Research Article - Neuropsychiatry (2018) Volume 8, Issue 4
The Signature of the Serotonin System in the Chronic Corticosterone Depression Model: A Study with [18F] MPPF, [18F] Altanserin and [11C] DASB
- Corresponding Author:
- Glenn Pauwelyn
Laboratory of Radiopharmacy, Ghent University
Ottergemsesteenweg 460, 9000 Ghent, Belgium
Tel: 003292648045
Abstract
Introduction
For more than six decades abnormalities in the serotonin system have been proposed to play a key role in the pathophysiology of major depressive disorder (MDD). An interesting pathway would be the role of a dysregulated hypothalamic-pituitary-adrenal (HPA) axis, seen in patients suffering from MDD, in the disturbed serotonergic neurotransmission. The present study aimed to further explore this role of the serotonergic system in the corticosterone (CORT) rodent depression model.
Methods
The CORT depression model was induced by means of chronic CORT administration (40 mg/kg) to male Long-Evans rats during three weeks. CORT-induced effects were investigated on the behavioral level, the total body weight and the plasma corticosterone levels, and compared to a healthy control group (N=18). Furthermore, in both the CORT and the control group, non-invasive imaging was performed using three positron emission tomography (PET) radiotracers. These included [18F]-2’-methoxyphenyl-(N-2’-pyridinyl)-p-fluoro-benzamidoethyipiperazine ([18F] MPPF), [18F] altanserin and 3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile ([11C] DASB), which allowed quantification of the binding potential (BPND) on the 5-HT1A receptor, the 5-HT2A receptor, and the serotonin transporter, respectively.
Results
The chronic CORT administration resulted in significantly lowered body weight, significantly elevated plasma corticosterone levels, and induced depression-like behavior. Compared to the control group, induction of the CORT depression model resulted in significantly decreased BPND of [18F] MPPF in the medial prefrontal cortex and anterior cingulate cortex (ACC). At the 5-HT2A receptor level, the [18F] altanserin BPND was significantly increased in all of the cortical regions of interest except for the ACC. No significant differences in regional BPND of [11C] DASB were detected.
Conclusion
This extended study with three radio-ligands emphasizes the potential influence of a dysregulated HPA-axis on the serotonin system. Furthermore, these results indicate the relevance and reliability of the corticosterone depression model in the investigation of the mode of action for current and novel antidepressant therapies on the 5-HT1A and 5-HT2A receptors.
Keywords
[18F] MPPF, [18F] Altanserin, [11C] DASB, Corticosterone, Depression, Long-Evans Rats, HPA-axis
Introduction
Major depressive disorder (MDD) is a severe psychiatric illness characterized by a persistent ( >2 weeks) lowered mood and/or anhedonia, combined with several other symptoms such as eating or sleeping disturbances, extreme fatigue, diminished ability to concentrate, feelings of worthlessness or suicidal ideation (DSM-V criteria according to the American Psychiatric Association) [1]. Unlike bipolar disorder, the depressive episodes in MDD are not interspersed with manic episodes. Intensive research generated the general hypothesis that MDD is not directly caused by a single gene polymorphism or a disturbance in a certain brain region or neurotransmitter system. Instead, it involves the disturbance of multiple discrete, but functionally integrated, pathways, leading to a failure of the remaining system to maintain homeostatic emotional control [2].
Stress is generally known to act as a vulnerability trait to develop MDD in subjects. An important hormonal response to stress is the activation of the hypothalamic-pituitary-adrenal (HPA)- axis, which causes the release of cortisol in the blood [3]. Chronic hypercortisolemia results in downregulation of the local glucocorticoid receptors, which causes a failure of the negative feedback regulation, inducing hyperactivity of the HPA-axis [4]. Prolonged reactivity of the HPA-axis has been observed in 43% of the patients suffering from MDD [5]. In addition, a growing amount of literature reporting on animal studies indicate that repeated subcutaneous administration of high doses of stress hormone corticosterone (analogue to cortisol in humans) causes a depressive-like behavior in both male and female rats [6,7]. Furthermore, the possible involvement of a dysregulated HPA-axis in the reported alterations of the serotonergic system in depression has been put forward by several authors [8-11]. For example, Savitz et al. reported acute stress or corticoids administration down-regulates or desensitizes the 5HT1a receptor [8]. Furthermore, a direct relationship between glucocorticoid receptor activation and increased 5-HT2A receptor levels was reported by Trajkowska et al. [10].
The role of serotonin and the serotonin system in the pathophysiology of MDD has been a major topic of investigation for more than six decades [12-15]. Most of the prominent contributions to the current knowledge have been provided by molecular imaging such as single photon emission computed tomography (SPECT) and positron emission tomography (PET) studies. These functional imaging techniques allow non-invasive investigation of neurotransmitter receptors and transporters in the brain. Unfortunately, the technological advantages of PET and SPECT have been partly overshadowed by the many discrepancies reported between imaging studies. To date, only few overall findings have been cautiously made concerning alterations in the serotonergic system in depression. As such, for the serotonin 5-HT1A receptor, a recent meta-analysis [16-18] reported a reduced 5-HT1A receptor binding in MDD, mainly in the mesial temporal cortex, but also to a lesser extent in the hippocampus, raphe nuclei, insular, anterior cingulate cortex (ACC), and occipital cortex. For the serotonin 5-HT2A receptor, generalizations could not yet be made, however, the most valuable information might probably originate from two imaging studies in unmedicated patients [19,20]. These studies both suggested that high prefrontal 5-HT2A receptor binding is implicated in MDD. For the serotonin transporter (SERT), a meta-analysis from eighteen PET and SPECT studies [21] revealed reduced SERT availabilities, mainly in the midbrain and the amygdala, and to a lesser extent in several other high binding regions including the thalamus, the striatum and the entire brainstem.
Several authors have put forward possible explanations for the many discrepancies observed between study results are described in the literature [21-23]. Hereby, a first group of confounding variables is related to the study group and includes differences in age, gender and sample size, the subject’s clinical phenotype of depression, the presence or absence of suicidal risk or comorbid disorders, the patient’s medication status and history, and the presence or absence of screening for familial vulnerability within the healthy control group. In this context, preclinical research, using a relevant and validated rodent depression model, might overcome several of these limitations and allows fundamental research of the pathophysiology of depression and the mode of action of antidepressant strategies. A second group of confounding variables is radiotracer-related and might concern differences in selectivity among the radiotracers, or differences in approach to analyze the images such as the choice of a particular compartmental or reference tissue model, and the risk of using a reference region with a displaceable fraction.
The aim of this study was to further explore the potential relationship between disturbances of the serotonin system and a dysregulated HPAaxis, meanwhile overcoming several of the above mentioned confounders by applying a backtranslational approach. Thereby using a rodent depression model with a good face, construct and predictive validity: the corticosterone depression model [6,7,24,25]. Disturbances of the serotonin system were explored using three highly selective PET-radiotracers. These included [18F]-2’- methoxyphenyl-(N-2’-pyridinyl) p-fluorobenzamidoethyipiperazine ([18F] MPPF), [18F] altanserin and 3-amino-4-(2 dimethylaminome thylphenylsulfanyl)-benzonitrile ([11C] DASB), which allowed the visualization of the serotonin 5-HT1A receptor, the 5-HT2A receptor, and the serotonin transporter, respectively. Secondarily, the CORT-induced effects on the behavioral level, the total body weight and the plasma corticosterone levels were examined. To our best knowledge, this is the first preclinical study using PET to evaluate the effects of chronic corticosterone administration on the serotonin system in rats.
Materials and Methods
▪ Experimental animals
This study was approved by the Ethical Committee of Ghent University (EC approval 15/63 and 15/86). Thirty-six male Long Evans rats were included in the study. At the beginning of the experiment all of the rats were approximately eight weeks old, weighing 285 ± 26 g. The animals were housed individually in type III cages (polycarbonate, bottom: 382 x 220 mm, height: 150 mm) at room temperature (21°C), had a standard 12-h light/dark cycle and had food and water available ad libitum.
