Review Article - Imaging in Medicine (2013) Volume 5, Issue 1
The role of urgent imaging in the diagnosis and management of patients with TIA and minor stroke
Negar Asdaghi1 & Shelagh B Coutts*2,31BC Centre for Stroke & Cerebrovascular Diseases, University of British Columbia, Vancouver, British Columbia, Canada
2Calgary Stroke Program, Departments of Clinical Neuroscience & Radiology, Hotchkiss Brain Institute, University of Calgary, Calgary, Alberta, Canada
3C1261, Foothills Medical Centre, 1403 29th Street NW, Calgary, Alberta, T2N 2T9, Canada
- Corresponding Author:
- Shelagh B Coutts
Calgary Stroke Program
Departments of Clinical Neuroscience & Radiology
Hotchkiss Brain Institute, University of Calgary
Calgary, Alberta, Canada
Tel: +1 403 944 1594
Fax: +1 403 283 2270
E-mail: scoutts@ucalgary.ca
Abstract
Keywords
CT ▪ disability ▪ minor stroke ▪ MRI ▪ recurrent stroke ▪ TIA
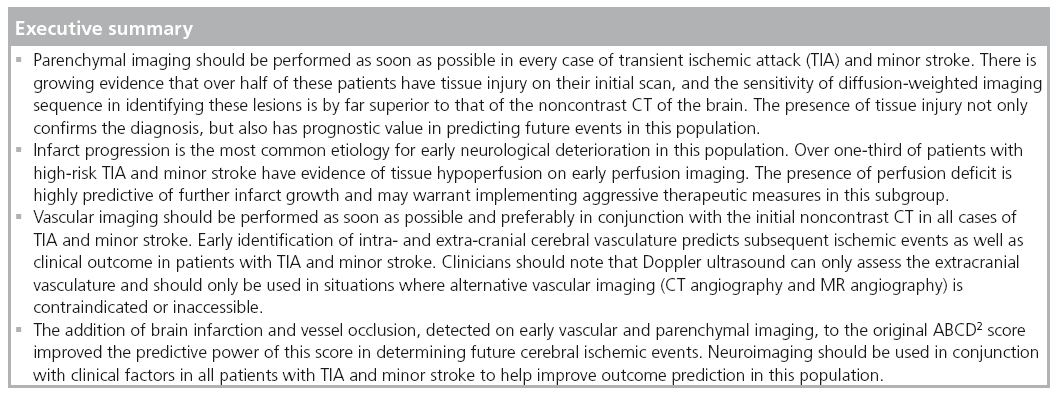
Patients with transient ischemic attack (TIA) and minor stroke have similar clinical and radiographic characteristics and represent disorders on the same ischemic continuum [1,2]. Furthermore, TIA and minor stroke patients have similar prognoses for survival and recurrent vascular events [1]. Moreover, TIA is defined as neurological symptoms lasting less than 24 h and, as such, the distinction between the two conditions is further irrelevant in the acute setting and should be abandoned. It is well established that despite their mild presentation, these patients are at high risk for early deterioration and subsequent recurrent ischemic events. A retrospective review of two population-based studies and two randomized controlled trials have shown that between 18 and 26% of patients who presented with ischemic stroke had a history of a preceding TIA within the past 7 days [3]. Similarly, in-hospital studies have demonstrated that a substantial number of patients who acutely present with minor or rapidly improving ischemic symptoms are in fact dead or disabled by the time of their hospital discharge [4,5]. Therefore, there is considerable interest in improving the ability to make a timely diagnosis and implement treatment strategies during the short period of time between TIA/minor stroke and subsequent events [6].
However, time constraint is only one of the many challenges that clinicians face in the management of these patients. In fact, TIA/minor stroke mimics comprise some of the most common neurological etiologies for which patients are referred to the emergency department [7]. Multiple studies have shown that reliance on clinical characteristics alone has only moderate accuracy for making the correct diagnosis in this patient population [8,9]. Similarly, despite the fact that clinical characteristics are important for identifying high-risk patients [10], the sensitivity of clinical scoring systems, such as the ABCD [11] and ABCD2 scores [10], in correctly predicting a recurrent event are far from perfect [12]. Therefore, over the past decade a number of studies have evaluated the ability of modern neuroimaging in isolation [13], or in combination with clinical features, in improving the accuracy of the currently used diagnostic tools in these patients [14,15].
In this article, the utility of different neuroimaging modalities in assessing patients with TIA and minor stroke and their value in accurately predicting outcome by differentiating high-risk patients with TIA/minor stroke from their low-risk counterparts is briefly discussed.
The role of parenchymal imaging in TIA & minor stroke
Brain CT scan
A noncontrast CT (NCCT) of the brain is the most widely available imaging modality for assessment of patients with suspected vascular events. It is fast, easily accessible and less expensive than all other imaging options. Despite this, NCCT has a relatively low sensitivity and inter-rater reliability in the detection of early infarct changes, even in patients with acute large ischemic syndromes [16]. Evidence of an acute infarct on NCCT alone has been shown to be predictive of recurrent stroke in TIA patients. However, the proportion of patients with evidence of acute infarcts was small (4%) [17]. A recent publication indicated that adding NCCT data increased the predictive value of the ABCD score [18]. These investigators rated a NCCT as abnormal if there was evidence of old or new infarction, or periventricular white matter disease. These abnormalities were assessed together and rated as present or absent. However, although there was a suggestion of improvement in the score with the addition of the NCCT information, the areas under the curves (AUCs) were similar to that of the ABCD score in this population (AUC: 0.76 for ABCD and 0.79 for ABCD + CT). This study has a number of limitations: there were no patients with evidence of an acute infarct on NCCT, which questions some of the radiological interpretation; the method of rating white matter disease on CT is not described; the exact timing of the NCCT related to the event is not clear; and diabetes was not included in the analysis.
