Review Article - Interventional Cardiology (2014) Volume 6, Issue 6
The role of echocardiography in transcatheter aortic valve implantation
- Corresponding Author:
- Daniela Cassar DeMarco
King’s College Hospital, Denmark Hill, London, SE5 9RS, UK
E-mail: daniela.cassardemarco@nhs.net
Abstract
Aortic stenosis is a common valve disease with increasing prevalence in the elderly. The presence of comorbidities in this population can make surgical aortic valve replacement challenging; therefore, transcatheter aortic valve implantation is increasingly being offered as a management option for these patients. Imaging with echocardiography has an important role through all aspects of the procedure from initial imaging and patient selection, guidance of the procedure and assessment of complications.
Keywords
aortic stenosis, echocardiography, transcatheter aortic valve implantation
Aortic stenosis (AS) is the most common native valve disease in Europe [1]. Its prevalence increases with age and it carries a poor prognosis [2]. Transcatheter aortic valve implantation (TAVI) is a relatively new interventional technique that offers a valid treatment option for patients with severe AS who are at high risk for conventional surgical aortic valve replacement (SAVR), when compared with standard therapy [3]. Echocardiography plays an important role in the initial assessment of patients with AS, peri-procedural monitoring and postprocedure evaluation [4].
Transcatheter aortic valve implantation
In 2002, Cribier et al. reported the first successful implantation of a bovine pericardial bioprosthesis mounted within a balloonexpandable stent, via an antegrade approach and trans-septal puncture in a 57-year-old man with calcific AS and cardiogenic shock [5]. Since that early experience, there were considerable technical improvements in size and maneuverability of the delivery system, and a retrograde transfemoral approach was developed. In patients whose lower limb vasculature is unsuitable, a transapical, subclavian or transaortic approach is possible [4].
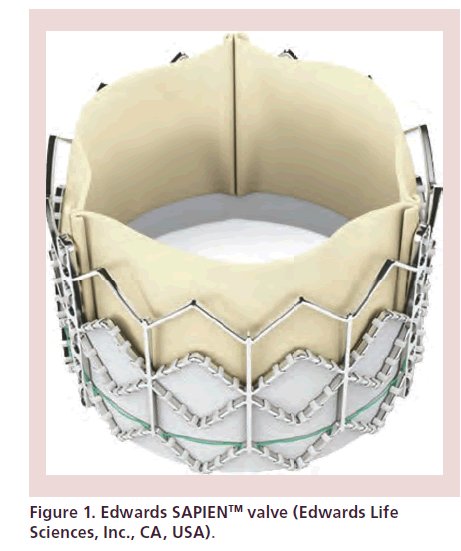
Currently, there are two specific devices that are licensed for use. The Edwards SAPIEN/SAPIEN XT valve (Edwards Lifesciences, Inc., CA, USA) (Figure 1) has bovine pericardial leaflets mounted in a balloonexpandable stent of chromium cobalt alloy. A polyethylene terephthalate fabric skirt covers the lower aspect of the stent in order to decrease paravalvular leaks. It is available in 20, 23, 26 and 29 mm in Europe. It may be delivered via both femoral and transapical approaches. The second device is the Medtronic CoreValve (Medtronic, Inc., MN, USA) (Figure 2), which consists of porcine pericardial leaflets mounted in a self-expandable nitinol frame. The lower portion fixes the valve to the left ventricular outflow tract (LVOT), the midportion should be positioned at the level of the sinuses of Valsalva and coronary ostia, while the upper segment fixes to the ascending aorta. It is available in 23, 26, 29 and 31 mm [4,6]. The CoreValve is mainly designed for vascular access but transapical implantation has been reported [7].
Transfemoral approach
This is a retrograde delivery technique where an introducer sheath with internal caliber of 18F to 24F is advanced into a femoral artery. Balloon aortic valvuloplasty (BAV) is performed during rapid right ventricular pacing. The crimped valve is advanced under fluoroscopic and transesophageal echocardiography (TOE) guidance to the native aortic valve and deployed during rapid pacing [4].