▪ Treatment groups
After an initial acclimatization period of one week, 36 rats were randomly assigned to one of two equal groups (n = 18), a CORT and a control group (Figure 1). To induce the depression model, rats in the CORT group received a daily s.c. injections (between 9 and 12 AM) of corticosterone (40 mg/kg) in the neck region for 21 consecutive days. Therefore, a uniform and fine corticosterone suspension (20 mg/ mL) was prepared by dissolving corticosterone- 21-acetate (Sigma Aldrich, Germany) in ethanol 100% (Sigma Aldrich, Germany) and subsequently mixing the solution with sesame oil (Sigma Aldrich, Germany). Finally, all of the ethanol phase was evaporated using a rotavapor (Buchi, Switzerland) to obtain a uniform and fine corticosterone suspension in sesame oil (20 mg/ml). Injections were given under light sedation (brief exposure to 5% isoflurane in medical oxygen) and were preceded by shaving and disinfection of the neck region. Rats in the control group underwent the same protocol as those in the CORT group, except for the corticosterone injections, which were replaced by vehicle injections (sesame oil, 2 mL/kg) only.
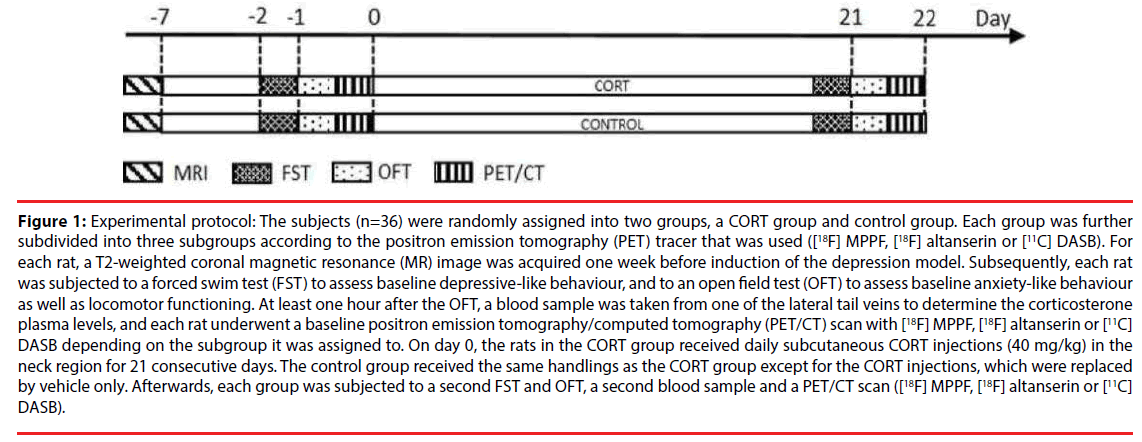
Figure 1: Experimental protocol: The subjects (n=36) were randomly assigned into two groups, a CORT group and control group. Each group was further subdivided into three subgroups according to the positron emission tomography (PET) tracer that was used ([18F] MPPF, [18F] altanserin or [11C] DASB). For each rat, a T2-weighted coronal magnetic resonance (MR) image was acquired one week before induction of the depression model. Subsequently, each rat was subjected to a forced swim test (FST) to assess baseline depressive-like behaviour, and to an open field test (OFT) to assess baseline anxiety-like behaviour as well as locomotor functioning. At least one hour after the OFT, a blood sample was taken from one of the lateral tail veins to determine the corticosterone plasma levels, and each rat underwent a baseline positron emission tomography/computed tomography (PET/CT) scan with [18F] MPPF, [18F] altanserin or [11C] DASB depending on the subgroup it was assigned to. On day 0, the rats in the CORT group received daily subcutaneous CORT injections (40 mg/kg) in the neck region for 21 consecutive days. The control group received the same handlings as the CORT group except for the CORT injections, which were replaced by vehicle only. Afterwards, each group was subjected to a second FST and OFT, a second blood sample and a PET/CT scan ([18F] MPPF, [18F] altanserin or [11C] DASB).
Within each group, rats were further randomly subdivided into three equal subgroups (n=6). One of the subgroups was scanned, before and after induction of the depression model, with [18F] MPPF, to investigate serotonin system disturbances at the 5-HT1A receptor level. A second group was scanned with [18F] altanserin, to explore alterations at the 5-HT2A receptor level, and a third group was scanned with [11C] DASB, to investigate depression related effects on the availability of the serotonin transporter
▪ Behavioural testing
Before and after induction of the depression model, each rat was subjected to a forced swim test (FST), to assess depression-like behaviour, and to an open field test (OFT), to assess anxietylike behaviour as well as locomotor functioning [6,26,27]. The OFT was performed at least one hour prior to radiotracer injection and the FST was performed the evening before the PET scan. The FST was conducted in a glass cylinder (40 cm high × 25 cm in diameter) filled with water at 23°C to a depth of 30 cm. Each rat was placed individually in the swim chamber for 5 min while its behavior was video-recorded. According to the method of Cryan et al. [28], predominant behaviour over 5-s intervals was noted by an experienced researcher (GP or NVL). Three characteristic behaviours could be distinguished: (1) climbing behaviour, defined as paddling movements of the forepaws along the side of the swim chamber; (2) swimming behaviour, defined as horizontal movements of the rat throughout the swim chamber, (3) immobility behaviour, where no activity is observed other than that required to keep the rat’s head above the water. To teach the rat that escaping is not possible; a 15 min induction phase was incorporated 24 h prior to the actual FST. Confounding odors were avoided by refreshing the water between each experiment. The OFT was conducted in a plasticized cardboard box with a square area of 70 × 70 cm and walls of 30 cm in height. The square area was further subdivided, with an indelible marker, into a centre zone (40 cm × 40 cm) and a peripheral zone. After first placing the rat into the centre of the box, it was free to move and its behavior was video-recorded during 15 min. SMART software 3.0 (Panlab S.L.U, Barcelona, Spain) was used to automatically analyse the recordings and calculate the total distance travelled (indicator of motor functioning) as well as the time spent in the centre zone (indicator of anxiety-like behavior). To habituate the rat to the box, a 15-min induction phase was incorporated 24 h prior to the actual OFT. Confounding odours were avoided by cleaning the box with 70% ethanol in water between each experiment.
▪ Plasma corticosterone levels
On scan days, approximately 200 μL venous blood was collected from each rat, immediately after a 26 G catheter was inserted in one of the lateral tail veins. Plasma corticosterone levels were measured in duplicate using a commercial ELISA kit (ALPCO, Salem, New Hampshire, USA) according to the manufacturer’s instructions.
▪ Radiosynthesis
[18F] MPPF and [18F] altanserin were both synthesized on a Synthra RNplus module (Synthra GmbH, Hamburg, Germany) in analogy with the chemical pathway described by Hayashi et al. [29] and Massarweh et al. [30] respectively.
[11C] DASB was synthesized on a TRACERlab FX C Pro synthesizer (GE Healthcare, Chicago, US) by methylation of the precursor, N-desmethyl-DASB (100 μg, ABX, Radeberg, Germany), with [11C] methyl triflate using established methods [31].
▪ Imaging protocols
Imaging was performed using a Flex Triumph II small animal PET/CT system (TriFoil Imaging, Northridge CA). Each radiotracer was used to acquire two scans from each rat in the respective subgroup. These scans comprised a baseline scan one day prior to the treatment procedure, and a second scan the day after the last corticosterone or vehicle injection. On scan days, rats were anesthetized using a mixture of isoflurane in medical oxygen (5% induction, 2% maintenance), and a 26 G catheter was placed in one of the lateral tail veins. Subsequently, the rats were positioned on the bed of the PET/CTscanner.
Within the [18F] MPPF subgroup, 60 min dynamic emission recordings were initiated on bolus injection of 34 ± 2 MBq [18F] MPPF. PET emission data were reconstructed in 12 images of 10 s, 6 images of 30 s, 10 images of 60 s, 9 images of 300 s, and 2 images of 600 s. Within the [18F] altanserin subgroup, 180 min dynamic emission recordings were initiated on bolus injection of 34 ± 3 MBq [18F] altanserin and PET emission data were reconstructed in 12 images of 10 s, 6 images of 30 s, 10 images of 60 s, 9 images of 300 s and 12 images of 600 s. Finally, within the subgroup receiving [11C] DASB, 90 min dynamic emission recordings were initiated on bolus injection of 24 ± 5 MBq [11C] DASB and emission data were reconstructed in 1 image of 20 s, 6 images of 5 s, 4 images of 15 s, 2 images of 35 s, 3 images of 60 s, 3 images of 180 s, 9 images of 300 s and 3 images of 600 s. PET images were iteratively reconstructed into a 200 × 200 × 63 matrix (0.5 x 0.5 × 1.175 mm voxels) by the Maximum- Likelihood Expectation-Maximization (MLEM) algorithm using 50 iterations. All PET scans were followed by a 5 min CT scan (50 kV, 640 μA, 650 ms exposure time, 85 μm focal spot, 256 projections over 360°, reconstructed in 512 x 512 × 512 matrix with 100 μm voxels).