In a pooled analysis of data collected independently from 12 centers on patients with TIA, the odds ratio (OR) of recurrent infarct was 4.2 (2.6–6.9) in patients with a positive CT profile relative to those with a negative CT profile [18]. However, the predictive power of CT remained significantly lower than that of the diffusion-weighted imaging (DWI).
In summary, a normal NCCT is reassuring but does not rule out the diagnosis of an ischemic event and similarly does not carry a high prognostic value in determining positive or negative outcome in patients with TIA and minor stroke. In practice, the main value of normal NCCT in this group is mostly to rule out other etiologies (e.g., tumor and blood, among others) that may present with TIA-mimicking symptoms. More advanced imaging is often required to help identify the etiology of the presenting symptoms and to predict disability and recurrent events.
Brain MRI
In contrast to NCCT, MRI has a much higher yield for the detection of small ischemic lesions in patients with mild or transient ischemic events. DWI is highly sensitive to ischemic changes, even within the hyperacute period after the infarct [19]. Several studies have demonstrated evidence of acute ischemia on early DWI in over 50% of patients who had complete symptom resolution within 24 h of onset [20–22]. DWI is more sensitive than NCCT in specifically detecting small-volume infarcts, regardless of their clinical severity [23]. Multiple clinical factors, including symptom duration, have been correlated with the presence of parenchymal lesions on MRI. A pooled analysis of MRI data in 808 patients with TIA from ten centers has demonstrated a linear correlation between duration of clinical symptoms and acute ischemia on imaging [24]. Acute DWI is also an important tool for correct localization of ischemic symptoms, especially in differentiating anterior versus posterior circulation strokes in those with small ischemic lesions [23]. Therefore, MRI has implications in determining symptomatic versus asymptomatic carotid disease and the need for urgent carotid revascularization [25]. Evidence of ischemic injury on MRI not only changed the ‘time-based’ definition to a ‘tissue-based’ definition in TIA patients [26], but also has important implications in predicting outcome. A prospective imaging study of 126 patients with high-risk TIA and minor ischemic stroke (NIHSS ≤3) [13], showed that patients with acute DWI lesions on MRI performed within 24 h of symptoms onset, were 2.6-times more likely to have a new stroke compared with those with normal DWI at baseline. This risk significantly increased by 8.9-fold in the presence of intracranial occlusion. In this study, the combination of baseline DWI lesion and intracranial occlusion also significantly increased the risk of 90-day functional dependence, as measured by Rankin score ≥3. Similarly, a retrospective analysis of 601 consecutive patients with TIA, who had an MRI within 24 h of symptom onset, showed that both high-risk clinical characteristics (ABCD2 ≥4) and imaging characteristics (acute DWI lesions) were independent predictors of early recurrent stroke in this patient population [27]. In this study, the 7-day risk of recurrent stroke was 0% with no predictors, 2% with ABCD2 ≥4, 4.9% with acute DWI and 14.9% with both clinical and imaging predictors. Similar high recurrent stroke rates have been found in a separate study of patients with TIA who had acute DWI lesions within 24 h of symptom onset [28].
Acute tissue injury is also predictive of development of silent ischemic lesions on follow-up imaging. In a study of 143 patients with high-risk TIA and minor stroke (defined as NIHSS <6) who had serial imaging, 9.8% showed evidence of new ischemic lesion on 30-day follow-up MRI [29]. It is important to note that close to half of these patients remained clinically asymptomatic. A trend to increased likelihood of new lesions at 30 days was seen with progressing baseline scan lesion number (none [2.2%], solitary [12.9%] and multiple [19.8%]; p = 0.046). Patients whose mechanism of stroke was large artery disease or cardioembolic were the most likely to have new lesions on 30-day follow-up MRI. Furthermore, the presence of multiple DWI lesions of varying ages (as defined by apparent diffusion coefficient maps) increased the risk of new lesion development on follow-up MR imaging (relative risk: 3.6; 95% CI: 1.9–6.8) [30]. Similarly, in an international multicenter collaborative study of patients with TIA, the OR of recurrent infarct was 18-fold higher in DWI-positive patients relative to DWI-negative patients [28]. Moreover, the extent of ischemic injury, as measured by the volume of acute DWI, has been shown to predict infarct progression in this population [31]. Furthermore, the presence of acute tissue injury can be used as an indicator of active ischemic process in patients with minor symptoms. In a prospective study of 693 patients with TIA and minor stroke, close to 50% of patients were classified as having cryptogenic events [32]. Follow-up imaging in these patients demonstrated a high rate of new ischemic lesions at day 30 (6.6%) and day 90 (14.5%), despite a very low clinical recurrent rate (1.2%) within 90 days. Therefore, the presence, the lesion volume and the number of acute DWI lesions all have positive predictive values in determining infarct progression and/or recurrence in this population.
