Research Article - Neuropsychiatry (2017)
The Seizure and Cognitive Outcome of Anterior Thalamic Nucleus Deep Brain Stimulation for Patients with Intractable Epilepsy
- Corresponding Author:
- Guoming Luan
Department of Neurosurgery
Beijing Sanbo Brain Hospital
Capital Medical University, Beijing, 100093, China
Tel: 86-10-62856718
Fax: 86-10-62856902
E-mail: luangm3@163.com
Abstract
Objectives:
The objective of this study was to evaluate the efficacy of anterior thalamic nucleus deep brain stimulation (ANT-DBS) for patients with intractable epilepsy based on seizure and cognitive outcomes.
Patients and Methods:
Seventeen patients suffering from intractable epilepsy who received ANT-DBS implants from April 2015 to October 2016 were retrospectively reviewed. The preoperative evaluation consisted of semiology, electroencephalogram (EEG), positron emission tomography (PET) and MRI. During follow-up over 0.6-1 years, the surgical effects were evaluated mainly based on the seizure outcome (McHugh classification); psychological examinations including Wechsler Adult Intelligence Scale (WAIS), the Hamilton Anxiety Scale (HAMA), the Hamilton Depression Scale (HAMD), and quality of life (QOL).
Results:
The results showed that there was complete seizure free in 1 case, and temporal seizure free in 1 case. The average seizure improvement was 56%. Five (29.4%), 4 (23.5%), 6 (35.3%), 1 (5.9%), and 1 (5.9%) patients were classified into McHugh classes I, II, III, IV, and V, respectively. Complex partial seizure and temporal lobe epilepsy may have better seizure outcomes. The average postoperative IQ was slightly higher than that before operation, and the quality of life was statistically improved after ANT-DBS.
Conclusions:
The outcomes showed obvious seizure improvement with cognitive gain. ANT-DBS appears to be an effective and promising therapy for intractable epilepsy.
Keywords
Intractable epilepsy, Deep brain stimulation (DBS), Anterior thalamic nucleus (ANT), Seizure outcome, Cognitive outcome
Introduction
Approximately 50 million people worldwide are affected by epilepsy [1], which has a significant impact on family and social life. Among these patients, 30-40% has drug-resistant epilepsy [1], and surgical intervention should therefore be considered. Drug-resistant epilepsy with generalized or multifocal ictal origins is still challenging for epilepsy specialists, as both drugs and surgical resection have little effect on seizure control [2]. In recent decades, neuromodulation therapies, such as vagus nerve stimulation (VNS), cortical stimulation and deep brain stimulation, have been used for the treatment of intractable epilepsy [3]. Among these therapies, deep brain stimulation was recently introduced and is utilized on adult patients.
The anterior nucleus of the thalamus connects to the cerebral cortex and limbic system anatomically. The ANT can influence seizure propensity, may inhibit the nerve impulse of the target area and has been shown to block the transmission route to control seizures in animal tests [4]. Many clinical trials have demonstrated that ANT-DBS has an effect on intractable epilepsy, mainly in patients with little cognitive improvement [5-7]. Convincing indications for ANT-DBS are still lacking, and some study has reported the occurrence of postoperative depression and memory impairment in some patients after ANT-DBS implantation [8].
As one of the most advanced epilepsy centers in China, we started to utilize neuromodulation for epilepsy patients more than 10 years ago, including VNS and ANT-DBS. In the last two years, 17 adult patients with frequent intractable seizures received ANT-DBS implants at our center after preoperative evaluation. The seizure and cognitive improvements varied. The present study reports the seizure and cognitive outcomes of this series from a single center.
Materials and Methods
▪ Clinical cohort
We retrospectively reviewed the medical records of 17 consecutive patients suffering from drugresistant epilepsy who underwent ATN-DBS at the Beijing Sanbo Brain Hospital, Capital Medical University between April 2015 and December 2016. Approval from the Sanbo Brain Hospital, Capital Medical University Internal Review Board was obtained before this retrospective analysis was conducted.