One week before induction of the depression model, for each rat a series of T2-weighted coronal anatomical images were acquired using a 7 tesla MRI-scanner (PharmScan 70/16, Bruker Biospin, Ettlingen, Germany), a rat brain RF volume coil, and a T2-weighted rapid acquisition with relaxation enhancement (RARE) sequence (TR 5200 ms, TE 37 ms, RARE factor 8, NA 4, 22 coronal slices, 109 μm in-plane resolution, 600 μm thickness, TA 11 min 47.2 s).
▪ PET data quantification
Using PMOD software version 3.405 (PMOD Technologies, Ltd., Zurich, Switzerland), each PET image was co-registered with its corresponding CT, and each CT with its corresponding MRI. Saving these transformation vectors and applying them to the original PET images finally resulted in co-registered PET/ MRI images. Based on information from the Px Rat W. Schiffer brain atlas, available in PMOD, and the Paxinos and Watson atlas [32], multiple volumes of interest (VOI) were manually delineated. These included (1) amygdala, ACC, cerebellum (caudal area), hippocampus, and medial prefrontal cortex for analysing the [18F] MPPF scans, (2) ACC, cerebellum, entorhinal cortex, insular cortex, medial prefrontal cortex (comprising prelimbic and infralimbic cortex), motor cortex and orbitofrontal cortex for analysing the [18F] altanserin scans, and (3) ACC, amygdala, caudate and putamen region (Cpu), cerebellum (caudal area, vermis excluded), hippocampus, midbrain, prelimbic cortex, and thalamus for analysing the [11C] DASB scans.
For [18F] MPPF, [18F] altanserin and [11C] DASB, non-displaceable binding potentials (BPND’s) were calculated for each VOI using the simplified reference tissue model 2 (SRTM2) [33]. For [18F] MPPF, based on previous studies in humans [34] and rats [35], the centre of the caudal area of the cerebellum was used as a reference region and a fixed k2’ was determined based on the mean k2’ estimated for [18F] MPPF binding in the amygdala, prelimbic cortex and hippocampus. For [18F] altanserin, referring to the results of Kroll et al. [36] and Riss et al. [37], the reference region consisted of the entire cerebellum and a fixed k2’ was determined as the mean k2 ’ value of all VOIs investigated. For [11C] DASB, based on previous literature [38- 41], it was opted to use the posterior half of the cerebellar hemispheres, thereby excluding the vermis, and the mean k2’ of the thalamus, CPu, and midbrain, was used to determine a fixed k2’.
▪ Statistical analysis
Rstudio 1.1.383 (R: A Language and Environment for Statistical Computing; R Core Team; R Foundation for Statistical Computing, Vienna, Austria, 2016, https://www.R-project.org/) with packages MASS (version 7.3-45), Sommer (version 3.0), nlme (version 3.1-131) and Hmisc (version 4.0-3) was used to compute all analyses.
Six datasets were acquired during this study containing the results of the total body weight, the FST, the OFT, [18F] MPPF, [18F] altanserin and [11C] DASB. Onto each data set, a multivariate linear mixed model with heterogeneous (unstructured) variances was set up. Each model was identified as E(Yt|T)=β0+β1t+β2T+β3tT with Yt as response variable. The response variables for the FST data set were immobility time and climbing time. Total distance travelled and percentage of time spent in the centre were the outcome variable of the OFT data set. For the [18F] MPPF, [18F] altanserin and [11C] DASB data sets, the delineated VOI’s were set as outcome variables. The models included the random factors time and animal as predictor values and treatment modality as fixed factor. The first predictor value t denotes the different scan moments. On the other hand, T denotes the treatment modalities (treatment variable, categorical), T (=1 if the treatment modality = “CORT” or 0 otherwise). In addition, a random intercept was included into each model. The degrees of freedom were calculated based on the Welsh-Satterthwaite equation and the type-I error α was set at 0.05 (two-tailed). The assumptions concerning the linearity of the regression function and the normality of the error term were assessed by making diagnostics plots and by statistical tests (Shapiro-Wilk). Finally, Pearson correlations (α set at 0.05) between all the outcome variables of the total body weight, the FST, the OST, and the [18F] MPPF, [18F] altanserin and [11C] DASB regional brain uptake, were calculated.
Results
▪ Total body weight
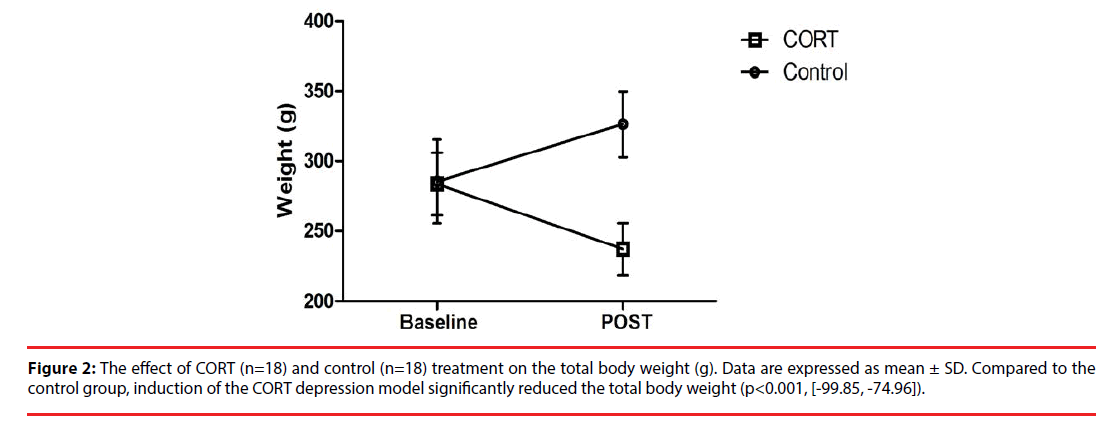
The effect of the chronic corticosterone (CORT group) or vehicle administrations (control group) on the total body weight is illustrated in Figure 2. The mean (± SD) baseline body weight of the rats in the CORT and control group was 284 ± 22 g and 285 ± 30 g, respectively. While in the control group, rats gained weight over time with a mean (± SD) increase of 13 ± 5% by the end of the protocol, rats in the CORT group were characterized by a 20 ± 9% weight loss. Therefore, chronic corticosterone injections significantly altered the total body weight compared to vehicle injections (p<0.001, 95% CI [-99.85, -74.96]).
Figure 2: The effect of CORT (n=18) and control (n=18) treatment on the total body weight (g). Data are expressed as mean ± SD. Compared to the control group, induction of the CORT depression model significantly reduced the total body weight (p<0.001, [-99.85, -74.96]).