Performing MRI studies in patients with TIA may increase the detection of unexpected brain abnormalities, such as tumors and cerebral aneurysms. Interestingly, the most common incidental finding on brain MRI is silent ischemia [33]. The presence of silent ischemia is a marker of vascular disease and has implications in prediction of future ischemic events [34]. Furthermore, there is growing evidence that links subclinical infarcts to cognitive and functional decline [35]. Therefore, new silent infarcts should not merely be considered as an epiphenomenon. In addition, the development of new silent ischemia can be used as a surrogate marker for inadequate secondary prevention measures and warrant more aggressive medical or surgical interventions in patients who remain clinically asymptomatic.
The role of perfusion imaging in TIA & minor stroke
Approximately one-third of patients with minor stroke and TIA have evidence of tissue hypoperfusion on CT perfusion or perfusionweighted MRI performed within 24 h of symptom onset [36–38]. Mismatch between a large perfusion deficit and a smaller ischemic core (demonstrated by DWI) is referred to as the ischemic penumbra. Penumbra is thought to represent a hypoperfused region that is at risk for infarction, but is also potentially amenable to salvage [39]. The most commonly used perfusion parameters to define ischemic penumbra are relative cerebral blood flow, relative cerebral blood volume and Tmax (the time to maximum residue function obtained by deconvolution) maps [40].
In the ABCD2 + MRI study [14], presence of ischemic penumbra was determined by assessment of relative sizes of mean transit time delay versus DWI lesions. Patients with mismatch (mean transit time >DWI) were significantly more likely to have recurrent stroke (27 vs 7%; p = 0.003) or functional impairment (29 vs 7%; p = 0.001) as compared with those without mismatch. Similarly, tissue hypoperfusion was predictive of both clinical and radiographic deterioration in the subgroup of patients with lacunar stroke [37]. In a prospective study of TIA and minor stroke patients who underwent serial imaging, tissue hypoperfusion (defined as regions with Tmax delay of ≥2 s) was identified in 34% of patients [31]. Those with baseline perfusion deficit were much more likely to develop new ischemic lesions on early follow-up MRI at day 7. All new ischemic lesions developed within the originally hypoperfused regions and, as such, represent infarct progression rather than infarct recurrence. A CT perfusion study in 65 patients with anterior circulation TIA, demonstrated that 33.8% of patients had focal perfusion deficit at baseline. Subsequent in-hospital events occurred more frequently in those with perfusion deficit compared with those without. Similarly, a detailed volumetric analysis of mismatch (defined as areas with Tmax delay of ≥4-s DWI) in 137 patients with TIA and minor stroke was found to be predictive of infarct growth in the originally hypoperfused regions of the brain on day 30 follow-up scans [41]. In this study, a 10-ml mismatch was found to have the highest sensitivity and specificity for predication of infarct growth on follow-up imaging at day 30. Therefore, a mismatch volume of ≥10 ml was therefore referred to as ‘critical mismatch’. To further validate these findings, the presence of critical mismatch (Tmax ≥4-s DWI ≥10ml) as a poor prognosticator of outcome was validated in a large consecutive cohort of 281 patients with minor stroke and TIA [42]. In this study, presence of critical mismatch at baseline was highly predictive of further infarct growth on follow-up imaging (OR: 10.3; 95% CI: 3.5–30.2) [42].
The role of vascular imaging in TIA & minor stroke
Accurate imaging of intra- and extra-cranial arteries is important in establishing the correct diagnosis, planning appropriate treatment strategies and prognosticating the recurrent ischemic rates in patients with TIA and nondisabling stroke. Noninvasive vascular imaging methods, now widely available, are replacing the need to perform the invasive intraarterial angiography (IAA) as the screening method for detection of vascular abnormalities in this population. Doppler ultrasound (DUS), trascranial Doppler (TCD), CT angiography (CTA) and MR angiography are currently available and are reasonable methods for initial vascular assessment in patients with a transient or minor neurological deficit.
DUS & TCD
Timely detection of carotid disease is arguably the most important step in secondary prevention of recurrent ischemic stroke in patients with TIA/minor stroke arising from carotid artery stenosis. Different methods of measuring carotid stenosis have been used [43]. Carotid endarterectomy has been shown in randomized controlled trials to reduce the risk of ipsilateral ischemic stroke due to significant carotid stenosis (70–99%) and in some cases with moderate stenosis, if the surgery is performed quickly after the index event [44,45]. The endarterectomy trials measured the degree of carotid stenosis from IAA, an invasive and expensive procedure that may also cause further delays in the care of these patients. Multiple noninvasive techniques, including carotid DUS, are now available and have largely replaced the need for performing IAA as the screening test for detection of carotid disease in patients with minor or completely resolved neurological symptoms. Carotid Doppler ultrasonography assesses the velocity changes in blood flow associated with stenosis in the carotid arteries using either a continuous wave, a single-gated pulsed wave or directional color modes [46,47]. Carotid DUS is less accurate than IAA, particularly at 50–69% stenosis; however, it does reduce the investigation risk and the time from clinical diagnosis to imaging, and ultimately surgery, in most cases [48]. Apart from reduced accuracy, carotid DUS has multiple other limitations including operator dependency and providing limited or no information on the posterior circulation and intracranial vascular status of the patients [49].
