Research Article - Clinical Investigation (2022) Volume 12, Issue 2
The Significant Role of Weekly Taxol for a Stable Disease and a Higher Progression-Free Interval in Recurrent Gynecological Malignancies: A Large Retrospective Monocentric Study
- Corresponding Author:
- Rosalba De Nola
Department of Biomedical and Human Oncological Science (D.I.M.O)
Division of Obstetrics and Gynecology, University of Bari “Aldo Moro”, Bari, Italy
E-mail: rosalba.denola@uniba.it
Received: 07-February-2022, Manuscript No. fmci-22-53782; Editor assigned: 10- February-2022, PreQC No. fmci-22-53782 (PQ); Reviewed: 21-Febuary-2022, QC No. fmci-22-53782 (Q); Revised: 23-Februay-2022, Manuscript No. fmci-22-53782 (R); Published: 28-February-2022, DOI: 10.37532/2041-6792.2022.12(2).13-22
Abstract
This is a monocentric retrospective study (N 96): All the patients had relapsed gynecological cancer (ovarian, peritoneal, endometrial, cervical) and received weekly taxol as salvage therapy. Platinum resistance and platinum partially-sensitivity were associated with a higher odds ratio to die, whereas the squamous histotype was characterized by higher survival rates. Cervical cancer predicted a higher progression-free interval. A number of previous chemotherapies ≤ 3 predicted a lower survival. The toxicity profile was acceptable with leucopenia (12.6%) and anemia (18.4%), rarely of grade 3. Weekly taxol is a valuable rescue therapy in relapsed advanced gynecological tumors with a good response outcome.
Keywords
Weekly taxol • Ovary • Cervix • Endometrium • Relapse
Introduction
One of the most concerning issues in the management of gynaecological cancer is the therapy of the recurrences. Despite the large use of weekly taxol, little data are available about its application as rescue therapy in case of recurrences, especially after multiple lines of previous chemotherapy. Therefore, we focused on the role of weekly taxol in the salvage treatment of recurrent gynaecological malignancies (ovary, endometrium, cervix, and peritoneum), evaluating the toxicity, the global response, the stable disease, the progression-free interval, and the overall survival. The present article analyzed the data taken from an observational retrospective monocentric study conducted at the Onco-gynecological Unit of the Obstetrics and Gynecology Division of the Policlinico of Bari (Italy).
Methods and Materials
In this paper, we reported the results of an observational retrospective monocentric study conducted at the Oncogynecological Unit of the Obstetrics and Gynecology Division of the Policlinico of Bari (Italy). The population under study had recurrent gynaecological malignancies (ovary, endometrium, cervix, and peritoneum) and underwent chemotherapy with weekly taxol (60 mg/m2-80 mg/m2) at our clinic in a global range of time from October 1994 to November 2020, counting for 96 women. The time of the first diagnosis covered an interval from October 1992 to July 2019. The data were collected and analyzed anonymously after informed consent acquisition at the first access to our hospital from all individual participants included in the study.
Statistical analysis
Most of the variables were complete, except for platinum sensitivity, histology, stable disease, stage, grading, and Progression-Free Interval (PFI) that registered respectively 6.25%, 10.41%, 44.79%, 8.33%, 16.66%, and 30.20% of missing data. Statistical analyses were performed using the R statistical environment (The R Foundation for Statistical Computing; Vienna, Austria), specifically the packages “fBasics”, “graphics”, “corrplot”, “lawstat”, “gmodels”, “survival” and “carData” [1-6].
Among all the quantitative variables, age at the diagnosis and age at the first taxol were normally distributed (Shapiro- Wilk test). Whereas, PFI, number of weekly taxols, months of follow-up, months of survival followed a non-normal distribution. Only age was homoscedastic (Bartlett test, modified robust Brown-Forsythe Levene-type test).