▪ Behavioral testing
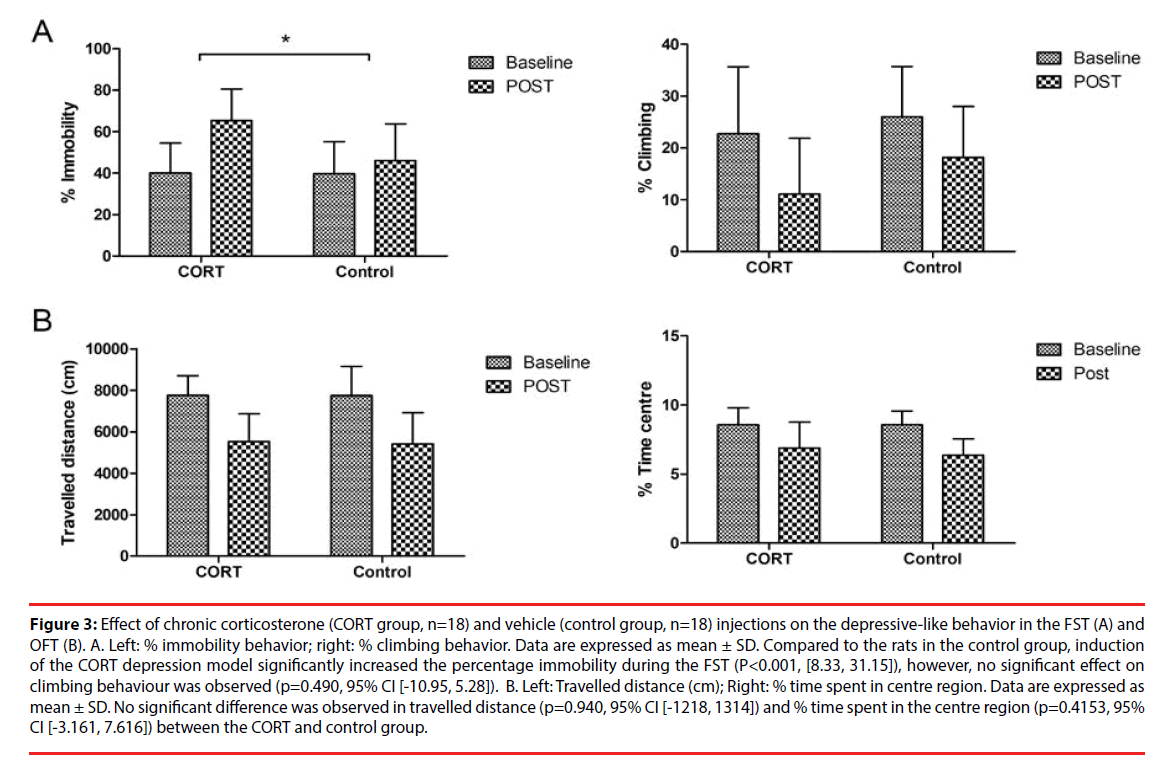
Figure 3A represents the mean (± SD) percentage of immobility and climbing behavior displayed during the FST in the CORT and control group. Compared to the observed alterations in the control group, the repeated corticosterone injections significantly increased the percentage immobility in the FST (p<0.001, [8.33, 31.15]), but did not significantly affect the climbing behaviour (p=0.490, 95% CI [-10.95, 5.28]).
Figure 3: Effect of chronic corticosterone (CORT group, n=18) and vehicle (control group, n=18) injections on the depressive-like behavior in the FST (A) and OFT (B). A. Left: % immobility behavior; right: % climbing behavior. Data are expressed as mean ± SD. Compared to the rats in the control group, induction of the CORT depression model significantly increased the percentage immobility during the FST (P<0.001, [8.33, 31.15]), however, no significant effect on climbing behaviour was observed (p=0.490, 95% CI [-10.95, 5.28]). B. Left: Travelled distance (cm); Right: % time spent in centre region. Data are expressed as mean ± SD. No significant difference was observed in travelled distance (p=0.940, 95% CI [-1218, 1314]) and % time spent in the centre region (p=0.4153, 95% CI [-3.161, 7.616]) between the CORT and control group.
Figure 3B represents the mean (± SD) distance travelled (cm) and the percentage time spent in the centre region during the OFT in the CORT and control group. Due to technical issues with the video camera, no OFT results were obtained for one rat in the CORT group and one rat in the control group. No significant differences in travelled distance (p=0.940, 95% CI [-1218, 1314]) and percentage time spent in centre region (p=0.4153, 95% CI [-3.161, 7.616]) were observed between the CORT and the control group.
FIG 3
▪ Plasma corticosterone levels
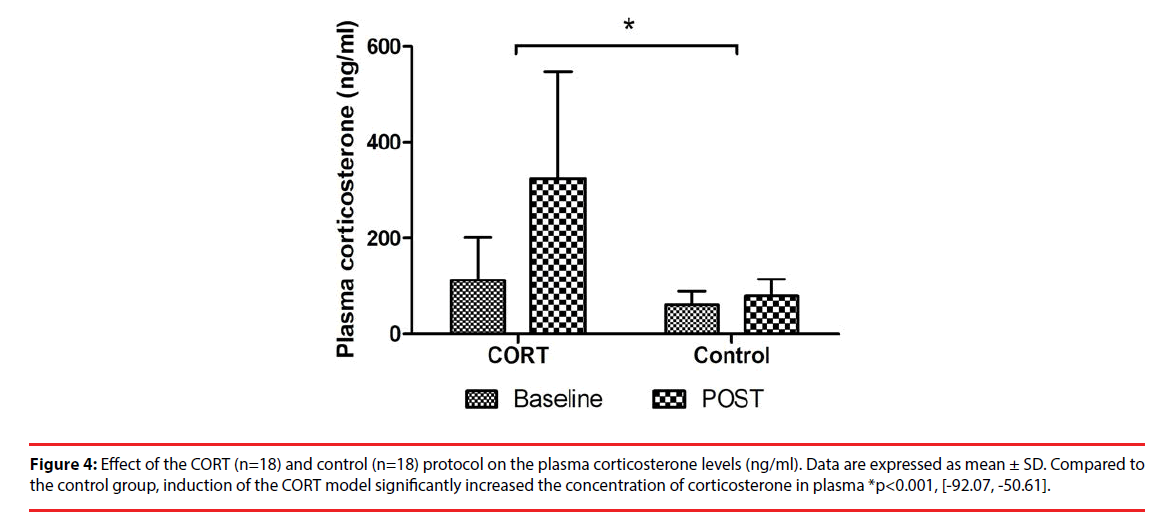
The mean (± SD) corticosterone plasma levels before and after chronic corticosterone or vehicle injections are presented in Figure 4. Compared to the control group, induction of the CORT model significantly increased the concentration of corticosterone in plasma (p<0.001, 95% CI [-92.07, -50.61]).
Figure 4: Effect of the CORT (n=18) and control (n=18) protocol on the plasma corticosterone levels (ng/ml). Data are expressed as mean ± SD. Compared to the control group, induction of the CORT model significantly increased the concentration of corticosterone in plasma *p<0.001, [-92.07, -50.61].
▪ PET imaging
Figures 5-7 represent the effects of the chronic corticosterone or vehicle injections on the regional BPND values of [18F] MPPF, [18F] altanserin, and [11C] DASB, respectively. For each radiotracer, the effects in the CORT group were compared to those in the control group.
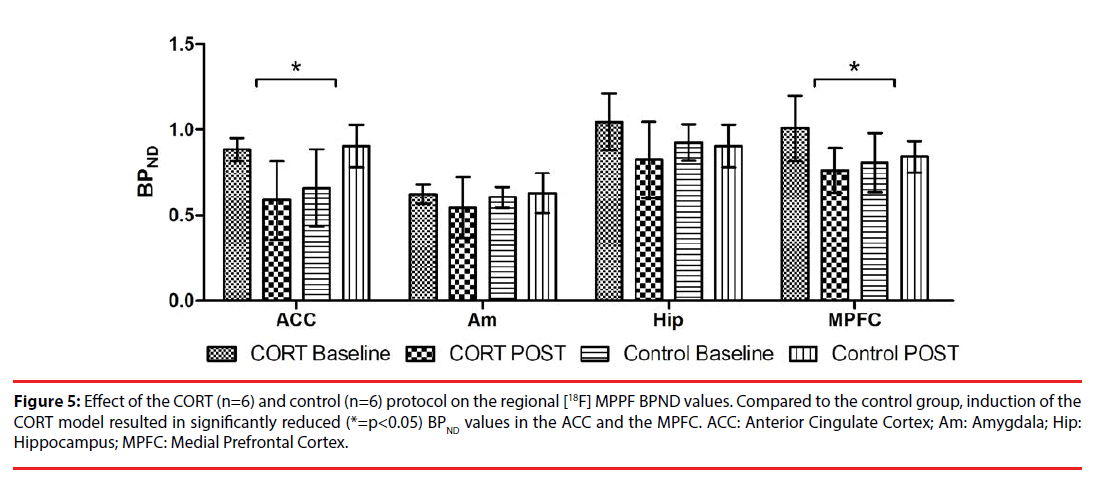
Figure 5: Effect of the CORT (n=6) and control (n=6) protocol on the regional [18F] MPPF BPND values. Compared to the control group, induction of the CORT model resulted in significantly reduced (*=p<0.05) BPND values in the ACC and the MPFC. ACC: Anterior Cingulate Cortex; Am: Amygdala; Hip: Hippocampus; MPFC: Medial Prefrontal Cortex.