Transapical approach
This is an antegrade approach. Access is obtained via a left anterior thoracotomy after anatomical localization of the apex with transthoracic echocardiography (TTE). The pericardium is opened and access to the left ventricular (LV) cavity is obtained. A guidewire is advanced to the native aortic valve under fluoroscopic and TOE guidance. BAV is performed under rapid pacing, followed by sheath insertion and valve deployment [4,6].
Patient selection & preoperative assessment
Patients with severe AS who are being considered for TAVI are screened and selected by a multidisciplinary team, consisting of interventional cardiologists, surgeons, anesthetists and imaging physicians, in order to assess feasibility and potential risks of the intervention. Imaging with echocardiography plays an essential role in patient selection and preparation, providing information about the severity of AS, valve anatomy and aortic annular size [4]. Further imaging of lower limb and abdominal vasculature with multislice computed tomography (MSCT) or MRI will help in selecting the most appropriate approach to the intervention [4,6,8].
Surgical risk may be quantified with the Logistic European System for Cardiac Operative Risk Evaluation, where a high surgical risk is defined by a score of ≥15–20% and/or the Society of Thoracic Surgeons Predicted Risk of Mortality Score, where a score of ≥10% is considered as high risk. Other comorbidities that favor TAVI over SAVR include prior cardiac surgery, previous chest radiation, porcelain aorta, liver cirrhosis, pulmonary hypertension, right ventricular failure or patient frailty. TAVI is contraindicated if the procedure is expected to not improve quality of life and in patients whose life expectancy is less than 1 year [4,9].
Role of transthoracic echocardiography
TTE is the initial investigation of choice to confirm the diagnosis of severe AS. It allows evaluation of valve morphology, degree of calcification, aortic valve area (AVA) by continuity equation, mean transaortic gradients and peak transvalvular velocity. Severe AS is defined by an AVA of less than 1 cm2 (<0.6 cm2/m2) or a mean aortic valve gradient of greater than 40 mmHg. The velocity ratio calculated by dividing LVOT velocity by aortic valve velocity, may be used if there is difficulty in measuring LVOT diameter accurately, and severe stenosis is present if the velocity ratio is ≤0.25 [10].
Full transthoracic assessment should be performed including evaluation of left and right ventricular size and function, associated aortic regurgitation (AR) or other valvular disease. The presence of septal hypertrophy causing severe LVOT obstruction should be identified as this may present a problem for TAVI deployment with risk of migration of the device. Pericardial calcification or LV thrombus is a contraindication to apical TAVI [4].
Patients with severe AS and LV systolic dysfunction may present a diagnostic challenge during assessment for TAVI. In the setting of low flow, low gradient AS, the calculated AVA suggests severe AS, however, the reduced stroke volume gives lower transvalvular pressure gradients [11]. These patients may have true-severe AS causing significant LV dysfunction, or pseudo-severe AS where an impaired LV fails to generate enough pressure to open the aortic valve, thereby contributing the calculation of a small valve area. Paradoxical low flow, low gradient AS describes an entity characterized by preserved ejection fraction and reduced stroke volume in the setting of severe AS. This condition is character by concentric LV remodeling and small cavity size leading to a reduced stroke volume with possible underestimation of AS severity based upon gradient measurements [11,12]. Patients with true AS may benefit from surgery, therefore it is important to distinguish between the two entities. Low-dose dobutamine stress echo (DSE) has been shown to distinguish between true-severe AS and pseudo-severe AS, and it also provides information about contractile reserve [4,11]. The valve is truly stenotic if dobutamine increases the stroke volume and increases maximum jet velocity to ≥4 m/s, while AVA remains less than 1.0 cm2. Conversely, AS is only mild to moderate, if stroke volume increases with minimal rise in gradient, causing increase in valve area, implying that LV dysfunction is due to other causes and not AS [12]. In a multicenter study using DSE, Monin et al. showed that patients with no contractile reserve defined as less than 20% increase in stroke volume during DSE have reduced survival compared with those with LV contractile reserve. AVR is associated with a significant improvement in survival in patients with LV contractile reserve (p = 0.001) and improved survival in those with no flow reserve (p = 0.07) [13]. In patients with low flow, low gradient AS, TAVI provides a similar outcome compared with SAVR and it improves survival compared with medical management [14].