Therefore, addition of TCD ultrasound is useful in the setting where DUS is the only available vascular imaging modality. TCD ultrasound is a noninvasive and reliable method for the detection of intracranial occlusion or stenosis through measurement of blood flow [50,51]. Increased flow velocities, as detected by TCD, have been shown to be strongly related to vascular risk factors including hypertension, hypercholerstolemia and diabetes [52]. The presence of intracranial stenosis, as detected by TCD, is associated with vascular risk factors, such as hypertension and diabetes. In a prospective study of 598 patients with recent TIA and nondisabling stroke, the mean flow velocity, the pulsitility index and their ratio, detected by TCD, showed strong prognostic value for recurrent vascular events [53]. An analysis of TCD findings on 1881 patients presenting with an acute TIA to the TIA-SOS clinic (a TIA clinic with round-the-clock access), showed presence of intracranial occlusion/stenosis in 8.8% of patients [54]. After 1-year follow-up on best preventative therapy, the incidence of recurrent vascular events was significantly higher in patients with baseline TCD abnormalities than those without. Apart from detection of intracranial stenosis and occlusion, TCD monitoring can identify asymptomatic microembolic signals (MESs). Emboli backscatter and appear as highintensity short-duration signals on continuous TCD monitoring [55]. In the acute phase of TIA/minor stroke, MESs have been reported between 9.3 and 71% of patients with a variety of arterial or cardiac embolic sources [56,57]. Multiple studies have shown that MES is an independent predictor of recurrent ischemic events in patients with TIA and and in stroke of all severity [58,59]. Furthermore, treatment with anti-thrombotic therapy has been shown to reduced MESs [60] and reduce the risk of early recurrence [61].
In summary, both carotid DUS and TCD monitoring are reliable and feasible vascular techniques in the assessment of TIA and minor stroke patients, and can be used as an alternative method in centers with limited access to other vascular imaging modalities.
MR angiography
It is well recognized that patients with mild or rapidly resolving neurological symptoms who do not receive thrombolytic therapy can have poor outcomes related to early deterioration [4]. The role of acute MR angiography in the detection of those potentially at risk for early deterioration has been studied. Minor stroke patients with documented large vessel occlusion are at the highest risk of deterioration when thrombolysis is withheld [4,62,63]. Together these studies report outcomes for only 241 patients in total, with 34 (14%) having evidence of large artery occlusion. A total of 44% (15 out of 34) of patients with occlusion had poor outcome versus 21% (44 out of 207) of patients with no occlusion (relative risk: 2.1; 95% CI: 1.3–3.3; p = 0.0085 Fisher’s exact test). All of these studies have used MRI to document large artery occlusion. The small number of patients is explained by the fact that it is challenging to obtain acute stroke MRI quickly and routinely.
CT angiography
The advantages of CT over MRI include its wider availability, lower cost, shorter scanning time intervals and better patient tolerability. CTA is a safe and feasible technique for vascular imaging and can be performed in most emergency departments in conjunction with NCCT as the initial imaging in acute stroke [64]. Intracranial large vessel occlusion identified by CTA was an independent factor to predict poor neurological outcome in a consecutive cohort of patients with stroke and TIA [65]. In a large consecutive cohort of patients with TIA and minor ischemic stroke, the utility of CT/CTA findings was assessed within 2 h of symptom onset [66].
In this cohort, persistent symptoms, CT/CTA abnormalities and DWI all positively predicted recurrent stroke. However, in the multivariable analysis, the only factor predictive of recurrent ischemic events was acute CT/CTA. CT/CTA abnormalities also predicted disability in this study, even in the absence of recurrent events [67].
The utility of brain imaging in outcome definition in TIA/minor stroke
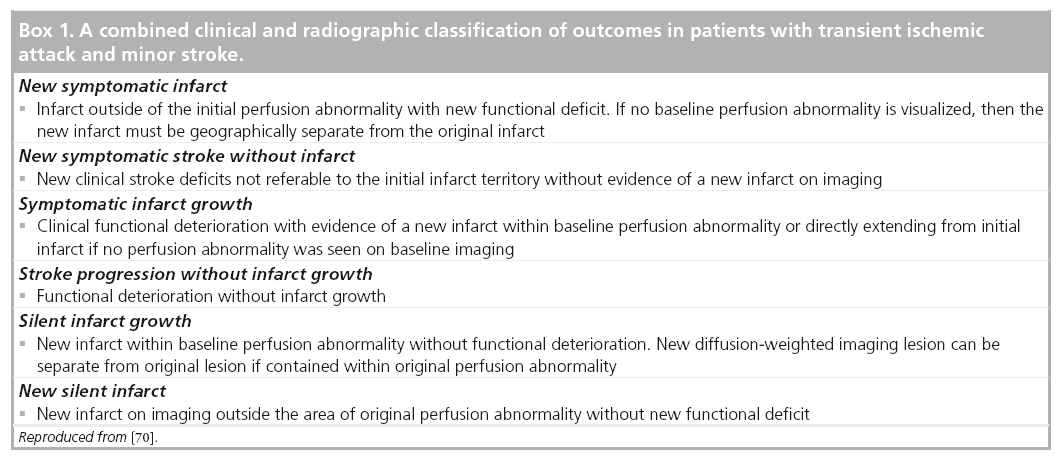
The definition of recurrent events in patients with ischemic stroke is challenging [68]. Infarct growth or development of a de novo ischemic lesion can occur on follow-up imaging with or without a change in patient’s clinical status. Furthermore, clinical deterioration can occur in the absence of apparent radiographic alterations. It has been well demonstrated that unlike infarct recurrence that occurs over time and almost at a linear rate, infarct progression commonly occurs early on, within a few days, after the index [69]. More importantly, the different outcomes are probably each correlated with separate etiologies, which may warrant different therapeutic measures. Therefore, it is important to develop a standardized event classification scheme that could both guide clinicians in dayto- day practice and perhaps be utilized in future research studies and clinical trials. Thus, we used baseline and follow-up MRI as a surrogate marker for disease activity in combination with clinical findings to create an event classification system that categorizes patient outcomes in six different groups (Box 1) [70]. In this substudy, most events in minor stroke and TIA patients were due to progression of the presenting event (either clinical or radiological progression) and were not secondary to an actual recurrence.