▪ Preoperative evaluation
All clinical records of this series were reviewed, and the preoperative data including the following variables were extracted: perinatal period events, gender, age of seizure onset, patterns of epilepsy (focal, general or uncertain), other surgical interventions, disease duration, MRI (generally normal/diffuse bilateral lesions), PET (generally normal/diffuse bilateral hypo metabolism), ictal EEG pattern (generalized or multifocal origin), age at DBS implantation, and any other symptoms.
Neuropsychological testing was also performed prior to surgery and included Intelligence Quotient (IQ) measurement using the Wechsler Adult Intelligence Scale (WAIS). Anxiety and depression status were measured using the Hamilton Anxiety Scale (HAMA) and the Hamilton Depression Scale (HAMD), respectively.
▪ Surgical procedures
During the surgery, a Leksell stereotactic head frame (Elekta Instruments, Atlanta, USA) was applied, and a 1 mm consecutive CT (Philips, The Netherlands) scan was performed. All the pictures were reconstructed for previously acquired 1.5T MRI (T1 sagittal image, 1 mm slices; T2 axial, coronal image 2 mm slices) using a surgical planning software system (Medtronic, Minn., USA). The preliminary target within the ANT was defined as 12 mm superior, 5–6 mm lateral and 0–2 mm anterior to the mid commissural point. According to the target location, stimulation electrodes (PINS, China; model L302, effective length 10.5 mm, 4 contacts) were stereotactically implanted into the bilateral ANTs, using a transventricular approach under general anesthesia. Then the thalamic stimulation electrodes were connected to a PC neurostimulator (PINS, China; model G102) located in a subcutaneous infraclavicular pouch. The stimulator was activated 14 days later using a unipolar cyclic (1 min ON/5 min OFF) stimulation with 1.5-4.0 V stimulation amplitude, 130 Hz stimulation frequency and a pulse width of 60 μs.
▪ Follow-up
The patients were asked to return for followups at 3 months, 6 months, 12 months, and annually thereafter. The assessment included seizure outcome (McHugh classification), a physical examination, 16 h VEEG, MRI, and a psychological examination including WAIS, HAMD, HAMA, and any other complaints.
Statistical analysis
Data analyses were performed using the SPSS computer software package (version 21, IBM Corp., Armonk, NY). Fisher’s exact tests were used to compare the categorical variables. For all results, a value of P<0.05 was considered statistically significant.
Results
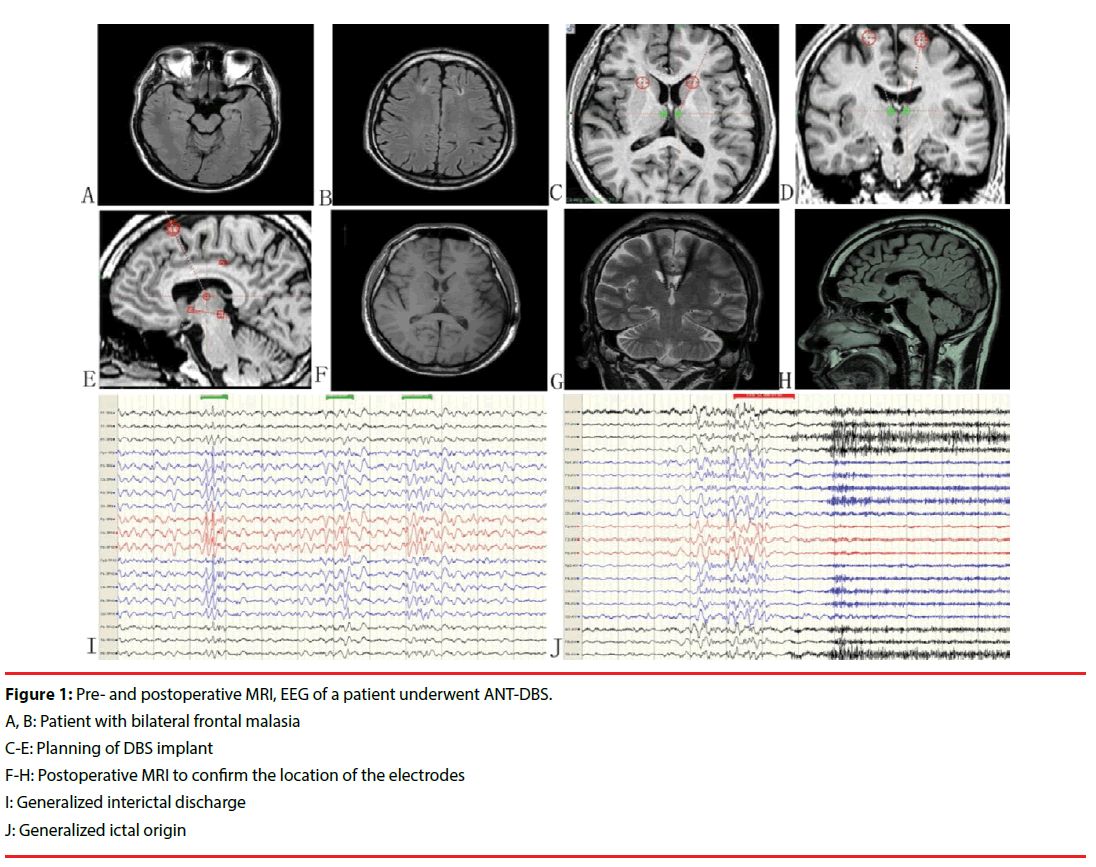