Once the diagnosis of severe valvular AS has been confirmed, further evaluation of the aortic valve and the aortic root are required. The combination of shortand long-axis views during TTE should be acquired to identify the number of aortic valve leaflets, and to describe leaflet mobility, thickness and degree of calcification [10]. Patients with a bicuspid aortic valve have been excluded from major trials and this anatomical finding is currently a contraindication for TAVI [4,6]. Bicuspid aortic valves present various problems for TAVI in that very elliptical aortic valve orifices may not be suitable for available devices. Also, asymmetric calcification, which is frequently associated with bicuspid valves, may preclude full expansion of prosthesis with resulting paravalvular AR and increased shear stress on the valve, thereby contributing to early degeneration [15]. Nevertheless, multiple reports of TAVI in patients with bicuspid aortic valves have been documented [15–17]. Both MSCT and TOE provide important information regarding the extent and distribution of calcification in and around the aortic valve leaflets and it is very important to evaluate this since extensive and eccentric calcification increases the risk of paravalvular regurgitation (PVR). Furthermore, patients with bulky aortic valve leaflets and small sinuses may be at increased risk of aortic root rupture. We recommend that this is evaluated using whichever imaging modality there is greatest local expertise in.
Annulus size
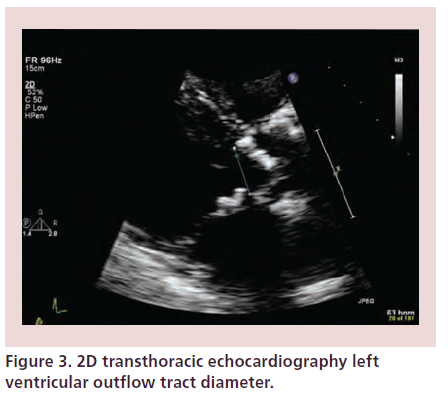
Accurate sizing of the aortic annular dimension is critical to the success of TAVI, as this will guide selection of valve type and size. Annular anteroposterior diameter is measured in the parasternal long-axis view, zoomed on the LVOT and aortic valve during midsystole (Figure 3). The aortic annulus is taken as the most basal point of the three leaflets continuing with the LVOT. Measurement of aortic annular diameter should be made at the lowest hinge points of the right coronary cusp to the noncoronary cusp, trailing edge to leading edge. Although is practice it is theoretically impossible to cut across the lowest hinge points of the right and noncoronary leaflets in the same 2D view because these two points are not directly opposite each other. As the annulus is usually elliptical, further measurements in an orthogonal plane should be performed. Calcification at the level of the aortic annulus may present difficulty in accurate assessment of annular diameter, therefore further assessment with TOE is necessary [4,6]. A prosthetic valve which is too small may result in migration of the device or paravalvular AR. It may also cause patient prosthesis-mismatch with associated valvular stenosis. Oversizing may cause redundancy of leaflet tissue, thereby causing increased tension with consequent central AR and reduced durability of the prosthesis [18]. A large device may pose difficulties in crossing the native valve with the delivery system or complications related to vascular access [4].
Figure 3: 2D transthoracic echocardiography left ventricular outflow tract diameter.