The categorical variables were illustrated as frequencies (%), whereas the statistical descriptive data were reported as mean ± standard deviation in the case of normal distribution and homoscedasticity. In absence of normal distribution and homoscedasticity, the variables were shown as a median and Interquartile Range (IQR). Due to the absence of homoscedasticity and normality, differences between more than two groups were studied with the nonparametric Kruskal-Wallis test, also known as the one-way analysis of variance (ANOVA) on ranks. Since the variable age at diagnosis and age at first taxol were normal and homoscedastic, we used also a one-way Analysis of Variance (ANOVA) in comparing differences between groups. Correlations between numerical variables were evaluated by Spearman’s coefficient using the R package “corrplot”. The relationship between categorical factors (i.e., histotypes and FIGO stage) was evaluated with a chi-squared test (package “gmodels”). Plots and graphs were realized using the R packages “graph”, “ggplot”, and “survival”. The independent predictors for a higher PFI were evaluated with linear regression analysis. Possible confounding factors were tested in univariate regression analysis and then used in multivariate linear regression analysis. A two-sided p-value <0.05 was considered to indicate statistical significance.
Kaplan-Meier curves comparing patients with different platinum-response and the number of previous chemotherapies is shown from the time of the diagnosis up to 166 months of follow-up. The log-rank test was used to test the differences between the survival curves. Cox proportional hazards regression was used for the odds ratios and 95% Confidence Intervals (CI) for the risk factors. The covariates in the case of different platinum responses were, as follows: histotype, number of weekly taxol cycles, and progression at the fourth month since the weekly taxol therapy began.
Kaplan-Meier curves comparing patients with different histotypes are shown from the time of the diagnosis up to 166 months of follow-up and from the beginning of taxol to the end of follow-up. The log-rank test was used to test the differences between the survival curves. Cox proportional hazards regression was used to calculate the hazard ratios and 95% Confidence Intervals (CI) for the risk factors. The covariates were histotypes and histological grading.
Results
The population under examination was mainly composed of postmenopausal women with a mean age of 59.00 ± 11.04 years (at first taxol) suffering from gynaecological tumours on weekly taxol therapy. The median number of weekly taxol was 14 (IQR 14), while the median of previous chemotherapy was 3 (IQR 1.25). Undoubtedly, the most frequent disease was ovarian cancer covering a percentage of 79.16%. Between the four types of cancer (ovary, endometrium, cervix, and peritoneum) there was a highly significant difference in the age of the patients (one-way ANOVA p 0.002) [Table 1].
| Total N=96 | Primitive site tumour | |||||
|---|---|---|---|---|---|---|
| Ovary | Endometrium | Cervix | Peritoneum | |||
| 79.16% | 9.37% | 6.25% | 5.20% | |||
| Age (diagnosis) | 55.68 ±11.65 | 54.08 ± 11.63 | 68.67 ± 5.96 | 51.33 ± 5.16 | 59.00 ± 7.57 | p=0.002 |
| Age (1st taxol) | 59.00 ± 11.05 | 57.55 ± 11.03 | 71.11 ± 5.99 | 54.17 ±4.79 | 65 ± 8.12 | p=0.001 |
| Histotype | Serous | Clear cells | Müllerian | Squamous | Endometrioid | Anaplastic |
| 72.22% | 5.55% | 1.11% | 6.66% | 13.33% | 1.11% | |
| Grading | I-7.79 % | II-15.58 % | III-76.62 % | |||
| Stage | I-5.68 % | II-9.09 % | III-67.04 % | IV-18.18 % | ||
| Histotype | FIGO STAGE | p=0.0002 | ||||
| I | II | III | IV | |||
| Serous | 0% | 5% | 58% | 10% | ||
| Clear cell | 1% | 0% | 4% | 1% | ||
| MMMT | 0% | 0% | 0% | 1% | ||
| Squamous | 2% | 2% | 2% | 0% | ||
| Endometrioid | 2% | 2% | 4% | 4% | ||
| Anaplastic | 0% | 0% | 1% | 0% | ||
Table 1. Main characteristics of the population under study. Age is indicated as mean ± SD and the p-value of the one-way ANOVA test according to the site of the primitive tumour was made explicit. The factorial variables are represented as integer numbers and frequency (%). The Fisher’s Exact Test was used to compare the different proportions of histotypes among the FIGO stages
The stage of the disease was assigned according to the Fédération Internationale de Gynécologie et d’Obstétrique (FIGO), 2009/2014/2017 respectively for endometrium, ovarian and peritoneum, cervical cancer [7-9].