Among the 17 patients, 13 (76.5%) were male, the mean age at seizure onset was 11.8 years (range 3–30 years), and the mean age at DBS implantation was 25.5 years (range 13–46 years). The average disease duration was 13 years (range 1–34 years) and the median monthly seizure frequency during the baseline period was 15.6 (range 0.4–600). Twelve (71.3%) patients had more than one seizure pattern, and 3 (18.2%) patients had a generalized ictal origin. Mostly normal MRI results were noted in 4 (23.5%) patients and the other 13 patients showed multifocal bilateral lesions. PET revealed multifocal hypometabolism in all patients. Two patients underwent vagus nerve stimulation before deep brain stimulation. There were no discontinuations in the long-term followup phase in this series. Clinical features are listed in Table 1 (Figure 1).
| No. | Gender (male) | Age at onset (years) | AEDs administeraction | Disease duration (years) | Seizure types | Surgical intervention before DBS | Age at DBS | Follow-up duration (months) | Seizure outcome (McHugh) |
Cognitive outcome | Quality of life | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WAIS | HAMD | HAMA | |||||||||||
| 1 | M | 30 | 4 | 1 | CPS+GTCS | N | 31 | 25 | Ⅰ | 92 | 38 | 39 | 59.63 |
| 2 | M | 16 | 2 | 5 | CPS | N | 21 | 25 | Ⅰ | 85 | 41 | 40 | 56.33 |
| 3 | F | 8 | 1 | 9 | SPS +CPS+GTCS | N | 17 | 24 | Ⅲ | 70 | 37 | 38 | 56.32 |
| 4 | M | 6 | 3 | 7 | CPS+ GTCS | N | 13 | 24 | Ⅱ | 81 | 38 | 36 | 63.33 |
| 5 | M | 12 | 1 | 34 | CPS | N | 46 | 23 | Ⅱ | 102 | 46 | 41 | 60.25 |
| 6 | M | 12 | 2 | 25 | CPS+ GTCS | N | 37 | 22 | Ⅲ | 93 | 39 | 41 | 58.66 |
| 7 | M | 8 | 3 | 7 | CPS | N | 15 | 22 | Ⅲ | 78 | 42 | 40 | 58.76 |
| 8 | M | 3 | 2 | 25 | CPS | N | 28 | 21 | Ⅱ | 80 | 41 | 38 | 65.38 |
| 9 | F | 23 | 3 | 3 | SPS +CPS+GTCS | N | 26 | 17 | Ⅲ | 75 | 38 | 37 | 68.33 |
| 10 | M | 30 | 1 | 16 | GTCS | N | 46 | 17 | Ⅰ | 104 | 37 | 38 | 66.75 |
| 11 | M | 10 | 4 | 6 | SPS+CPS+GTCS | N | 16 | 17 | Ⅱ | 74 | 38 | 39 | 63.66 |
| 12 | F | 9 | 1 | 5 | SPS +GTCS | N | 14 | 16 | Ⅰ | 65 | 40 | 41 | 61.00 |
| 13 | M | 15 | 1 | 15 | CPS | N | 30 | 15 | Ⅴ | 70 | 39 | 38 | 59.66 |
| 14 | M | 13 | 2 | 17 | CPS+GTCS | VNS | 30 | 13 | Ⅰ | 125 | 37 | 41 | 68.75 |
| 15 | F | 10 | 2 | 10 | GTCS | N | 20 | 13 | Ⅲ | 78 | 39 | 40 | 66.25 |
| 16 | M | 3 | 3 | 21 | CPS | N | 24 | 12 | Ⅲ | 90 | 41 | 40 | 64.50 |
| 17 | M | 10 | 2 | 15 | SPS +GTCS | VNS | 25 | 12 | Ⅳ | 92 | 39 | 38 | 61.75 |
M=male, F=female; SPS (simple partial seizure), complex partial seizure (CPS), secondary generalized tonic clonic seizure (GTCS), VNS=Vagusneuve stimulation.