Role of 2D & 3D TOE
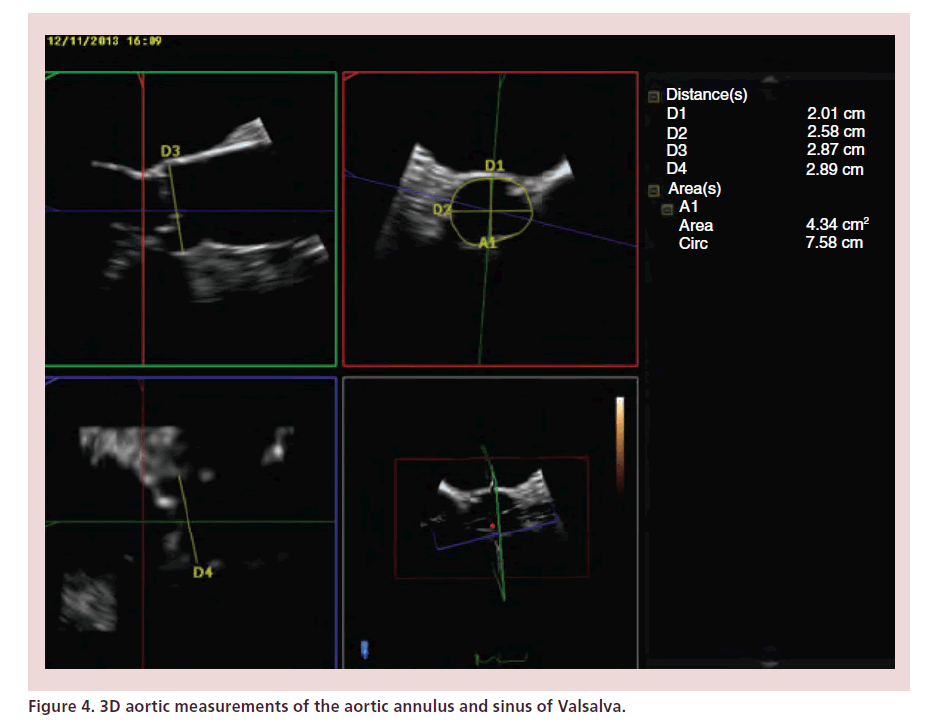
While TTE allows better Doppler alignment and hence measurement of transvalvular gradients, TOE is superior in that it allows better image quality and more accurate measurement of the aortic annular diameter. Although TTE and TOE annular dimension measurements are comparable [8], TOE may give larger values, with a mean difference between the two modalities of 1.36 mm (two standard deviations range of -4.48 and 1.75 mm) [19]. Similarly to the technique used during TTE, TOE measurements at the level of the aortic root should be taken in a systolic frame of a zoomed mid-esophageal long-axis view. Annular diameter is measured from the leaflet insertion of the noncoronary cusp to the right coronary cusp [20]. As may be seen with images from MSCT, the aortic root has a complex 3D structure. The LVOT and aortic annulus may be ovalor elliptical-shaped rather than circular [21]. Annular diameter measurements taken during 2D TOE may not be perpendicular to the true anteroposterior diameter of the aortic annulus. 3D TOE overcomes this problem as it allows online multiplanar reconstruction of the aortic root, thereby allowing measurement of anatomically correct minimum and maximum annular diameters, perimeter and cross-sectional area (Figure 4) [4,22]. Measurements taken with 3D TOE are comparable to those of MSCT [22] and are our recommended echo imaging modality for annulus sizing.
During TAVI, the native aortic valve leaflets are crushed against the walls of the aortic root. This can cause aortic rupture and/or coronary ostial obstruction with potentially life-threatening complications [4,23]. Measurement of the distance between the aortic annulus and ostia of the coronary arteries will help appropriate valve selection. Measurement of right coronary ostial distance can be measured on 2D TOE, but left coronary requires 3D TOE or MSCT [4]. In addition, it is important to measure the size of the coronary sinuses to ensure that there is enough space to accommodate the calcified aortic valve leaflets.
Full assessment of the aortic valve is necessary. The number of leaflets, degree of calcification, classification of aortic valve opening as central or eccentric, and degree, if any, of AR should be documented [4]. During TOE, the characteristics of the aorta should also be assessed as heavily atheromatous changes may pose risk of stroke or embolic phenomena during transfemoral TAVI [4].
It is also necessary to exclude significant mitral valve disease, thrombus in the left atrium or left ventricle and to document baseline LV function.
Peri-procedural assessment
Role of 2D TTE
TTE plays an important part in the selection of candidates for TAVI. It has limited use of peri-procedure. Its main function is to locate the apex during a transapical TAVI. Images of the left ventricle should be obtained in two orthogonal views, and the cardiothoracic surgeon who will perform the thoracotomy should be present to agree the optimal intercostal space for the thoracotomy [4,23].