Two-thirds of the tumours showed serous histology and a low degree of differentiation (G3). Notably, 85.22% of the population under weekly taxol had a diagnosis of advanced cancer (FIGO stage III and IV, 67.04% and 18.18%, respectively). In detail, squamous, endometrioid, and clear cell histotypes were diagnosed also at early stages. Serous tumours were diagnosed mainly in advanced stages (68% III and IV stages).
Proportions of histo-types across all FIGO stages were significantly different (Fisher’s Exact Test, p=0.0002), as summarized in table 1.
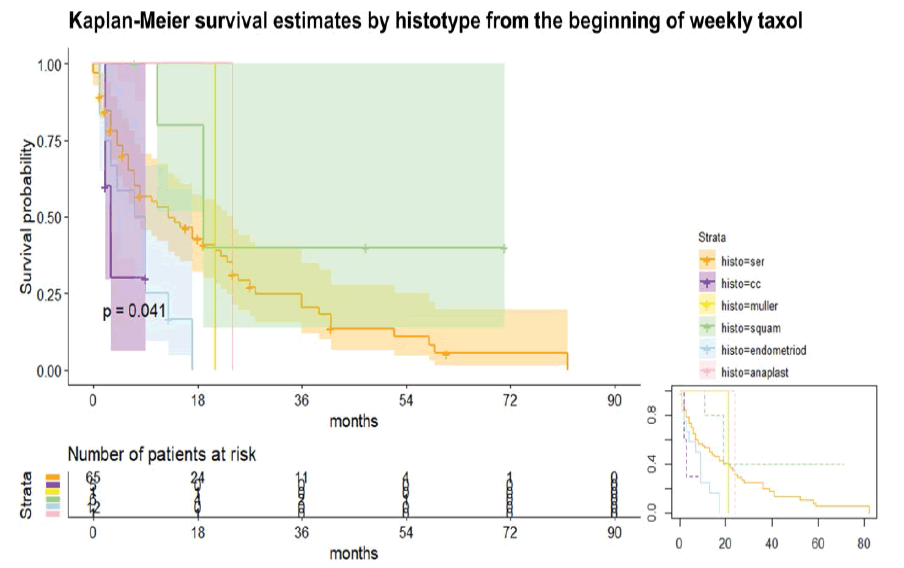
The Kaplan-Meier curve estimated different survival depending on histotypes (log-rank test p<0.05), respectively from the beginning of weekly taxol and the diagnosis (not shown). The histotypes were six, as follows:
• Serous
• Clear Cell (CC)
• Malignant Mixed Müllerian Tumor (MMMT)
• Squamous
• Endometrioid
• Anaplastic (see table 1) [Figure 1]
Considering the timeline starting from the beginning of taxol (figure 1), the RR to die of CC is 2.28 higher than serous, whereas the RR to die of serous is 2.42 higher than squamous. The HR of the different histotypes used the serous histotypes as a comparison. Endometrioid histotype is characterized by a higher RR to die, in detail 2.7 and 5.02 compared to serous and squamous respectively. Notably, CC had a Hazard Ratio (HR) to die of 2.73 (95% CI 0.81, 9.15), whereas the endometrioid had HR to die of 2.37 (95% CI 1.19, 4.72). The squamous had the lowest HR: 0.40 (95% CI 0.12, 1.28). Note that the comparison was made to the serous histotype. The MMMT and anaplastic had similar HR than serous, considered as 1 from the Cox regression model (p<0.05).
Similar results were found in the case of Overall Survival (OS) estimated on histotypes (figure 1) from the time of diagnosis. At the Cox regression (p<0.05), the CC histotypes had the highest HR to die (4.37, 95% CI 1.29, 14.75) followed by endometrioid with an HR of 2.29 (95% CI 1.16, 4.55). Same results for MMMT and anaplastic, approximately 1, as the serous histotypes. Surprisingly, we have demonstrated the crucial role of weekly taxol in “rescue therapy” for major gynaecological malignancies. In detail, we found extremely high significant differences in the number of weekly taxol between patients with stable disease and patients with disease progression during chemotherapy: The stable disease group (PR, CR, and/or SD ≥ 3 months) had a significantly higher median number of weekly taxol than the non-stable disease group (PD or SD<3 months). In detail, they respectively had a median number of 18 (IQR 15,5) vs 8 (IQR 10) taxol cycles (Wilcoxon rank-sum test, p=0.0002).