Table 1: Clinical characteristics of the cohort.
▪ Seizure outcome
At the last follow-up, one patient was found to be completely seizure-free, and one patient reported being seizure-free in the first year. The average seizure frequency was 7.3 times a month compared with 15.6 times before the operation (p=0.012). The average seizure improvement was 56%. Five (29.4%), 4 (23.5%), 6 (35.3%), 1 (5.9%), and 1 (5.9%) patients were classified into McHugh classes I, II, III, IV, and V, respectively. A subgroup analysis showed that patients with temporal lobe (n=6) epilepsy, frontal lobe epilepsy (n=5), and parietal and occipital lobe epilepsy (n=3) showed reductions of 67%, 51%, and 35% in the number of seizures, respectively, and patients with all other seizure onset zones showed a 48% reduction. Single factor analysis showed that among gender, age of seizure onset, disease duration, EEG pattern and seizure type, complex partial seizure and temporal lobe epilepsy may have better seizure outcomes. The single factor analysis is shown in Table 2.
| Total | Favorable outcome* (n, %) | P value | |
|---|---|---|---|
| Gender | 0.241 | ||
| Male | 13 | 8(61.5%) | |
| Female | 4 | 1(25.0%) | |
| MRI | 0.563 | ||
| Negative | 5 | 3(60.0%) | |
| Bilatera lesion | 12 | 6(50.0%) | |
| Semiology | 0.036 | ||
| Complex partial seizure | 7 | 6 (85.7%) | |
| Other pattern | 10 | 3(30%) | |
| Duration from seizure onset to operation | 0.335 | ||
| Less than 5 years | 4 | 3 (75.0%) | |
| More than 5years | 13 | 6(46.2%) |
*Favorable outcome: Mchugh class I or II
Table 2: Factors related to seizure outcome.
▪ Cognitive status and quality of life
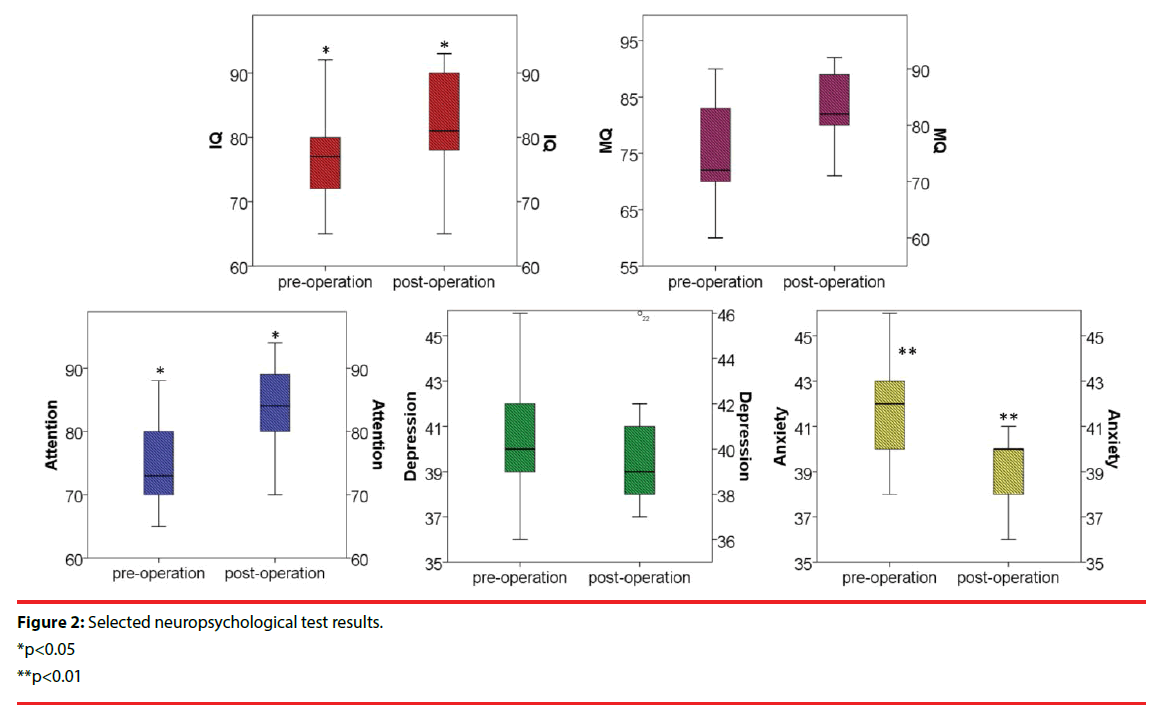
The average postoperative IQ was 81.9 (range 65-93) compared with 77.6 (range 65-92) before the operation. Neuropsychological test composite scores showed statistically significant improvement from baseline to 5 years including improvements in attention (p<0.05), executive functions (p<0.05), depression (p<0.01), tension/anxiety (p>0.05), total mood disturbance (p>0.05), and subjective cognitive functions (p<0.05). The neuropsychological test results are presented in Figure 2. QOL-31 was statistically improved after ANT-DBS (53.9±5.5 vs. 62.3±3.9, p=0.015). Two patients with severe depression showed significant improvements after ANT-DBS implantation, coincident with seizure reduction. Single factor analysis showed that no factor was statistically related to the cognitive outcomes among gender, age of seizure onset, disease duration, EEG pattern and seizure type.