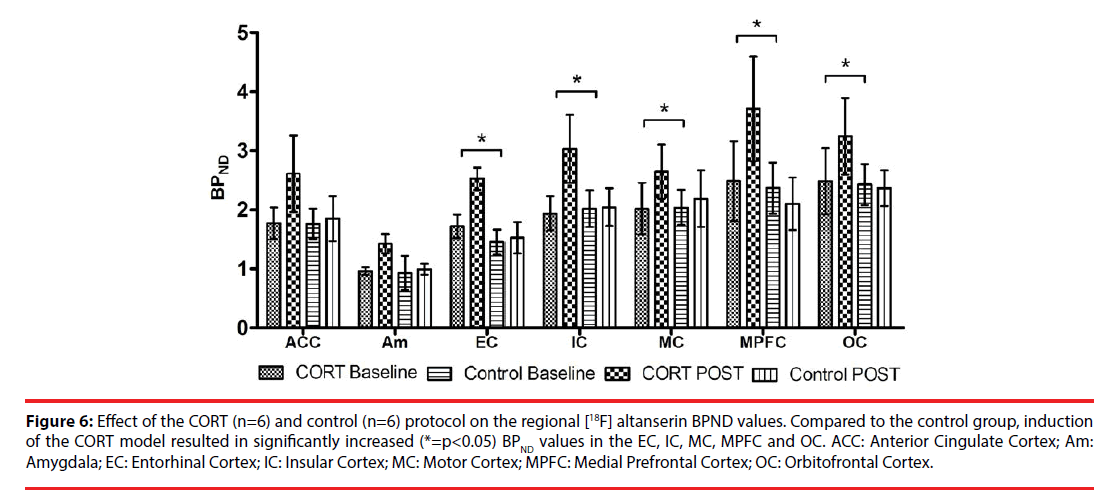
Figure 6: Effect of the CORT (n=6) and control (n=6) protocol on the regional [18F] altanserin BPND values. Compared to the control group, induction of the CORT model resulted in significantly increased (*=p<0.05) BPND values in the EC, IC, MC, MPFC and OC. ACC: Anterior Cingulate Cortex; Am: Amygdala; EC: Entorhinal Cortex; IC: Insular Cortex; MC: Motor Cortex; MPFC: Medial Prefrontal Cortex; OC: Orbitofrontal Cortex.
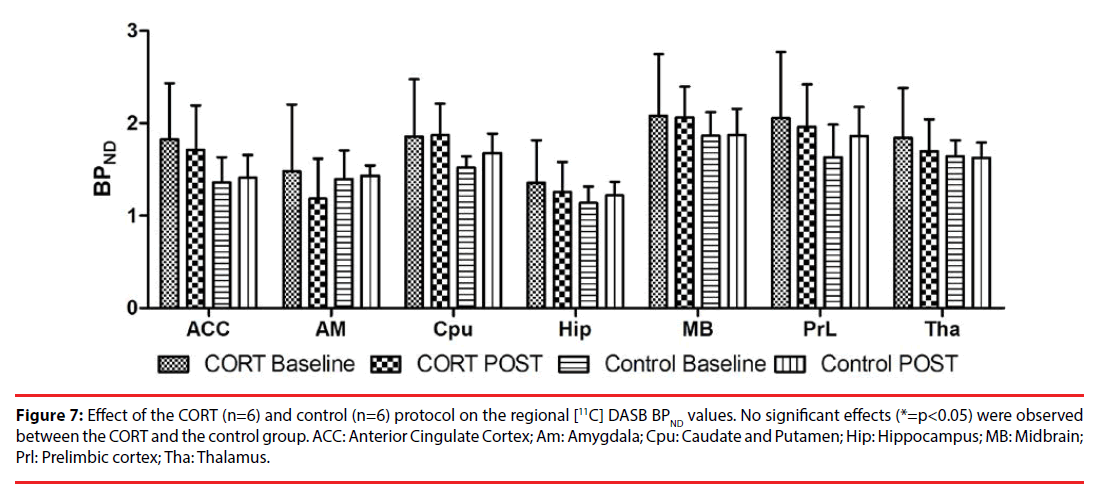
Figure 7: Effect of the CORT (n=6) and control (n=6) protocol on the regional [11C] DASB BPND values. No significant effects (*=p<0.05) were observed between the CORT and the control group. ACC: Anterior Cingulate Cortex; Am: Amygdala; Cpu: Caudate and Putamen; Hip: Hippocampus; MB: Midbrain; Prl: Prelimbic cortex; Tha: Thalamus.
At the 5-HT1A receptor level, induction of the CORT model resulted in a significantly decreased BPND of [18F] MPPF in the ACC (p=0.005, 95% CI [-0.778, -0.148]) and in the medial prefrontal cortex (p=0.032, 95% CI [-0.549, -0.026]). A tendency towards a decreased BPND was also observed in the hippocampus, however, this effect missed significance (p=0.059, 95% CI [-0.532, 0.010]). No significant effect was found in the amygdala (p=0.541, 95% CI [-0.240, 0.127]).
At the 5-HT2A receptor level, induction of the CORT model significantly increased the [18F] altanserin binding potential in all except one of the investigated cortical regions: the entorhinal cortex (p<0.001, 95% CI [0.569, 1.792]), the insular cortex (p=0.007, 95% CI [0.309, 1.907]), the medial prefrontal cortex (p=0.015, 95% CI [0.291, 2.624]), the motor cortex (p=0.011, 95% CI [0.224, 1.632]), and the orbitofrontal cortex (p=0.022, 95% CI [0.152, 1.882]). No significant treatment effect was observed in the ACC (p=0.212, 95% CI [-1.254, 0.282]) and in the amygdala (p=0.525, 95% CI [-0.260, 0.505]).
For the serotonin transporter, induction of the CORT model resulted in a trend towards an increased BPND of [11C] DASB in the ACC (p=0.055, 95% CI [-1.388, 0.015]), however, this effect missed significance (Figure 7). No significant differences in regional BPND values were observed in the remaining VOIs: amygdala (p = 0.366, 95% CI [-1.042, 0.388]), caudate and putamen region (p=0.811, 95% CI [-0.674, 0.528]), hippocampus (p=0.136, 95% CI [-0.837, 0.116]), midbrain (p=0.804, 95% CI [-0.740, 0.370], prelimbic cortex (p=0.307, 95% CI [-1.177, 0.375]), and thalamus (p=0.536, 95% CI [-0.707, 0.370]).
▪ Correlations
For the [18F] MPPF and the [18F] altanserin subgroups, correlations between the significant treatment outcome parameters were investigated and are presented in Tables 1 and 2, respectively. At the 5-HT1A receptor level, induction of the CORT depression model resulted in a significant correlation between the [18F] MPPF binding potential in the ACC and the one in the medial prefrontal cortex (correlation coefficient 0.86, p = 0.027). Furthermore, the CORT-related effects on total body weight and plasma corticosterone levels in the [18F] MPPF subgroup were also significantly correlated (correlation coefficient - 0.88, p=0.022). No significant correlations were observed in the [18F] MPPF control group.
| [18F] MPPF | Body weight | Plasmacorticosterone | FSTimmobility | ACC | MPFC |
|---|---|---|---|---|---|
| CORT group | |||||
| Body weight | 0.022 | 0.825 | 0.102 | 0.217 | |
| Plasmacorticosterone | 0.022 | 0710 | 0.060 | 0.142 | |
| FSTimmobility | 0.825 | 0.710 | 0.356 | 0.447 | |
| ACC | 0.102 | 0.060 | 0.356 | 0.027 | |
| MPFC | 0.217 | 0.142 | 0.447 | 0.027 | |
| Control group | |||||
| Body weight | 0.845 | 0.846 | 0.615 | 0.342 | |
| Plasmacorticosterone | 0.845 | 0.514 | 0.754 | 0.964 | |
| FSTimmobility | 0.846 | 0.514 | 0.152 | 0.132 | |
| ACC | 0.615 | 0.754 | 0.152 | 0.393 | |
| MPFC | 0.342 | 0.964 | 0.132 | 0.393 | |
ACC: Anterior Cingulate Cortex; MPFC: Medial Prefrontal Cortex
Table 1: Correlations between the significant treatment outcome parameters in the [18F] MPPF CORT and control group.