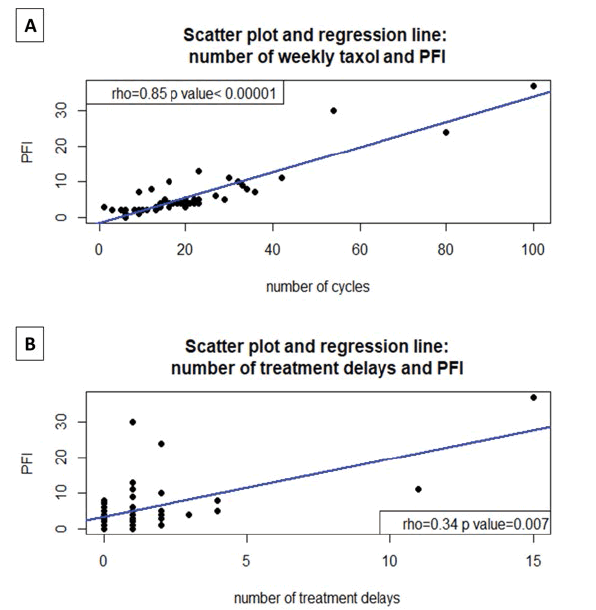
Furthermore, the number of weekly cycles of taxol had an extremely high significant positive correlation with the progression-free interval (PFI), Spearman’s correlation index (rho)=0.85, p<0.00001 [Figure 2A] .
Figure 2: A: Scatter Plot: the extremely high significant positive correlation between the number of weekly taxol cycles and the Progression-Free interval (PFI), Spearman correlation index (rho)=0.85, p<0.00001; B: Scatter Plot the extremely high significant positive correlation between the number of treatment delays and the Progression-Free interval (PFI), Spearman correlation index (rho) =0.34, p<0.007
Notably, the PFI correlated positively also with the number of treatment delays, rho=0.34 (p=0.007), as shown in figure 2B. PFI was expressed in months of progressionfree survival at serum Ca125 and/or advanced imaging techniques (CT, PET, MRI) according to Gynecological Cancer Intergroup (GCIG) criteria and RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1 and starting from the beginning of weekly taxol [10-13].
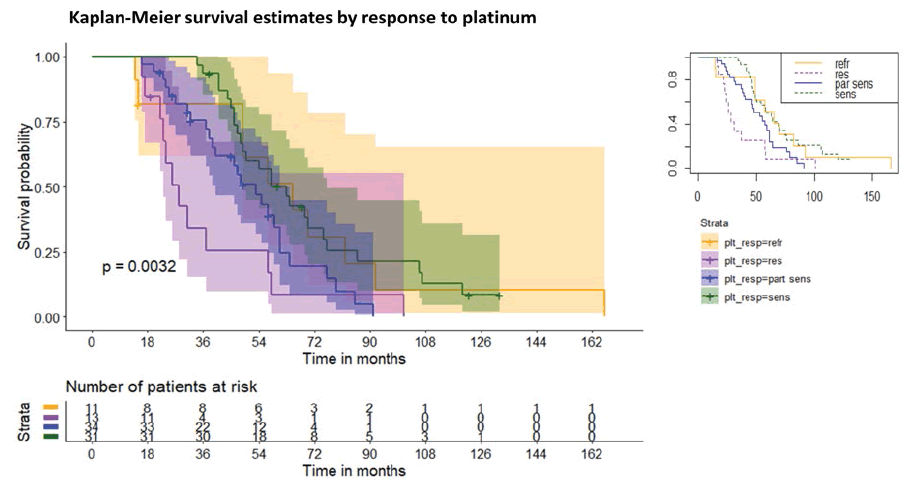
The mean follow-up was 56.40 ± 36.25 months, ranging from a minimum of 14 months to a maximum of 183 months. During the follow-up, 79 patients died and 17 were still alive at the end of the follow-up. We investigated the possible influence of the platinum response before weekly taxol administration on subsequent survival. Therefore, four groups were identified and were composed as follows:
• Platinum-refractory patients (progression during platinum therapy or within 4-6 weeks after the last platinum)
• Platinum-resistant patients (relapse before 6 months from the last platinum)
• Partially sensitive platinum patients (relapse between 6 and 12 months from the last platinum)
• Platinum-sensitive patients (relapse after 12 months from the last platinum) according to the classical classification of platinum response [14,15].