The clinical outcome of either recurrent TIA versus stroke at 1 year was assessed in a subgroup of VISION patients with high-risk TIA [71]. Highrisk TIA patients who were DWI negative on their baseline scan were 4.6-times (27.4 vs 5.9%; p < 0.05) more likely to have a subsequent TIA at 1 year than patients with a DWI lesion, but were 4.3-times (2.1 vs 9.1%; p = 0.19) less likely to have a subsequent stroke. The implication that DWI-negative patients have recurrent transient events rather than recurrent strokes suggests that some of these patients have an alternative pathophysiological explanation than ischemia (e.g., migraine, epilepsy and somatiform disorder, among others) [71]. However, the question remained as to what proportion and what type of patients were falsely DWI negative on baseline imaging. In an analysis of 403 patients of all stroke severity who were enrolled in the VISION study, 103 (25.6%) were DWI negative [72]. In this group, the final diagnosis was stroke in 26 (25.2%), TIA in 63 (61.2%) and nonischemic in 14 (13.6%) patients (seizures and migraine, among others). Of the stroke patients, six out of 26 (23.1%) had infarcts on 30-day follow-up MRI on fluid attenuated inversion recovery (FLAIR) sequences in clinically relevant regions (four lacunar syndromes and two posterior circulation syndromes). The majority of patients with a final clinical diagnosis of stroke, but no evidence of infarction on follow-up imaging (13 out of 26, 65%) had either brainstem or lacunar strokes as the clinical diagnosis.
Prediction of outcome in TIA & minor stroke
Previous work in TIA has used the ABCD2 score [10] to predict recurrent stroke. The ABCD2 score uses clinical and ischemic event details to predict clinical outcome and does not include brain imaging. We proposed that brain imaging may be a way of improving the prediction of outcome. The new score ABCD2 + MRI was created by adding evidence of acute infarct on DWI to the ABCD2 score. The predictive accuracy of the ABCD2 + MRI score was significantly higher than ABCD2 (AUC: 0.88 vs 0.78; p = 0.01). Those with a high score (7–9) had a 90-day recurrent stroke risk of 32.1%, a moderate score (5–6) had a risk of 5.4% and a low score (0–4) had a risk of 0.0%. Unlike the ABCD2 score (p = 0.33), the ABCD2 + MRI score (p = 0.02) predicted functional impairment at 90 days. Interestingly, in the multivariate analysis, vessel occlusion and perfusion (see ‘The role of perfusion imaging in TIA & minor stroke’ section for discussion on perfusion) were substitutable in the model, and in the final model only occlusion was chosen, as it is more widely applicable [14]. When considering evidence of acute infarct on either CT or MRI (ABCD2 I score) given the wider accessibility of CT scan, the prognostic yield of the score also improved. Pooled AUC improved from 0.66 (0.53–0.78) for the ABCD2 score to 0.78 (0.72–0.85) for the ABCD2 I score. This was confirmed in a multicenter study that included 4574 patients with TIA and demonstrated a significant improvement in the yield of ABCD2 score in predicting recurrent strokes at day 7 and 90 with addition of brain infarct on NCCT or DWI [12]. To further increase the yield of scoring system, the ABCD3 I score was developed that considered two points for dual TIA, two points for acute infarction on DWI and two points for carotid stenosis of at least 50% [15]. Considering these high-risk features, the predictive value of ABCD2 score significantly improved in the ABCD3 I score for early recurrent events by day 30 (0.90 at day 2; p = 0.035, 0.92 at day 7; p = 0.001, 0.85 at day 28; p = 0.028 and 0.79 at day 90; p = 0.073).
Conclusion
A significant proportion of patients with minor or completely resolved neurological symptoms show evidence of vascular or tissue abnormalities on acute neuroimaging studies. These factors have been proven to be invaluable in risk stratification, treatment planning and outcome prediction in these patients. Furthermore, despite best medical management, approximately one-third of these patients have evidence of radiographic deterioration on sequential MRI. The majority of these patients remain clinically unchanged. This not only emphasizes the dynamic pathophysiologic changes that occur in the brain of patients with TIA and minor ischemic stroke, but also argues that more sensitive surrogates (such as serial MRI scans) should be used both at baseline and follow-up assessment of these patients in conjunction with clinical and functional outcome measures.
