), no pupil reaction, hypotension or hypertension, elevated ICP, hypoxia, hypoglycemia or hyperglycemia, hypothermia or hyperthermia, low level of hemoglobin, coagulopathy, high level of lactate, electrolyte derangement, high level of Marshall CT classification and presence of subarachnoid, epidural or subdural hemorrhage were identified as predictors for poor GOS outcome. Even though, it is recommended that GCS should be serially assessed even after emergency department stabilization and evaluated in company with the computed tomography (CT) finding for an accurate prognosis [12]. It is notable that the majority of studies for Marshal CT classification show that patients with diffuse brain injury (I) had good outcomes, whereas patients ingroup IV or V having a mass lesion on CT had unfavorable outcome. Given that, there is a very strong relationship between Marshall CT classification and outcome. Future studies need to focus on combining the CT characteristics and other predictors for increasing the prognostic value [31].
The primary role of CT scanning has been the acute identification of focal injuries that may require emergent neurosurgical interventions, such as extra-axial or parenchymal hemorrhage, midline shift and incipient herniation. Approximately 10% of patients with severe TBI require a craniectomy based on the findings from an initial CT scan. According to the Brain Trauma Foundation guidelines, these findings include extra-axial hematomas (epidural or subdural hemorrhages) larger than 30 mL in size or associated with greater than 5 mm of midline shift and parenchymal hematomas in a noneloquent cortex greater than 20 mL in size [32]. Kim and Gean [13] showed that individual predictors of poor outcome on conventional CT include compressed or absent basilar cisterns, the presence of subarachnoid hemorrhage, midline shift, and intracranial hemorrhagic lesions (e.g. subdural or epidural hematomas, shear injury, and contusions). Compressed or absent basilar cisterns indicate a threefold higher risk of increased ICP and are associated with a twoto three-fold increase in mortality. Traumatic subarachnoid hemorrhage is associated with a twofold increase in mortality, and hemorrhage in the basilar cisterns has a 70% positive predictive value for poor outcome. Midline shift indicates increased ICP and is similarly associated with a poor clinical outcome, although this association is somewhat complicated by the fact that midline shift is caused by intracranial hemorrhage that also negatively impacts outcome. Finally, intracranial hemorrhage has an approximately 80% positive predictive value for poor functional outcome, with prognosis worsening as the hematoma volume increases in size. Of note, mortality is higher in patients with acute subdural hematomas compared with those with epidural hematomas [13,32].
Lemcke et al. [14] studied the outcome of patients with severe head injury after decompressive craniectomy and found that the shift of the midline on CT was significantly correlated with the quality of outcome. Patients with a poor outcome (GOS 1-3) had a mean midline shift of 9.8 ± 6.7 mm, whereas patients with a good outcome (GOS4-5) showed a mean midline shift of 6.7 ± 5.0 mm. The important predictive value of this factor has been confirmed by Marshall et al. among others. The visibility of the basal cisterns was also shown in this study to be very significantly connected to the quality of outcome at follow-up [32].
It is important to recognize that Marshall CT classification has been extensively evaluated and it is considered as many studies have showed a tool in the prognosis. Nevertheless a number of other CT classifications exist [33-35], which can be an interesting alternative to examine the elements that Marshall CT classifications do not consider.
Zhu et al. [15] described how in 2005, a study by Maas et al. compared alternative CT models with the Marshall CT classification and found it was preferable to use combinations of individual CT predictors rather than the Marshall CT classification for prognostic purposes in TBI [36]. Later in 2007, Maas et al. [37] again evaluated the prognostic value of CT scan characteristics in TBI and found that individual CT characteristics added substantially to the prognostic value of the CT classification alone. They found that making greater use of individual CT characteristics allowed them to improve on the already sizeable predictive value of the original Marshall CT classification scheme. These findings need to be corroborated through further prospective investigations.
Limitations
Gray and non-English literature was excluded. In this review study, a comparison of a new classification for the use of tomography as a prognosis was not proposed or made.
Conclusion
Brain imaging gives a landscape in outcome; the application of CT classifications can be helpful in the prognosis of TBI. Nevertheless, many elements may be consider in the evaluation of imaging and the development of new scores can improve the predictive value on the outcome taking into account the CT findings.
Disclosure
The authors declare that there is no conflict of interest, financial or otherwise, regarding the publication of this paper.
References
- http://gpc.minsalud.gov.co/gpc_sites/Repositorio/Conv_563/GPC_trauma_craneo/CPG_TBI_professionals.pdf
- https://emedicine.medscape.com/article/433855-overview#a4
- Byass P, de Courten M, Graham WJ et al. Reflections on the global burden of disease 2010 estimates. PLoS. Med. 10, 1477 (2013).
- Hassan Z, Smith M, Littlewood S et al. Head injuries: A study evaluating the impact of the NICE head injury guidelines. Emerg. Med. J. 22, 845-849 (2005).
- Metting Z, Rödiger LA, De Keyser J et al. Structural and functional neuroimaging in mild-to-moderate head injury. Lancet. Neurol. 6, 699-710 (2007).
- David T. Head injury: Triage, assessment, investigation and early management of head injury in children, young people and adults (NICE guideline CG 176). Arch. Dis. Child. Educ. Pract. Ed. 100, 97-100 (2015).
- Josh LD, Robert DS. Imaging brain trauma. Curr. Opin. Crit. Care. 16, 92-97 (2010).