| [18F]altanserin | Body weight | Plasmacorticosterone | FSTimmobility | EC | IC | MPFC | MC | OFC |
|---|---|---|---|---|---|---|---|---|
| CORT group | ||||||||
| Body weight | 0.939 | 0.223 | 0.407 | 0.734 | 0.671 | 0.517 | 0.057 | |
| Plasmacorticosterone | 0.939 | 0.612 | 0.861 | 0.633 | 0.921 | 0.312 | 0.723 | |
| FSTimmobility | 0.223 | 0.612 | 0.165 | 0.441 | 0.112 | 0.819 | 0.312 | |
| EC | 0.407 | 0.861 | 0.165 | 0.817 | 0.788 | 0.498 | 0.390 | |
| IC | 0.734 | 0.633 | 0.441 | 0.817 | 0.038 | 0.088 | 0.517 | |
| MPFC | 0.671 | 0.921 | 0.112 | 0.788 | 0.038 | 0.474 | 0.938 | |
| MC | 0.517 | 0.312 | 0.819 | 0.498 | 0.088 | 0.474 | 0.051 | |
| OFC | 0.057 | 0.723 | 0.312 | 0.390 | 0.517 | 0.938 | 0.051 | |
| Control group | ||||||||
| Body weight | 0.229 | 0.070 | 0.278 | 0.608 | 0.770 | 0.433 | 0.603 | |
| Plasmacorticosterone | 0.229 | 0.322 | 0.675 | 0.227 | 0.843 | 0.666 | 0.415 | |
| FSTimmobility | 0.070 | 0.322 | 0.719 | 0.988 | 0.769 | 0.779 | 0.772 | |
| EC | 0.278 | 0.675 | 0.719 | 0.064 | 0.875 | 0.052 | 0.006 | |
| IC | 0.608 | 0.227 | 0.988 | 0.064 | 0.609 | 0.036 | 0.038 | |
| MPFC | 0.770 | 0.843 | 0.769 | 0.875 | 0.609 | 0.574 | 0.785 | |
| MC | 0.433 | 0.666 | 0.779 | 0.052 | 0.036 | 0.574 | 0.016 | |
| OFC | 0.603 | 0.415 | 0.772 | 0.006 | 0.038 | 0.785 | 0.016 | |
EC: Entorhinal Cortex; IC: Insular Cortex; MPFC: Medial Prefrontal Cortex; MC: Motor Cortex; OFC: Orbitofrontal Cortex.
Table 2: Correlations between the significant treatment outcome parameters in the [18F] altanserin CORT and control group.
At the 5-HT2A receptor level, CORT induced alterations of the [18F] altanserin binding potential in the insular cortex were significantly correlated with the BPND alterations observed in the medial prefrontal cortex (correlation coefficient 0.84, p<0.038). Although this particular correlation was not observed in the [18F] altanserin control group, several other correlations were observed in this control group. These included a significant correlation between the [18F] altanserin BPND in the orbitofrontal cortex on the one hand and the entorhinal cortex (correlation coefficient 0.94, p=0.006), the insular cortex (correlation coefficient 0.84, p=0.038), and the motor cortex (correlation coefficient 0.89, p=0.016) on the other hand, as well as a significant correlation between the BPND in the insular cortex and the motor cortex (correlation coefficient 0.84, p=0.036).
Discussion
The current study investigated the effects of the chronic corticosterone depression model on total body weight, depressive- and anxiety-like behavior, and plasma corticosterone levels in male Long-Evans rats. Furthermore, PET was used in combination with [18F] MPPF, [18F] altanserin, and [11C] DASB to investigate the effects of this depression model on the regional availability of the serotonin 5-HT1A and 5-HT2A receptors, as well as the serotonin transporter in the brain.
The 3-week procedure of daily corticosterone or vehicle injections resulted in a normal growth curve of the rats in the control group, which comprised an increase of 13 ± 5% in total body weight [42]. Corresponding to the literature, induction of the CORT depression model resulted in a 20 ± 9% decrease in total body weight [6,7]. Next to the possibility of a reduced food intake caused by the state of depression [43], this weight loss is mainly evoked by the direct action of corticosterone, as this hormone favors the gluconeogenesis to support the nutrient requirements during periods of stress [44]. Induction of the CORT model significantly increased the plasma corticosterone levels compared to the levels observed in the control group. This further confirms the supply of subcutaneously injected corticosterone to the blood stream as well as the dysregulation of the HPA-axis [45,46].
Compared to the effects in the control group, induction of the CORT model resulted in a significantly increased immobility behavior during the FST. This indicates a depressivelike behavior and aligns with the findings of previous chronic corticosterone studies in rats [7,47-49]. The absence of a CORT-related effect on the total distance travelled during the OFT demonstrates an intact locomotor functioning. This emphasizes the results of the FST as it assures that the depressive-like behavior was not confounded by the corticosterone induced weight loss of the rats [7]. Furthermore, no CORT-related effect could be detected on the percentage of time spent in the center region of the open-field box. Taken together, these results indicate that chronic corticosterone injections cause’s depressive-like, but not anxiety-like behavior, which confirms the findings of previously published studies [6,7,50].
At the serotonin 5-HT1A receptor level, CORTrelated effects resulted in a significantly decreased [18F] MPPF binding potential in the ACC and the medial prefrontal cortex. BPND alterations in these regions were also significantly correlated in the CORT group, but not in the control group. Therefore, these findings might contribute to the hypothesis of an enhanced coupling between the salience network (which encompasses the ACC) and the (anterior) default mode network (which comprises parts of the medial prefrontal cortex) found in clinical studies [51,52]. Other CORTrelated effects included a tendency towards a decreased BPND in the hippocampus, however, this effect missed significance (p=0.059, 95% CI [-0.532, 0.010]). This lack of significance in the hippocampus is probably caused by an impaired corticosterone uptake in one of the six rats (indicated by lower post-treatment corticosterone plasma levels and a reduced weight loss), which increased the variability within the CORT group. Overall, our CORT-related in vivo imaging findings at the 5-HT1A receptor level are in line with previous ex vivo studies in rodents subjected to chronic mild stressors or to chronic corticosterone administration, where a reduced 5-HT1A receptor related serotonin response (e.g. attenuated 8-OH-DPAT hypothermia) or reduced 5-HT1A receptor binding (e.g. autoradiography with [3H]-8-OHDPAT) has been reported [53-56]. Furthermore, as indicated in the review by Savitz et al. [57], the retrospective analysis by Nikolaus et al. [58], and the meta-analysis by Wang et al. [18], a diminished density of the 5-HT1A receptors has also been repeatedly observed in patients suffering from MDD. Hereby, the effect was not limited to the ACC and (pre)frontal cortex, it was also observed in other cortical regions (e.g. insular, parietal, occipital and temporal cortex), as well as in the amygdala, the hippocampus and the raphe nuclei. A possible hypothesis for this 5-HT1A receptor downregulation is based on the activation of glucocorticoid receptors (GRs) by high concentrations of stress hormone (cortisol in humans or corticosterone in rats) and the subsequent formation of glucocorticoidmineralocorticoid receptor heterodimers. These heterodimers are transported to the nucleus where they bind to glucocorticoid response elements (GREs) on the promotor of the Htr1a gene (encoding for the 5-HT1A receptor), inhibiting its expression [8,9].