Surprisingly, the KM curves of groups 1 and 4, after an initial decrease in the survival rate in group 1 at 50 months of follow-up, almost overlapped. Overall, however, the Relative Risk (RR) of dying for group 1 compared to group 4 is greater than 1.25. The four groups have a survival trend and have a statistically significant difference (log-rank test p=0.0032), see figure 3.
Therefore, the presence of platinum resistance (group 2) or partial platinum sensitivity (group 3) can be considered a risk factor for death in patients receiving weekly taxol therapy. A subsequent multifactorial Cox regression (p<0.00001) adjusted hazard ratios (HRs) for survival based on platinum sensitivity and confirmed the presence of platinum resistance (group 2) or partial platinum sensitivity (group 3) can be considered a risk factor for death in patients receiving weekly taxol regardless of histotype, number of weekly taxol cycles and progression to the fourth month after starting weekly taxol therapy. Compared to the group 1, the adjusted HR were, respectively for 2,3 and 4, as follows: 2.84 (95% CI 0.49, 16.33), 2.19 (95% CI 0.43, 11.06), 0.21 (95% CI 0.04, 1.17).
The multivariate linear regression analysis (number of taxol cycles, OS in months, and tumor type) showed that only the number of weekly taxol cycles (OR 0.320 (95% CI: 0.258; 0.382), p<0.00001) can be considered an independent predictor of a major PFI (Table 2).
| Univariate and Multivariate Analysis of Linear | ||||
|---|---|---|---|---|
| Regression Illustrating The Predictors of PFI | ||||
| Variables | Univariate | Multivariate | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Clear cell | -3.021 (-8.455,2.412) | 0.27 | -0.292 (-3.803,3.218) | 0.8 |
| MMMT | -1.02 (-11.564,9.521) | 0.84 | -1.219 (-6.814,4.375) | 0.66 |
| Endometrioid | -1.221 (-6.129, 3.68) | 0.62 | -1.522 (-4.971,1.927) | 0.37 |
| Anaplastic | -1.021 (-11.564, 9.521) | 0.84 | -1.509 (-7.106,4.088) | 0.59 |
| N.Taxol Cycles | 0.355 (0.314,0.396) | <0.00001 | 0.320 (0.258,0.382) | <0.00001 |
| Survival (months) | 0.068 (0.027,0.108) | 0.001 | 0.015 (-0.008 ,0.038) | 0.19 |
| Endometrial tumor | -1.691 (-7.162,3.780) | 0.93 | 1.402 (-2.337,5.140) | 0.45 |
| Cervical Tumour | 14.609 (6.178, 23.041) | <0.001 | NA | NA |
| Peritoneal tumor | 0.109 (-5.957, 6.175) | 0.57 | -1.545 (-7.280,4.190) | 0.59 |
Legend: |
||||
Table 2. Univariate and multivariate analysis of linear regression as a model to predict PFI (variable answer) based on the following predictors: Histotype, cycles of weekly taxol, survival, and site of the primitive tumour. Significant p-values are in bold
The univariate regression revealed that the presence of cervical cancer is an excellent good predictor for high PFI (OR 14.609 (95% CI 6.178, 23.041), p<0.001). Concerning the toxicity, the data were complete for 87 patients out of 96, as summarized in table 3. In 8/87 cases there was a need for blood transfusion and 12/87 required growth factors (Darbaepoietin alfa, Filgrastim, Lenograstim) with a median treatment delay of only one per patient. However, 47 out of 96 patients had never experienced a treatment delay. Notably, 6/87 cases needed a dose reduction during therapy due to weight loss and not to drug toxicity.