Future perspective
The presence of infarct progression on follow-up assessments (in both symptomatic and asymptomatic patients), despite implementation of best medical therapies, raises the question of whether alternative therapies are required for these patients. Are certain patients with TIA/minor stroke potential candidates for acute revascularization treatments such as intravenous thrombolytics? The safety of thrombolysis in these patients with intracranial occlusion is currently under investigation in the TEMPO-1 trial [101]. The early use of dual antiplatelet agents (aspirin and plavix) is currently being tested in the POINT trial [102]. Hopefully the results of these trials will provide further evidenced-based treatment options for TIA and minor stroke patients in the near future.
Financial & competing interests disclosure
S Coutts receives salary support from Alberta Innovates – Health Solutions and the Heart and Stroke Foundation of Canada Distinguished Clinician Scientist award, supported in partnership with the Canadian Institute of Health Research (CIHR) Institute of Circulatory and Respiratory Health (ICRH) and AstraZeneca Canada Inc. N Asdaghi receives salary support from the Vancouver General Hospital and University of British Columbia Hospital Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Dennis MS, Bamford JM, Sandercock PA, Warlow CP. A comparison of risk factors and prognosis for transient ischemic attacks and minor ischemic strokes. The Oxfordshire Community Stroke Project. Stroke 20(11), 1494–1499 (1989).
- Winbeck K, Bruckmaier K, Etgen T, Von Einsiedel HG, Rottinger M, Sander D. Transient ischemic attack and stroke can be differentiated by analyzing early diffusionweighted imaging signal intensity changes. Stroke 35(5), 1095–1099 (2004).
- Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology 64(5), 817–820 (2005).
- Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke 36(11), 2497–2499 (2005).
- Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 56(8), 1015–1020 (2001).
- Rothwell PM, Buchan A, Johnston SC. Recent advances in management of transient ischaemic attacks and minor ischaemic strokes. Lancet Neurol. 5(4), 323–331 (2006).
- Amort M, Fluri F, Schafer J et al. Transient ischemic attack versus transient ischemic attack mimics: frequency, clinical characteristics and outcome. Cerebrovasc. Dis. 32(1), 57–64 (2011).
- Sheehan OC, Merwick A, Kelly LA et al. Diagnostic usefulness of the ABCD2 score to distinguish transient ischemic attack and minor ischemic stroke from noncerebrovascular events: the North Dublin TIA Study. Stroke 40(11), 3449–3454 (2009).
- Josephson SA, Sidney S, Pham TN, Bernstein AL, Johnston SC. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke 39(11), 3096–3098 (2008).
- Johnston SC, Rothwell PM, Nguyen- Huynh MN et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 369(9558), 283–292 (2007).
- Rothwell PM, Giles MF, Flossmann E et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet 366(9479), 29–36 (2005).
- Giles MF, Rothwell PM. Systematic review and pooled analysis of published and unpublished validations of the ABCD and ABCD2 transient ischemic attack risk scores. Stroke 41(4), 667–673 (2010).
- Coutts SB, Simon JE, Eliasziw M et al. Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann. Neurol. 57(6), 848–854 (2005).
- Coutts SB, Eliasziw M, Hill MD et al. An improved scoring system for identifying patients at high early risk of stroke and functional impairment after an acute transient ischemic attack or minor stroke. Int. J. Stroke 3(1), 3–10 (2008).
- Merwick A, Albers GW, Amarenco P et al. Addition of brain and carotid imaging to the ABCD2 score to identify patients at early risk of stroke after transient ischaemic attack: a multicentre observational study. Lancet Neurol. 9(11), 1060–1069 (2010).
- Wardlaw JM, Mielke O. Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment – systematic review. Radiology 235(2), 444–453 (2005).
- Douglas VC, Johnston CM, Elkins J, Sidney S, Gress DR, Johnston SC. Head computed tomography findings predict shortterm stroke risk after transient ischemic attack. Stroke 34(12), 2894–2898 (2003).
- Giles MF, Albers GW, Amarenco P et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke 41(9), 1907–1913 (2010).
- Saini M, Butcher K. Advanced imaging in acute stroke management – part II: magnetic resonance imaging. Neurol. India 57(5), 550–558 (2009).
- Ay H, Oliveira-Filho J, Buonanno FS et al. ‘Footprints’ of transient ischemic attacks: a diffusion-weighted MRI study. Cerebrovasc. Dis. 14(3–4), 177–186 (2002).
- Redgrave JN, Coutts SB, Schulz UG, Briley D, Rothwell PM. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke 38(5), 1482–1488 (2007).
- Rovira A, Rovira-Gols A, Pedraza S, Grive E, Molina C, Alvarez-Sabin J. Diffusionweighted MR imaging in the acute phase of transient ischemic attacks. AJNR Am. J. Neuroradiol. 23(1), 77–83 (2002).
- Asdaghi N, Jeerakathil T, Hameed B et al. Oxfordshire community stroke project classification poorly differentiates small cortical and subcortical infarcts. Stroke 42(8), 2143–2148 (2011).
- Shah SH, Kidwell CS, Albers G et al. A multicenter pooled, patient-level data analysis of diffusion-weighted MRI in TIA patients. Stroke 38, 463a (2007).
- Rovira A, Grive E, Rovira A, Alvarez-Sabin J. Distribution territories and causative mechanisms of ischemic stroke. Eur. Radiol. 15(3), 416–426 (2005).