At the serotonin 5-HT2A receptor level, CORTrelated effects comprised a significantly increased [18F] altanserin BPND in multiple cortical brain regions including the entorhinal cortex, insular cortex, medial prefrontal cortex, motor cortex, and orbitofrontal cortex. Therefore, these results are indicative for a 5-HT2A receptor upregulation following dysregulation of the HPA-axis. Similar to the observations at the 5-HT1A receptor level, a significant correlation between the alterations of the [18F] altanserin binding potential in the medial prefrontal cortex and the ones in the insular cortex (also part of the salience network) was observed for the rats in the CORT group, but not for those in the control group. Overall, our in vivo imaging findings at the 5-HT2A receptor level are consistent with previous ex vivo studies in rats, which reported an increased 5-HT2A receptor binding in the neocortex [59] or the parietal cortex [60] of rats following chronic corticosterone treatment as well as an enhanced cortical 5-HT2A expression following chronic mild stressors [61]. Human PET studies mention both increased and decreased 5HT2A receptor densities in the cortico-limbic regions in patients with MDD, although some of these inconclusive PET results are possibly related to differences in therapy resistance level of the included patients [62], or to the presence of confounders such as remaining effects of antidepressant therapies, alcohol abuse and obesity [63]. In contrast, clinical imaging studies with selective radiotracers in MDD patients that were not recently medicated [19,64], as well as studies based on post-mortem findings in suicide victims [65,66], are carefully converging to support an association between 5-HT2A receptor upregulation and MDD [23]. This increase has been particularly observed in the frontal cortical regions (Brodmann area 8, 9 and 10), but has also been found in the parietal or occipital cortex [14,19,20,63,67]. However, it cannot be ruled out that this receptor upregulation is only present in a subgroup of MDD patients, particularly those with a dysregulation of the HPA axis, observed in 43% (or 64% when over 60 years of age) of the patients suffering from MDD [5]. A direct relationship between glucocorticoid receptor activation and increased 5-HT2A receptor levels was reported by Trajkowska et al. [10] using mature organotypic hippocampal cell cultures derived from rats. While a seven-day exposure of this culture to 3 μM corticosterone significantly increased the 5-HT2A receptor levels, this receptor upregulation was blocked by addition of the GR receptor antagonist mifepristone.
Except for a trend towards a reduced [11C] DASB binding potential in the ACC, no significant CORT-related effects were observed at the level of the serotonin transporter. This can be partially explained by the large individual variabilities in the groups, as both increases and decreases up to more than 50% of the baseline SERT availability have been observed before and after the three-week protocol. Compared to other studies in rodents, contradictory findings have been reported concerning the effects of chronic corticosterone administration or chronic mild stress on SERT availability. While Fernandes et al. [60] reported an increased hippocampal SERTavailability after one week of corticosterone administration, Lopez et al. [11] observed no SERT-related effect in the hippocampus after a two-week exposure to chronic mild stress, and Tang et al. reported a decrease in hippocampal SERT expression after stress induced anhedonia [68]. Furthermore, no SERT-related effect was observed in the raphe nuclei after administration of glucocorticoid receptor agonists in rats [69], and mice susceptible to the chronic mice stress procedure revealed an increased expression of SERT in the prefrontal area [70]. While SERTrelated discrepancies between clinical study results can be partially explained by the presence of confounding variables such as the patient’s clinical phenotype of depression or the patient’s medication status and history, further research is required to investigate and elucidate the high variability in preclinical studies.
A limitation of this study is the relative small number of subjects that was included in each subgroup (N=6). It’s possible that this study was underpowered in order to detect additional significant SERT-related differences. Additional multi-centre studies might help to achieve sufficient statistical power to detect small alterations in neurotransmitter systems or other neurobiological changes.
Conclusion
This extended study with three radioligands emphasizes the potential influence of a dysregulated HPA-axis, observed in 43% of the patients suffering from MDD, on the serotonin system. In summary, downregulation of the 5HT1A receptors in the ACC and medial prefrontal cortex, and a widespread up-regulation of the 5HT2A receptors in the cortex were found following chronic CORT treatment. Furthermore, although additional research is required to explore the role of the serotonin transporter in this depression model, the current results indicate that corticosterone depression model is a relevant and reliable model to investigate the mode of action in current and novel antidepressant therapies on the serotonin 5-HT1A and 5-HT2A receptors in the brain.
Acknowledgements
The authors would like to thank Marlies Vanthournout, Mona Bové and Lynn Marchand for their assistance with the experiments.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edthn (2013).
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br. Med. Bull 65(1), 193-207 (2003).
- Herman JP, Mcklveen JM, Ghosal S, et al. Regulation of the Hypothalamic‐Pituitary‐Adrenocortical Stress Response. Compr. Physiol 6(1), 603‐621 (2016).
- Aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. Can. Med. Assoc. J 180(3), 305-313 (2009).
- Varghese FP, Brown ES. The Hypothalamic-Pituitary-Adrenal Axis in Major Depressive Disorder: A Brief Primer for Primary Care Physicians. Prim. Care. Companion. J. Clin. Psychiatry 3(4), 151-155 (2001).
- Gregus A, Wintink AJ, Davis AC, et al. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav. Brain. Res 156(1), 105-114 (2005).
- Kott JM, Mooney-Leber SM, Shoubah FA, et al. Effectiveness of different corticosterone administration methods to elevate corticosterone serum levels, induce depressive-like behavior, and affect neurogenesis levels in female rats. Neuroscience 312(1), 201–214 (2016).
- Savitz J, Lucki I, Drevets WC. 5-HT1A receptor function in major depressive disorder. Prog. Neurobiol 88(1), 17-31 (2009).
- Ou X-M, Storring JM, Kushwaha N, et al. Heterodimerization of Mineralocorticoid and Glucocorticoid Receptors at a Novel Negative Response Element of the 5-HT1A Receptor Gene. J. Biol. Chem 276(1), 14299-14307 (2001).
- Trajkovska V, Kirkegaard L, Krey G, et al. Activation of glucocorticoid receptors increases 5-HT2A receptor levels. Exp. Neurol 218(1), 83-91 (2009).
- Lopez JF, Chalmers DT, Little KY, et al. Mineralocorticoid Receptor in Rat and Human Hippocampus : Implications for the Neurobiology of Depression. Psychoneuroendocrinology 1998(1), 3223 (1998).
- Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol. Psychiatry 12(1), 331-359 (2007).
- Pletscher A, Laubscher A. Blood platelets as models for neurons: uses and limitations. J. Neural. Transm. Suppl 16(1), 7-16 (1980).
- Cryan JF, Leonard BE. 5-HT(1A) and beyond: The role of serotonin and its receptors in depression and the antidepressant response. Hum. Psychopharmacol 15(1), 113-135 (2000).
- Sullivan GM, Oquendo MA, Huang Y, et al. Elevated cerebrospinal fluid 5-hydroxyindoleacetic acid levels in women with comorbid depression and panic disorder. Int. J. Neuropsychopharmacol 9(5), 547-556 (2006).
- Sekiduka-Kumano T, Kawayama T, Ito K, et al. Positive association between the plasma levels of 5-hydroxyindoleacetic acid and the severity of depression in patients with chronic obstructive pulmonary disease. BMC. Psychiatry 13(1), 159 (2013).
- Arango V, Underwood MD, Boldrini M, et al. Serotonin 1A Receptors, Serotonin Transporter Binding and Serotonin Transporter mRNA Expression in the Brainstem of Depressed Suicide Victims. Neuropsychopharmacology 25(6), 892-903 (2001).
- Wang L, Zhou C, Zhu D, et al. Serotonin-1A receptor alterations in depression : a meta-analysis of molecular imaging studies. BMC. Psychiatry 16(319), 1-9 (2016).
- Bhagwagar Z, Hinz R, Taylor M, et al. Increased 5-HT 2A Receptor Binding in Euthymic, Medication-Free Patients Recovered From Depression: A Positron Emission Study With [11C] MDL 100,907. Am. J. Psychiatry 163(9), 1580-1587 (2006).
- Meyer JH, McMain S, Kennedy SH, et al. Dysfunctional Attitudes and 5-HT 2 Receptors During Depression and Self-Harm. Am. J. Psychiatry 160(1), 90-99 (2003).
- Gryglewski G, Lanzenberger R, Kranz GS, et al. Meta-analysis of molecular imaging of serotonin transporters in major depression. J. Cereb. Blood. Flow. Metab 34(7), 1096-1103 (2014).
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 64(3), 327-337 (2007).
- Ruhé HG, Visser AKD, Frokjaer VG, et al. Molecular Imaging of Depressive Disorders. PET SPECT Psychiatry, Berlin, Heidelberg (2014).
- Brummelte S, Galea LAM. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience 168(3), 680-90 (2010).
- Levinstein MR, Samuels B. Mechanisms underlying the antidepressant response and treatment resistance. Front. Behav. Neurosci 8(1), 208 (2014).
- Abelaira HM, Réus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Rev. Bras. Psiquiatr 35(S2), S112–120 (2013).
- Morme P. Stress and Emotionality : a Multidimensional and Genetic Approach. Neurosci. Biobehav. Rev 22(1), 33-57 (1998).
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents : recent developments and future needs. Trends. Pharmacol. Sci 23(5), 238-245 (2002).