▪ Device-related adverse events
One patient reported a feeling of lower limb weakness with no abnormal findings on neurological examination. One patient suffered from a mild menstruation disturbance, and 1 patient suffered frequent but short dizzy spells. Two patients had poor appetites.
Discussion
A number of clinical studies demonstrated that electrical stimulation of the anterior thalamic nucleus has favorable effects in many forms of seizure, such as partial seizures, generalized tonicclonic seizures and complex partial epileptic seizures [5,8]. Salanove, et al. [7] conducted a multi-center randomized clinical trial, and the results showed that the average control rate of anterior thalamic nucleus stimulation for partial seizures was 43% after 1 year and 68% after 5 years. Another study found that ANT-DBS was also beneficial for cognitive function. In the present series, 70% of the patients had more than 50% seizure reduction, and 2 patients were seizure-free during 2 years of follow-up.
In this series, patients with complex partial seizures had better seizure outcomes than those with GTCS. Although a recent meta-analysis [3] found no association between seizure pattern and DBS outcome, many studies have demonstrated that ANT-DBS has a better effect on partial seizures. Animal tests showed that chronic ANT stimulation could exert a neuroprotective effect on hippocampal neurons [4]. On the contrary, Valentin, et al. [9] reported that centro-median thalamic nucleus DBS was particularly efficacious for patients with refractory generalized epilepsy. We speculated that this would be correlated with the adjustment of PAZS circles [6], and further studies should be designed to elucidate the mechanism.
We know that epilepsy patients are likely to have mood disorders, such as depression [10]. By the middle of the follow-up period, most patients reported a decrease in depression and an improvement in memory. We speculated that the improved seizure control likely played a role in the reduction of anxiety and depression. However, some recent studies reported that bilateral ANTDBS is associated with subjective depression and memory impairment in a minority of patients [5]. Many studies have revealed that ANT plays an important role in the memory neuro-circle. Combined with our results [11], determination of the exact impact of ANT-DBS on memory and mood requires a larger patient group and longer follow-up duration.