Role of TOE
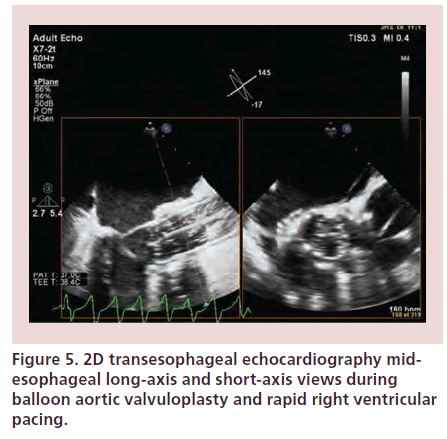
TOE may be used during TAVI to confirm the echo-cardiographic findings during work-up. It assists monitoring during different stages of the procedure from positioning of guidewire and delivery system, BAV to prosthesis positioning and implantation and rapid detection of complications (Figure 5) [4]. The disadvantages of peri-procedural TOE include the need for general anesthesia and potential obstruction of the fluoroscopic view by the TOE probe. Both 2D and 3D techniques have a complimentary role during TAVI [23]. 3D TOE provides better spatial visualization than 2D TOE, therefore it allows better appreciation of the guidewire path and permits improved evaluation of the prosthesis position on the balloon, relative to the native valve annulus and surrounding structures [23,24].
Figure 5: 2D transesophageal echocardiography mid-esophageal long-axis and short-axis views during balloon aortic valvuloplasty and rapid right ventricular pacing.
Intra-procedural guidance
Guidewire/delivery system positioning
Once the aortic annular diameter has been confirmed, the prosthetic valve size selected and the guidewire advanced across the aortic valve, TOE allows direct visualization of the wire in the left ventricle. During this stage of the procedure, it is important to ensure that the wire does not get entangled in the mitral valve apparatus and that it has a clear path to or from the apex and aortic valve [23].
Balloon aortic valvuloplasty
Balloon dilatation (BAV) is obviously performed during rapid right ventricular (RV) pacing in order to predilate the annulus and split the commissures of the aortic valve [4]. Real-time 3D TOE monitoring during this stage confirms the position of the balloon, especially in valves with less calcification that are not easily seen fluoroscopically. TOE (especially using X-plane imaging) monitors the behavior of the aortic valve leaflets as they are pushed back into the coronary sinuses [4,23]. It is important to know the size of the valvuloplasty balloon because this helps one understand how the prosthesis will fit into the annulus and sinuses. After BAV, the aortic valve should be reassessed for native cusp mobility and AR.
TAVI valve positioning & deployment
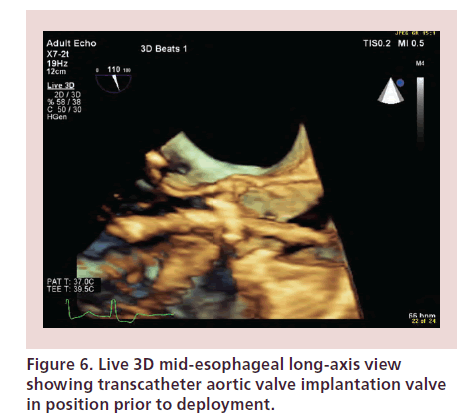
Together with fluoroscopy, TOE confirms the ideal position of the valve prior to deployment. Real-time 3D TOE allows direct visualization of the proximal and distal margins of the valve mounted and also the delivery system (Figure 6). The valve should be deployed once the interventionalist and echocardiographer agree that the position is optimal. If the position of the valve is too low, it may impinge on the anterior mitral valve leaflet and cause valve dysfunction or perforation at a later stage. If the valve is implanted too high, there is a risk of migration in the aorta. It can also cause coronary ostial occlusion and PVR [24].
Post-procedural assessment
Immediately after deployment, TOE is used to confirm the position and circular appearance of the valve. Leaflet mobility should be assessed and color Doppler will identify any valvular or paravalvular AR. A transverse (short-axis) view across the LVOT, just beneath the prosthesis, is very helpful in differentiating transvalvular from PVR. In addition, examining the circumferential extent of regurgitant jets appears to be a practical method for preliminary evaluation of the degree of PVR and to decide if postdilatation is necessary. Transient mild transvalvular AR may be detected while the guidewire is still crossing the aortic valve. Prosthetic valve function is assessed with continuous-wave, pulsed-wave and color Doppler from transgastric TOE views. A complete TOE assessment should be carried out and the mitral valve and LV function should also be assessed before peri-procedure imaging is considered complete [24].