- Samuel SS, James WB, Edward CD et al. Structural imaging of mild traumatic brain injury may not be enough: Overview of functional and metabolic imaging of mild traumatic brain injury. Brain. Imaging. Behav. 11, 591-610 (2017).
- Christopher AM, Jason FT, GeanA. Imaging evaluation of acute traumatic brain injury. Neurosurg. Clin. N. Am. 27, 409-439 (2016).
- David LB, Christine LMD, Joshua SS. Current and future diagnostic tools for traumatic brain injury: CT, conventional MRI and diffusion tensor imaging. Handb. Clin. Neurol. 127, 267-275 (2015).
- Jill VH, Elisabeth AW, Karen AT et al. Emerging imaging tools for use with traumatic brain injury research. J. Neurotrauma. 29, 654-671 (2012).
- Kim YJ. A systematic review of factors contributing to outcomes in patients with traumatic brain injury. J. Clin. Nurs. 20, 1518-1532 (2011).
- Kim JJ, Gean AD. Imaging for the diagnosis and management of traumatic brain injury. Neurotherapeutics. 8, 39-53 (2011).
- Lemcke J, Ahmadi S, Meier U. Outcome of patients with severe head injury after decompressive craniectomy. Acta. Neurochir. Suppl. 106, 231-233 (2010).
- Zhu GW, Wang F, Liu WG. Classification and prediction of outcome in traumatic brain injury based on computed tomographic imaging. J. Int. Med Res. 37, 983-995 (2009).
- Menon DK, Zahed C. Prediction of outcome in severe traumatic brain injury. Curr. Opin. Crit. Care. 15, 437-441 (2009).
- MRC CRASH Trial Collaborators. Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. Patients. BMJ. 28, 336-425 (2007).
- Uttam KB, Chandler S, Jiachen Z et al. Imaging of traumatic brain injury. Radiol. Clin. N. Am. 53, 695-715 (2015).
- Ambrose J. Computerized transverse axial scanning (tomography)-2. Clinical applications. Br. J. Radiol. 46, 1023-1047 (1973).
- Hounsfield, GN. Computerised transverse axial scanning (tomography)-1. Description of system. Br. J. Radiol. 46, 1016-1022 (1973).
- Abdalla MR, Rubiano AM. Basic anatomy applied to the interpretation of axial tomography of the brain in emergency medicine in: Godoy DA. Int. Care. Neurol. Neurosurg. 2, 27-51 (2013).
- http://docplayer.net/10707822-Ferne-emra-2009-mid-atlantic-emergency-medicine-medical-student-symposium-abcs-of-head-ct-interpretation-heather-m-prendergast-md-mph.html
- Hawryluk GW, Manley GT. Classification of traumatic brain injury: Past, present and future. Handb. Clin. Neurol. 127, 15-21 (2015).
- Wei SC, Ulmer S, Lev MH et al. Value of coronal reformations in the CT evaluation of acute head trauma. Am. J. Neuroradiol. 31, 334-339 (2010).
- Zacharia TT, Nguyen DD. Subtle pathology detection with multi-detector row coronal and sagittal CT reformations in acute head trauma. Emerg. Radiol. 17, 97-102 (2010).
- Haacke EM, Mittal S, Wu Z et al. Susceptibility-weighted imaging: Technical aspects and clinical applications, Part 1. Am. J. Neuroradiol. 30, 19-30 (2009). https://goo.gl/AWSFCV
- Steyerberg EW, Mushkudiani N, Perel P et al. Predicting outcome after traumatic brain injury: Development and international validation of prognostic scores based on admission characteristics. PLoS. Med. 5, 165 (2008).
- Stein SC, Fabbri A, Servadei F et al. A critical comparison of clinical decision instruments for computed tomographic scanning in mild closed traumatic brain injury in adolescents and adults. Ann. Emerg. Med. 53, 180-188 (2009).
- Ro YS, Shin SD, Holmes JF et al. Comparison of clinical performance of cranial computed tomography rules in patients with minor head injury: A multicenter prospective study. Acad. Emerg. Med. 18, 597-604 (2011).
- Zhu GW, Wang F, Liu WG. Classification and prediction of outcome in traumatic brain injury based on computed tomographic imaging. J. Int. Med. Res. 37, 983-995 (2009).
- The Brain Trauma Foundation. The American Association of Neurological Surgeons. The joint section on neurotrauma and critical care. Computed tomography scan features. J. Neurotrauma. 17, 597-627 (2000).
- Marshall LF, Gautille T, Klauber MR et al. The outcome of severe closed head injury. J. Neurosurg. 75, 28-36 (1991).
- Azian AA, Nurulazman AA, Shuaib L et al: Computed tomography of the brain in predicting outcome of traumatic intracranial haemorrhage in Malaysian patients. Acta. Neurochir. 143, 711-720 (2001).
- Teasdale G, Teasdale E, Hadley D. Computed tomographic and magnetic resonance imaging classification of head injury. J. Neurotrauma. 9, 249-257 (1992).
- Schreiber MA, Aoki N, Scott BG et al. Determinants of mortality in patients with severe blunt head injury. Arch. Surg. 137, 285-290 (2002).
- Maas AI, Hukkelhoven CW, Marshall LF et al. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurg. 57, 1173-1182 (2005).
- Maas AI, Steyerberg EW, Butcher I, et al. Prognostic values of computerized tomography scan characteristics in traumatic brain injury: Results from the IMPACT study. J. Neurotrauma. 24, 303-314 (2007).