The target puncture procedure utilized for anterior thalamic stimulation can be challenging because the electrode may shift when passing the lateral ventricle, leading to surgical failure. The following conclusions were drawn from this series. Firstly, the origin of the segmental support should be changed to 45 mm, and the target should be set as 10 mm on the micro propeller to increase the insertion depth of the rigid casing. Secondly, a microelectrode should be used to form the tunnel between the ventricular wall and the target. Thirdly, if necessary, a trocar can be placed directly in the target or 5 mm above the target point to maximally reduce the deviation of the electrode implant. Regarding the target point, Wu, et al. concluded that a better understanding of thalamic anatomy is necessary to optimize the outcome [12].
Compared with vagus nerve stimulation, DBS is mainly used for patients over the age of 14. The main consideration is that the stability of the electrode position will be affected by the development of a child’s brain. For patients with intractable epilepsy under the age of 14, VNS is recommended as the first option. A few studies reported that DBS has a greater side effect profile compared to VNS, and the DBS procedure is more complicated [13]. More research should be conducted to find better indications for each procedure.
Conclusion
As the primary method for neuromodulation and control of epilepsy, ATN-DBS provides more options for patients with intractable seizures, as it can effectively reduce the seizure frequency, especially for complex partial seizures, and even improves memory, depression and anxiety.
Funding
This study was supported by the following sources: the Research Fund for the Doctoral Beijing Nova Program (Z141107001814042), Collaboration between Clinical and Basic Research in Capital Medical University (16JL09), the Capital Applied Clinic Research Programs of Science and Technology (Z131107002213171), and the Beijing Municipal Natural Science Foundation (7144217).
References
- Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology 88(3), 296-303 (2017).
- Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 128(Pt 5), 1188-1198 (2005).
- Sprengers M, Vonck K, Carrette E, et al. Deep brain and cortical stimulation for epilepsy. Cochrane. Database. Syst. Rev (6), CD008497 (2014).
- Meng DW, Liu HG, Yang AC, et al. Stimulation of Anterior Thalamic Nuclei Protects Against Seizures and Neuronal Apoptosis in Hippocampal CA3 Region of Kainic Acid-induced Epileptic Rats. Chin. Med. J (Engl) 129(8), 960-966 (2016).
- Troster AI, Meador KJ, Irwin CP, et al. Memory and mood outcomes after anterior thalamic stimulation for refractory partial epilepsy. Seizure 45(1), 133-141 (2017).
- Krishna V, King NK, Sammartino F, et al. Anterior Nucleus Deep Brain Stimulation for Refractory Epilepsy: Insights Into Patterns of Seizure Control and Efficacious Target. Neurosurgery 78(6), 802-811 (2016).
- Salanova V, Witt T, Worth R, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 84(10), 1017-1025 (2015).
- Lim SN, Lee ST, Tsai YT, et al. Electrical stimulation of the anterior nucleus of the thalamus for intractable epilepsy: a long-term follow-up study. Epilepsia 48(2), 342-347 (2007).
- Valentin A, García Navarrete E, Chelvarajah R, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 54(10), 1823-1833 (2013).
- Maguire MJ, Weston J, Singh J, et al. Antidepressants for people with epilepsy and depression. Cochrane. Database. Syst. Rev (12), CD010682 (2014).
- tillova K, Jurák P, Chládek J, et al. The Role of Anterior Nuclei of the Thalamus: A Subcortical Gate in Memory Processing: An Intracerebral Recording Study. PLoS. One 10(11), e0140778 (2015).
- Wu C, D'Haese PF, Pallavaram S, et al. Variations in Thalamic Anatomy Affect Targeting in Deep Brain Stimulation for Epilepsy. Stereotact. Funct. Neurosurg 94(6), 387-396 (2016).
- Gooneratne IK, Green AL, Dugan P, et al. Comparing neurostimulation technologies in refractory focal-onset epilepsy. J. Neurol. Neurosurg. Psychiatry 87(11), 1174-1182 (2016).