Complications of TAVI
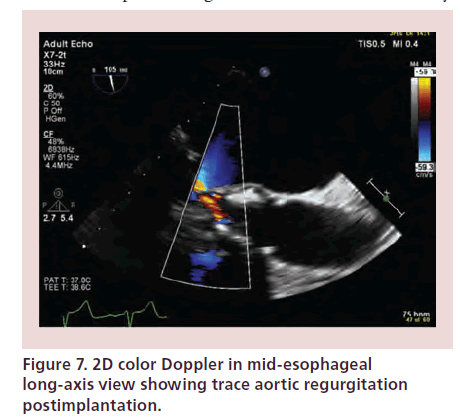
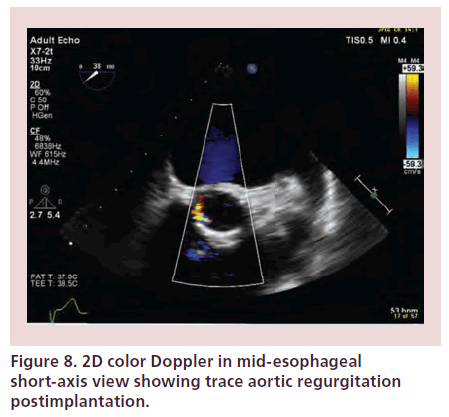
Immediate TOE imaging after TAVI allows detection of transvalvular regurgitation or PVR. Severe AR may occur secondary to undersizing of the prosthesis, restricted cusp motion, incomplete expansion or incorrect positioning of the device [4,25]. PVR may result secondary to lack of expansion of the valve at areas of heavy calcification of the native valve. Trace or mild paravalvular leaks usually have a benign course (Figures 7 & 8) [4]. As previously mentioned, PVR may require further ballooning to achieve maximal valve expansion, taking care to avoid overexpansion that may result in rupture of the aortic root and cusp trauma leading to worsening AR. Prosthesis mismatch may also result in regurgitation and device migration.
During TAVI, the valve itself or the native leaflets may obstruct the coronaries or any dislodgment of a fragment of calcium into the coronary arteries may result in severe hemodynamic disturbance and new LV wall motion abnormality [4]. An enlarging pericardial effusion secondary to perforation of the right ventricle with the temporary wire may also cause hemodynamic compromise and cardiac tamponade. This is easily detected with echo, and it will require drainage.
Sudden worsening of mitral regurgitation (MR) may occur as a consequence of pacing or implantation of the device that is too low within the LVOT. Damage to the mitral subvalvular apparatus may occur during apical TAVI resulting in MR [4]. Imaging of the aortic root after TAVI is mandatory to detect tear or rupture and the integrity of the ascending aortic wall should also be assessed.
Although some centers are performing TAVI procedures without peri-procedure TOE, there are data which show that TOE-guided procedures are quicker and use less contrast medium, which can be important in patients with renal insufficiency [26].
Postimplantation follow-up
TTE follow-up post-TAVI is similar to that of postsurgical AVR. Calculation of gradients across the valve and effective orifice area (EOA) should be performed with awareness that the gradients tend to be lower than through equivalent size surgical AVRs. TAVI valves exhibit two regions of flow acceleration, proximal to the valve cusps and at the level of the cusps. LVOT diameter and prevalvular velocity should be recorded immediately proximal to the TAVI valve, while postvalvular velocity with continuous- wave Doppler distal to the stent. If LVOT velocity is recorded within the stent but proximal to the cusp, the result will be an overestimation of the valve area [4,27,28].