- Latchaw RE, Alberts MJ, Lev MH et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke 40(11), 3646–3678 (2009).
- Ay H, Arsava EM, Johnston SC et al. Clinical- and imaging-based prediction of stroke risk after transient ischemic attack: the CIP model. Stroke 40(1), 181–186 (2009).
- Giles MF, Albers GW, Amarenco P et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology 77(13), 1222–1228 (2011).
- Coutts SB, Hill MD, Simon JE, Sohn CH, Scott JN, Demchuk AM. Silent ischemia in minor stroke and TIA patients identified on MR imaging. Neurology 65(4), 513–517 (2005).
- Sylaja PN, Coutts SB, Subramaniam S, Hill MD, Eliasziw M, Demchuk AM. Acute ischemic lesions of varying ages predict risk of ischemic events in stroke/TIA patients. Neurology 68(6), 415–419 (2007).
- Asdaghi N, Hameed B, Saini M, Jeerakathil T, Emery D, Butcher K. Acute perfusion and diffusion abnormalities predict early new MRI lesions 1 week after minor stroke and transient ischemic attack. Stroke 42(8), 2191–2195 (2011).
- Bal S, Patel SK, Almekhlafi M, Modi J, Demchuk AM, Coutts SB. High rate of magnetic resonance imaging stroke recurrence in cryptogenic transient ischemic attack and minor stroke patients. Stroke 43(12), 3387–3388 (2012).
- Vernooij MW, Ikram MA, Tanghe HL et al. Incidental findings on brain MRI in the general population. N. Engl. J. Med. 357(18), 1821–1828 (2007).
- Ong CT, Sung KC, Sung SF, Wu CS, Hsu YC, Su YH. Impact of silent infarction on the outcome of stroke patients. J. Formos. Med. Assoc. 108(3), 224–230 (2009).
- Vermeer SE, Prins ND, Den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N. Engl. J. Med. 348(13), 1215–1222 (2003).
- Krol AL, Coutts SB, Simon JE, Hill MD, Sohn CH, Demchuk AM. Perfusion MRI abnormalities in speech or motor transient ischemic attack patients. Stroke 36(11), 2487–2489 (2005).
- Poppe AY, Coutts SB, Kosior J, Hill MD, O’Reilly CM, Demchuk AM. Normal magnetic resonance perfusion-weighted imaging in lacunar infarcts predicts a low risk of early deterioration. Cerebrovasc. Dis. 28(2), 151–156 (2009).
- Abul-Kasim K, Brizzi M, Petersson J, Sundgren PC. Added diagnostic utility of CT perfusion and CT angiography in acute ischemic stroke. Evaluation of three different patient categories. Funct. Neurol. 24(2), 93–98 (2009).
- Davis SM, Donnan GA, Butcher KS, Parsons M. Selection of thrombolytic therapy beyond 3 h using magnetic resonance imaging. Curr. Opin. Neurol. 18(1), 47–52 (2005).
- Butcher KS, Parsons M, Macgregor L et al. Refining the perfusion-diffusion mismatch hypothesis. Stroke 36(6), 1153–1159 (2005).
- Asdaghi N, Butcher K, Qazi A et al. Perfusion imaging predicts outcome in TIA and minor stroke. Cerebrovasc. Dis. 31(Suppl. 2), 67 (2011) (Abstract).
- Asdaghi N, Coulter J, Butcher K et al. Perfusion imaging predicts outcome in TIA and minor stroke. A prospective derivationvalidation study. Cerebrovasc. Dis. 33(Suppl. 2), 39, (2012) (Abstract).
- Wardlaw JM, Lewis S. Carotid stenosis measurement on colour Doppler ultrasound: agreement of ECST, NASCET and CCA methods applied to ultrasound with intra-arterial angiographic stenosis measurement. Eur. J. Radiol. 56(2), 205–211 (2005).
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 351(9113), 1379–1387 (1998).
- Barnett HJ, Taylor DW, Eliasziw M et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 339(20), 1415–1425 (1998).
- Steinke W, Kloetzsch C, Hennerici M. Carotid artery disease assessed by color Doppler flow imaging: correlation with standard Doppler sonography and angiography. AJR Am. J. Roentgenol. 154(5), 1061–1068 (1990).
- Van Merode T, Lodder J, Smeets FA, Hoeks AP, Reneman RS. Accurate noninvasive method to diagnose minor atherosclerotic lesions in carotid artery bulb. Stroke 20(10), 1336–1340 (1989).
- Wardlaw JM, Stevenson MD, Chappell F et al. Carotid artery imaging for secondary stroke prevention: both imaging modality and rapid access to imaging are important. Stroke 40(11), 3511–3517 (2009).
- Horrow MM, Stassi J, Shurman A, Brody JD, Kirby CL, Rosenberg HK. The limitations of carotid sonography: interpretive and technology-related errors. AJR Am. J. Roentgenol. 174(1), 189–194 (2000).
- Kenton AR, Martin PJ, Abbott RJ, Moody AR. Comparison of transcranial color-coded sonography and magnetic resonance angiography in acute stroke. Stroke 28(8), 1601–1606 (1997).