- Hayashi K, Furutsuka K, Ito T, et al. Fully automated synthesis and purification of Fully automated synthesis and purification of 4‐(2′‐methoxyphenyl)‐1‐[2′‐(N‐2″‐pyridinyl)‐p [18F] fluorobenzamido] ethylpiperazine. J. Labelled. Comp. Radiopharm 2012(1), 6-7 (2012).
- Massarweh GÃ, Kovacevic M, Evans AC, et al. Time-efficient and convenient synthesis of [ 18 F ] altanserin for human PET imaging by a new work-up procedure. Appl. Radiat. Isot 67(1), 2040-2043 (2009).
- Laeken NV, Kersemans K, De Meestere D, et al. Improved HPLC purification strategy for [ 11 C ] raclopride and [11 C] DASB leading to high radiochemical yields and more practical high quality radiopharmaceutical formulations. Appl. Radiat. Isot 78(1), 62-7 (2013).
- Paxinos H, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th edtn. (2007).
- Wu Y, Carson RE. Noise Reduction in the Simplified Reference Tissue Model for Neuroreceptor Functional Imaging. J. Cereb. Blood. Flow. Metab 22(12), 1440-1452 (2002).
- Costes N, Zimmer L, Reilhac A, et al. Test – Retest Reproducibility of 18 F-MPPF PET in Healthy Humans : A Reliability Study. J. Nuclear. Med 48(8), 1279-1289 (2007).
- Millet P, Moulin M, Bartoli A, et al. In vivo quantification of 5-HT1A-[18F] MPPF interactions in rats using the YAP-(S)PET scanner and a beta-microprobe. Neuroimage 41(3), 823-834 (2008).
- Kroll T, Elmenhorst D, Matusch A, et al. Suitability of [18F]altanserin and PET to determine 5-HT2A receptor availability in the rat brain: in vivo and in vitro validation of invasive and non-invasive kinetic models. Mol. Imaging. Biol 15(4), 456-467 (2013).
- Riss PJ, Hong YT, Williamson D, et al. Validation and quantification of [18F]altanserin binding in the rat brain using blood input and reference tissue modeling. J. Cereb. Blood. Flow. Metab 31(12), 2334-2342 (2011).
- Meyer JH. Review paper Examen critique Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J. Psychiatry. Neurosci 32(2), 86-102 (2007).
- Beliveau V, Svarer C, Frokjaer VG, et al. Functional connectivity of the dorsal and median raphe nuclei at rest. Neuroimage 116(1), 187-195 (2015).
- Frankle WG, Slifstein M, Gunn RN, et al. Estimation of serotonin transporter parameters with 11C-DASB in healthy humans: reproducibility and comparison of methods. J. Nucl. Med 47(5), 815-826 (2006).
- Hoekzema E, Rojas S, Herance R, et al. [11 C] -DASB microPET imaging in the aged rat : Frontal and meso-thalamic increases in serotonin transporter binding. Exp. Gerontol 46(12), 1020-1025 (2011).
- Charles river - Long-Evans rats n.d. http://www.criver.com/products-services/basic-research/find-a-model/long-evans-rat.
- Tucci V. Handbook of neurobehavioral genetics and phenotyping. n.d.
- Stephens MAC, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol. Res 34(4), 468-483 (2012).
- Bundgaard C, Larsen F, Jørgensen M, et al. Pharmacokinetic / Pharmacodynamic Feedback Modelling of the Functional Corticosterone Response in Rats after Acute Treatment with Escitalopram. Basic. Clin. Pharmacol. Toxicol 100(3), 182-189 (2007).
- Scherer IJ, Holmes PV, Harris RBS. The importance of corticosterone in mediating restraint-induced weight loss in rats. Physiol. Behav 102(2), 225-233 (2011).
- Fenton EY, Fournier NM, Lussier AL, et al. Imipramine protects against the deleterious effects of chronic corticosterone on depression-like behavior, hippocampal reelin expression, and neuronal maturation. Prog. Neuro-Psychopharmacology. Biol. Psychiatry 60(1), 52-59 (2015).
- Johnson SA, Fournier NM, Kalynchuk LE. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Beha. Brain. Res 168(2), 280-288 (2006).
- Sterner EY, Kalynchuk LE. Progress in Neuro-Psychopharmacology & Biological Psychiatry Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats : Relevance to depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 34(5), 777-790 (2010).
- Demuyser T, Deneyer L, Bentea E, et al. In-depth behavioral characterization of the corticosterone mouse model and the critical involvement of housing conditions. Physiol. Behav 156(1), 199-207 (2016).
- Jennings JR, Sheu LK, Kuan DC-H, et al. Resting state connectivity of the medial prefrontal cortex covaries with individual differences in high-frequency heart rate variability. Psychophysiology 53(4), 444–454 (2016).
- Mulders PC, van Eijndhoven PF, Schene AH, et al. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev 56(1), 330-344 (2015).
- Watanabe Y, Sakai RR, Mcewen BS, et al. Stress and antidepressant effects on hippocampal and cortical 5 - H T I A and 5-HT 2 receptors and transport sites for serotonin. 61(5), 87-94 (1998).
- McAllister-Williams RH, Anderson AJ, Young AH. Corticosterone selectively attenuates 8-OH-DPAT-mediated hypothermia in mice. Int. J. Neuropsychopharmacol 4(1), 1-8 (2001).
- van Riel E, Meijer OC, Steenbergen PJ, et al. Chronic unpredictable stress causes attenuation of serotonin responses in cornu ammonis 1 pyramidal neurons. Neuroscience 120(3), 649-658 (2003).
- Savitz J, Lucki I, Drevets WC. Progress in Neurobiology 5-HT 1A receptor function in major depressive disorder. Prog. Neurobiol 88(1), 17-31 (2009).
- Savitz JB, Drevets WC. Neuroreceptor imaging in depression. Neurobiol. Dis 52(1), 49-65 (2013).
- Nikolaus S, Hautzel H, Heinzel A, et al. Key players in major and bipolar depression—A retrospective analysis of in vivo imaging studies. Behav. Brain. Res 232(2), 358-390 (2012).
- Kuroda Y, Mikuni M, Ogawa T, et al. Effect of ACTH, adrenalectomy and the combination treatment on the density of 5-HT2 receptor binding sites in neocortex of rat forebrain and 5-HT2 receptor-mediated wet-dog shake behaviors. Psychopharmacology 108(1-2), 27-32 (1992).
- Fernandes C, McKittrick CR, File SE, et al. Decreased 5-HT(1A) and increased 5-HT(2A) receptor binding after chronic corticosterone associated with a behavioural indication of depression but not anxiety. Psychoneuroendocrinology 22(7), 477-491 (1997).
- Ossowska G, Nowak G, Kata R, et al. Brain monoamine receptors in a chronic unpredictable stress model in rats. J. Neural. Transm 108(3), 311-319 (2001).
- Baeken C, De Raedt R, Bossuyt A, et al. The impact of HF-rTMS treatment on serotonin2A receptors in unipolar melancholic depression. Brain. Stimul 4(2), 104-111 (2011).
- Savitz JB, Drevets WC. Neurobiology of Disease Neuroreceptor imaging in depression. Neurobiol. Dis 52(1), 49-65 (2013).
- Meyer JH, McMain S, Kennedy SH, et al. Dysfunctional Attitudes and 5-HT 2 Receptors During Depression and Self-Harm. Am. J. Psychiatry 160(1), 90-99 (2003).
- Arango V, Underwood MD, Mann JJ. Postmortem findings in suicide victims. Implications for in vivo imaging studies. Ann. N. Y. Acad. Sci 836(1), 269-287 (1997).
- Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J. Psychiatr. Res 37(5), 357-373 (2003).
- Shelton RC, Sanders-bush E. Elevated 5-ht 2a receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience 158(4), 1406-1415 (2009).
- Tang M, Lei J, Sun X, et al. Stress-induced anhedonia correlates with lower hippocampal serotonin transporter protein expression. Brain. Res 151(3), 127-134 (2013).
- Kuroda Y, Watanabe Y, Albeck DS, et al. Effects of adrenalectomy and type I or type II glucocorticoid receptor activation on 5-HT1A and 5-HT2 receptor binding and 5-HT transporter mRNA expression in rat brain. Brain. Res 648(1), 157-161 (1994).
- Couch Y, Anthony DC, Dolgov O, et al. Microglial activation, increased TNF and SERT expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain. Behav. Immun 29(3), 136-146 (2013).