AR post-TAVI may be seen as a central jet or PVR. Quantification of severity of AR may be difficult; however, known qualitative measures to evaluate native AR may be used with prosthetic valves [29]. Color-flow Doppler may be used to assess the regurgitant jet size. For central jets of AR, the proximal jet width or cross-sectional area of the jet beneath the prosthesis (within the LVOT) may be used to grade severity of AR according to the percentage LVOT diameter occupied: ≤25% indicates mild, 26–64% indicates moderate and greater than 65% indicates severe [4]. This method cannot be used for PVR as these jets are commonly multiple and eccentric. Vena contracta size may be difficult to assess in prosthetic heart valves due to artefact and acoustic shadowing, and there has not been validation for adding vena contracta of multiple jets encountered post-TAVI. Also, adding the proportion of the circumference occupied by multiple jets in TAVI valves as one would for prosthetic valves may overestimate the severity of PVR [4].
3D echocardiography may be considered for assessment of AR volume. In vitro studies by Pirat et al., have shown that measurement of the proximal isovelocity surface area using real-time 3D color Doppler is feasible as the geometric assumptions one would encounter in 2D imaging are overcome [30]. The utility of this modality in prosthetic heart valves has yet to be validated.
In the assessment of AR, one should also measure the pressure half-time of a continuous-wave Doppler signal, the density of the spectral display and the presence or absence of diastolic flow reversal in the aorta. The stability of the valve and assessment of any rocking motion of the device or cusp dehiscence will allow identification of valve degeneration [4].
Conclusion
As the number of patients undergoing TAVI continues to increase, it is clear that both 2D and 3D echocardiography play an important role throughout all stages of the procedure. The success of the procedure depends on a multidisciplinary approach and multi-imaging modalities. Furthermore, it is expected that the development of new hybrid catheterization laboratories will facilitate all aspects of the intervention including the role of the imaging physician by creating an appropriate environment that allows maneuvering of the echocardiographic machines and increased radiation safety.
Future perspective
There is an increasing trend toward the use of local anesthesia and sedation for TAVI procedures to minimize hemodynamic instability and recovery times. This would limit peri-procedural TOE. Therefore, it is likely that intracardiac echo or transnasal TOE incorporating 3D will be increasingly used. In addition, there will be increasing use of semi-automated 3D analysis software for aortic anatomy analysis from 3D echo images, this will be analogous to the software available for MSCT.
Furthermore, development of hybrid catheterization laboratories will allow interventional procedures such as TAVI and cardiac surgery to be performed in the same location. Such laboratories will have the advantage of providing high-quality imaging and they will facilitate care of the patient should complications arise.
Executive summary
• Short description of some of the commercially available transcatheter aortic valve implantation (TAVI) valves and different approaches to TAVI procedure.
• Role of the multidiscplinary team in patient selection and risk score assessment.
• Imaging with echocardiography and other modalities to assess the size of the aortic annulus and other anatomical features.
• Echocardiographic assessment during all stages of TAVI to assist the operators with supplemental imaging.
• Immediate assessment post-TAVI to assess results and possible complications.
• Follow-up postimplantation to calculate gradients across the TAVI valve and to quantify any aortic regurgitation.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Iung B, Baron G, Butchart EG et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular disease. Eur. Heart J. 24, 1231–1243 (2003).
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 368, 1005–1011 (2006).
- Leon MB, Smith CR, Mack M et al. Transcatheter aorticvalve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363, 1597–1607 (2010).
- Zamorano JL, Badano LP, Bruce C et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur. J. Echocardiogr. 12, 557–584 (2011).
- Cribier A, Eltchaninoff H, Bash A et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106, 3006–3008 (2002).
- Holmes DR, Mack MJ, Kaul S et al. 2012 ACCF/AATS/ SCAI/STS Expert Consensus document on transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 59(13), 1200–1254 (2012).
- Lange R, Schreiber C, Gotz W et al. First successful transapical aortic valve implantation with the corevalve revalving system: a case report. Heart Surg. Forum 10, E478–E479 (2007).
- Vahanian A, Alfieri O, Andreotti F et al. Guidelines on the management of valvular heart disease (version 2012): the joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 33, 2451–2496 (2012).
- Mylotte D, Martucci G, Piazza N. Patient selection for transcatheter aortic valve implantation: an interventional cardiology perspective. Ann. Cardiothorac. Surg. 1(2), 206–215 (2012).