- Alexandrov AV, Demchuk AM, Wein TH, Grotta JC. Yield of transcranial Doppler in acute cerebral ischemia. Stroke 30(8), 1604–1609 (1999).
- Wijnhoud AD, Koudstaal PJ, Dippel DW. Relationships of transcranial blood flow Doppler parameters with major vascular risk factors: TCD study in patients with a recent TIA or nondisabling ischemic stroke. J. Clin. Ultrasound 34(2), 70–76 (2006).
- Wijnhoud AD, Koudstaal PJ, Dippel DW. The prognostic value of pulsatility index, flow velocity, and their ratio, measured with TCD ultrasound, in patients with a recent TIA or ischemic stroke. Acta Neurol. Scand. 124(4), 238–244 (2011).
- Meseguer E, Lavallee PC, Mazighi M et al. Yield of systematic transcranial Doppler in patients with transient ischemic attack. Ann. Neurol. 68(1), 9–17 (2010).
- Markus HS, Droste DW, Brown MM. Detection of asymptomatic cerebral embolic signals with Doppler ultrasound. Lancet 343(8904), 1011–1012 (1994).
- Droste DW, Ritter M, Kemeny V, Schulte-Altedorneburg G, Ringelstein EB. Microembolus detections at follow-up in 19 patients with acute stroke: correlation with stroke etiology and antithrombotic treatment. Cerebrovasc. Dis. 10(4), 272–277 (2000).
- Georgiadis D, Grosset DG, Kelman A, Faichney A, Lees KR. Prevalence and characteristics of intracranial microemboli signals in patients with different types of prosthetic cardiac valves. Stroke 25(3), 587–592 (1994).
- Valton L, Larrue V, Le Traon AP, Massabuau P, Geraud G. Microembolic signals and risk of early recurrence in patients with stroke or transient ischemic attack. Stroke 29(10), 2125–2128 (1998).
- Gao S, Wong KS, Hansberg T, Lam WW, Droste DW, Ringelstein EB. Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis. Stroke 35(12), 2832–2836 (2004).
- Goertler M, Baeumer M, Kross R et al. Rapid decline of cerebral microemboli of arterial origin after intravenous acetylsalicylic acid. Stroke 30(1), 66–69 (1999).
- Markus HS, Droste DW, Kaps M et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation 111(17), 2233–2240 (2005).
- Nedeltchev K, Schwegler B, Haefeli T et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke 38(9), 2531–2535 (2007).
- Rajajee V, Kidwell C, Starkman S et al. Early MRI and outcomes of untreated patients with mild or improving ischemic stroke. Neurology 67(6), 980–984 (2006).
- Lev MH, Farkas J, Rodriguez VR et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J. Comput. Assist. Tomogr. 25(4), 520–528 (2001).
- Smith WS, Tsao JW, Billings ME et al. Prognostic significance of angiographically confirmed large vessel intracranial occlusion in patients presenting with acute brain ischemia. Neurocrit. Care 4(1), 14–17 (2006).
- Coutts SB, Modi J, Patel SK, Demchuk AM, Goyal M, Hill MD. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the prospective CATCH study. Stroke 43(4), 1013–1017 (2012).
- Coutts SB, Modi J, Patel SK et al. What causes disability after transient ischemic attack and minor stroke?: results from the CT and MRI in the triage of TIA and minor cerebrovascular events to identify high risk patients (CATCH) Study. Stroke 43(11), 3018–3022 (2012).
- Coull AJ, Rothwell PM. Underestimation of the early risk of recurrent stroke: evidence of the need for a standard definition. Stroke 35(8), 1925–1929 (2004).
- Coutts SB, Hill MD, Eliasziw M, Fischer K, Demchuk AM. Final 2 year results of the vascular imaging of acute stroke for identifying predictors of clinical outcome and recurrent ischemic events (VISION) study. BMC Cardiovasc. Disord. 11(1), 18 (2011).
- Coutts SB, Hill MD, Campos CR et al. Recurrent events in transient ischemic attack and minor stroke: what events are happening and to which patients? Stroke 39(9), 2461–2466 (2008).
- Boulanger JM, Coutts SB, Eliasziw M, Subramaniam S, Scott J, Demchuk AM. Diffusion-weighted imaging-negative patients with transient ischemic attack are at risk of recurrent transient events. Stroke 38(8), 2367–2369 (2007).
- Sylaja PN, Coutts SB, Krol A, Hill MD, Demchuk AM. When to expect negative diffusion-weighted images in stroke and transient ischemic attack. Stroke 39(6), 1898–1900 (2008).
- TNK-tPA Evaluation for Minor Ischemic Stroke With Proven Occlusion (TEMPO-1). http://clinicaltrials.gov/ct2/show/NCT01654 445?cond=stroke&intr=TNK&titles=TEMP O&rank=1
- Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) Trial. http://clinicaltrials.gov/ct2/show/ NCT00991029?term=POINT&rank=1
• • Seminal paper on the added value of vascular and parenchymal imaging to clinical factors in predicting recurrent events in transient ischemic attack (TIA).
• • Large pooled analysis of TIA patients with TIA showing that the risk of recurrence after MRI-negative TIA is very low.
• • Important paper demonstrating that the silent accumulation of brain lesions increases the risk of dementia.
• • Demonstrates that vascular imaging is the key in identifying TIA and minor stroke patients at risk of recurrent events.
Websites