| Toxicity | Global frequency (%) | Grade of toxicity, relative frequency (%) |
|---|---|---|
| Leucopenia | 12.6 | ≤ 2, 27.3 |
| 3, 63.6 | ||
| 4, 9.1 | ||
| Anemia | 18.4 | ≤ 2, 62.5 |
| 3, 37.5 | ||
| Joint Pain and/or Myalgia | 2.3 | ≤ 2, 100 |
| Peripheral Neuropathy | 1.1 | 1, 100 |
| Cardiotoxicity | 1.1 | 1, 100 |
| Oculotoxicity | 1.1 | 1, 100 |
| Thrombocytopenia | 2.3 | ≤ 2, 100 |
Table 3. Toxicity of the weekly taxol among the population under study. Missing data for 9 patients out of 96 (N=87). The grade refers to the CTCAE v 3.0 criteria
Among our population, 35 patients were tested for overall (germinal and somatic) BRCA 1 and 2 with the following distribution: wild type 63%, BRCA1 23%, BRCA2 14%. The PFI tended to be higher in BRCA1 (5.5 ± (7.5) months) and BRCA2 (5.5 ± (1.5) months) mutated than in the wild-type patients (4 ± (3) months), see Figure 4.
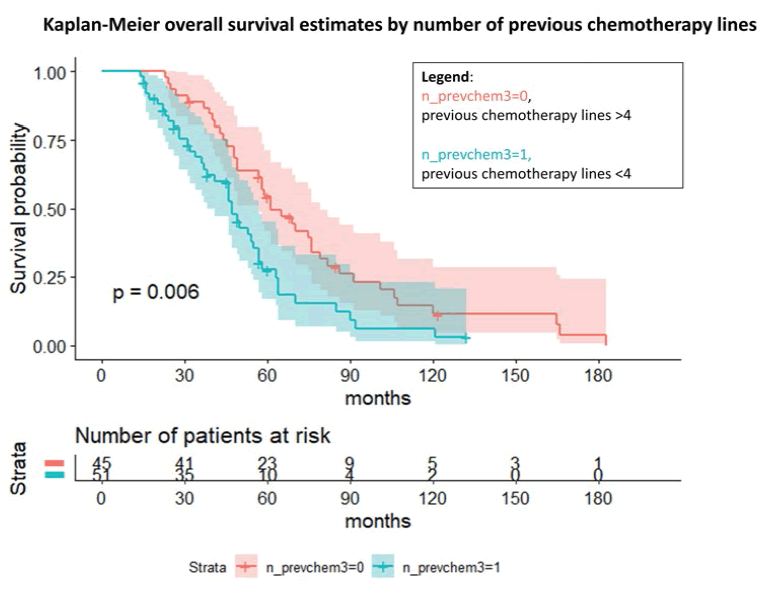
Figure 4: Kaplan-Meier overall survival estimates by the number of previous chemotherapy lines with gynaecological malignancies treated with weekly taxol as rescue therapy
However, these findings were not statistically significant, probably due to the little information available about the BRCA status, with 66 missing data. Notably, we investigated the number of previous chemotherapy lines and the possible influence on the overall survival and the survival during the weekly taxol. The log-rank test and the Cox regression for the OS (p=0.006) showed an overall OR to die of 1.88 (95% C.I., 1.19; 2.98) in case of a low number of previous chemotherapy (≤ 3) compared to the other group. Considering the survival from the first taxol administration, we observed a similar trend even if nonsignificant (not shown).