- Baumgartner H, Hung J, Bermejo J et al. Echocardiographic assessment of valve stenosis; EAE/ASE recommendations for clinical practice. Eur. J. Echocardiogr. 10, 1–25 (2009).
- Awtry E, Davidoff R. Low-flow/low-gradient aortic stenosis. Circulation 124, e739–e741 (2011).
- Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J. Am. Coll. Cardiol. 60, 1845–1853 (2012).
- Monin JL, Quéré JP, Petit H et al. Low-gradient aortic stenosis: operative risk stratification and predictors for longterm outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 108, 319–324 (2003).
- Herrmann HC, Pibarot P, Hueter I et al. Predictors of mortality and outcomes of therapy in low-flow severe aortic stenosis: a placement of aortic transcatheter valves (PARTNER) trial analysis. Circulation 127, 2316–2326 (2013).
- Himbert D, Pontnau F, Messika-Zeitoun D et al. Feasibility and outcomes of transcatheter aortic valve implantation in high-risk patient with stenotic bicuspid aortic valves. Am. J. Cardiol. 110, 877–833 (2012).
- Baralis G, Di Gregorio O, Riva L, Steffenino G, Grossi C, Locatelli A. Transcatheter aortic valve implantation in bicuspid aortic valve: never say never. G. Ital. Cardiol. (Rome) 13, 67–70 (2012).
- Wijesinghe N, Ye J, Rodés-Cabau J et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis. J. Am. Coll. Cardiol. Intv. 3, 1122–1125 (2010).
- Piazza N, De Jaegere P, Schultz C, Becker AE, Serruys PW, Anderson RH. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ. Cardiovasc. Interv. 1, 74–81 (2008).
- Moss RR, Ivens E, Pasupati S et al. Role of echocardiography in percutaneous valve intervention. J. Am. Coll. Cardiol. Img. 1, 15–24 (2008).
- Chin D. Echocardiography for transcatheter aortic valve implantation. Eur. J. Echocardiogr. 10, i21–i29 (2009).
- Messika-Zeitoun D, Serfaty JM, Brochet E et al. Multimodal assessment of the aortic annulus diameter. Implications for transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 55, 186–194 (2010).
- Smith LA, Dworakowski R, Bhan A et al. Real-time three-dimensional transesophageal echocardiography adds value to transcatheter aortic valve implantation. J. Am. Soc. Echocardiogr. 26, 359–369 (2013).
- Moss RR, Ivens E, Pasupati S et al. Role of echocardiography in percutaneous aortic valve implantation. JACC Cardiovasc. Imaging 1, 15–24 (2008).
- Smith LA, Monaghan MJ. Monitoring of procedures: periinterventional echo assessment for transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Imaging 14, 840–850 (2013).
- Zamorano JL, Gonçalves A, Lang R. Imaging to select and guide transcatheter aortic valve implantation. Eur. Heart J. 35(24), 1578–1587 (2014).
- Bagur R, Rodés-Cabau J, Doyle D et al. Usefulness of TEE as the primary imaging technique to guide transcatheter transapical aortic valve implantation. J. Am. Coll. Cardiol. Img. 4, 15–24 (2011).
- Clavel MA, Rodés-Cabau J, Dumont E et al. Validation and characterization of transcatheter aortic valve orifice are measured by Doppler echocardiography. J. Am. Coll. Cardiol. Img. 4, 1053–1062 (2011).
- Shames S, Koczo A, Hahn R et al. Flow characteristics of the SAPIEN aortic valve: the importance of recognizing in-stent flow acceleration for the echocardiographic assessment of valve function. J. Am. Soc. Echocardiogr. 25, 603–609 (2012).
- Zoghbi WA, Chambers JB, Dumesnil JG et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 22, 975–1014 (2009).
- Pirat B, Little SH, Igo SR et al. Direct measurement of proximal isovelocity surface area by real-time three-dimensional color Doppler for quantitation of aortic regurgitant volume: an in vitro validation. J. Am. Soc. Echocardiogr. 22, 306–313 (2009).