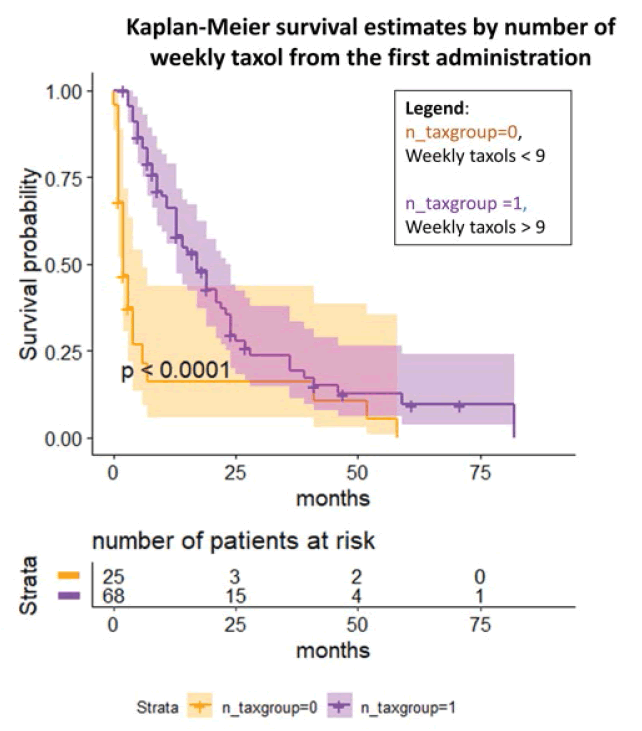
Lastly, we investigated the number of weekly taxol and the possible influence on the after the first administration until the last follow-up [Figure 5]. The log-rank test and the Cox regression (p<0.0001) showed an OR to die of 0.008 (95% C.I. 0.0005, 0.13) in case of a high number of weekly taxol (≥ 9) compared to the other group even after adjustment for the number of previous chemotherapies, BRCA status, and previous platinum sensitivity.
Discussion
The present study focused on the role of weekly taxol in recurrent gynaecological malignancies as salvage therapy. Our principal goal was to evaluate the global response, the stable disease, the progression-free interval, the overall survival, and the toxicity of the weekly taxol in recurrent gynaecological cancers.
Little information is available in the current literature that offers mainly retrospective studies with a small sample size [16-20]. The present study is retrospective and enrolled a wider population (N 96) than before in the literature [16- 21].
Most of the patients had a high FIGO stage disease, serous histology with a grading G3, and a diagnosis of ovarian tumour (79.16%), as expected considering its natural behaviour of cyclic recurrences even after optimal surgery and complete response to first-line chemotherapy [17]. The patients were all affected by relapsed gynaecological cancer already treated with a median number of 3 ± 1.25 lines of previous chemotherapies.
The age distribution varied significantly among all the tumours: the youngest patients were affected by cervical cancer (mean age 51.33 ± 5.16 years), the women with ovarian cancer stayed in the middle, whereas the patients with peritoneum and endometrial cancer were significantly older.
Interestingly, the survival from the time of diagnosis and the first taxol differed significantly among the histotypes at the Cox regression. A possible explanation could be found in the different FIGO stages at the diagnosis of the various histotypes leading to lower survival in the case of histotypes presenting at advanced disease and the different chemosensitivity among them. In the current literature, the endometrioid histotypes have better survival rates, whereas high grades of serous histotypes are characterized by the worse outcome in both endometrial and ovarian cancers [22,23]. Notably, the endometrioid histotypes had a higher HR to die at our survival analysis than serious ones. A possible explanation could be linked to the high prevalence of IV stage among the endometrioid subtype under weekly taxol (see table 1) and the absence of a sharp distinction of the site (endometrium/ovary), even if the sample was mainly composed by ovarian cancers, high-grade tumours, serous histotypes. As in current literature, an even lower survival characterized clear cell histotypes [22,23]. Only in the case of carcinosarcoma (also known as MMMT) and anaplastic subtypes, we reported different findings than in the literature, probably due to their rarity and consequently their low representation in the sample, showing similar HR to death than serous histotypes [22]. In other words, we reported the HR to die in decrescent order: CC, endometrioid, anaplastic, MMMT, serous, squamous. Interestingly, the squamous histotype was a good predictor for a better response to weekly taxol in terms of PFI (since all the cervical cancers were squamous) and OS with low HR to die at the survival curve, even if most of the squamous subtypes were at the II stage and associated to the cervical carcinoma that correlated with younger age and a significant OR for higher PFI. The choice of weekly taxol as “rescue therapy” for second and further chemotherapy lines in recurrent cervical cancer is common. We used the standard scheme, even if some authors prefer the modified dose-dense scheme skipping the fourth administration due to a better profile of tolerance and a non-inferior response [19].
The rationale for the dose-dense chemotherapy is to destroy small volume-tumours via frequent and low exposure to taxol in the most sensitive cell-cycle phase (G2/M) [24], leading to gain a lower tumour burden in G0 according to the growth kinetics model developed by Gompertzian (small tumours grow faster than bigger ones) [20], determining atypical mitotic exit and cell death via apoptosis after mitotic block due to the disruption of spindle microtubules dynamics [24-26]. The positive correlation between PFI and the number of treatment delays may probably suggest that skipping some dose of weekly taxol is linked to better tolerance and response. Indeed, the dose-dense scheme could be modified without the last dose on day 22 with the following scheme 1-8-15 as other authors suggest [19], even after twelve weeks of consecutive therapy with taxol (1-8- 15-21) [21].
The achievement of disease stability, a higher survival probability, a greater progression-free interval was directly related to the number of weekly taxol cycles. Considering its efficacy and good tolerability, weekly taxol can be considered a valuable option for salvage and maintenance therapy in relapses of advanced gynaecological tumours, with higher PFI in cervical cancer, even after multiple lines of previous chemotherapy, as already seen in literature also in comparison with the q21 taxol [16,18-20]. Even in the case of highly pre-treated re-current gynaecological cancers, the palliative goal of reaching a stable disease is crucial for patients and their families. However, this population can also benefit from weekly taxol thanks to increased PFI and survival.
Notably, we found significant results about the platinum response in terms of overall survival. The platinum-sensitive group had a significantly better OS than the platinumpartially sensitive one, confirming previous findings [20]. However, other authors found different results without any statistically significant differences in OS based on platinum-response [16]. The platinum-resistant group experienced the least OS, whereas the platinum-refractory group followed an unexpected curve. After 50 months of follow-up, the platinum-refractory Kaplan-Meir curve tended to the platinum-sensitive one probably due to the survival benefit of the dose-dense regimen. The comparison between the curves demonstrated that the presence of platinum resistance or platinum-partially sensitivity is a risk factor to die in patients under weekly taxol therapy. The metronomic administration of chemotherapy, in this case weekly, could lead to an anti-angiogenic effect thanks to a microtubule disruption, a downregulation of VEGF and IL8 [20]. Moreover, as already evaluated in the GOG phase II study, the objective response rate of the chemoresistant population (after combined platinum-taxol q21) to weekly taxol (80 mg/m2) was 20.9% with acceptable toxicity and rare serious adverse events [21].
Surprisingly, the number of previous chemotherapies predicted a lower survival when equal o less than three. Therefore, the use of a rescue therapy (i.e., weekly taxol) in the enrolled population along the timeline of possible chemotherapies at a previous point (≤ 3) could drive towards a worse prognosis than the woman who took already benefit from more than 3 lines of previous chemotherapy mainly based on platinum in a different combination, especially with q21 taxol. The woman who experienced a better OS in case of weekly taxol may benefit from the BRCA status and a previous platinum response even in case of platinum rechallenge. However, it is difficult to discriminate the effect of previous platinum and taxol alone since they are usually administered together.
The principal limitation of our study is its retrospective nature and its wide time frame together with the presence of possible confounding factors hypothetically due to the pooled analysis including any line of chemotherapy (salvage and maintenance therapy), any tumour site (ovary, peritoneum, endometrium, cervix) with different FIGO stages. Therefore, further studies are needed to understand these results, increase the number of BRCA tests, and create different subsets based on different types of chemotherapies and biological drugs, such as PARP-inhibitors.
Conclusion
The weekly taxol is a valuable and safe option for rescue therapy in recurrent gynaecological malignancies. The achievement of disease stability and a higher progressionfree interval is on the number of weekly taxol cycles, with a better outcome in the patients affected by cervical cancer. Interestingly, the survival from the diagnosis and the beginning of taxol was influenced by histotype, with higher survival estimates for squamous. The endometrioid and serous histotypes had similar records, whereas the clear cell carcinoma was confirmed to behave like the worst at the survival curve. Notably, the overall survival is influenced by the previous response to platinum-based chemotherapy and the previous number of chemo-therapy lines. The platinumresistant patients experienced the least OS, whereas the platinum-sensitive and surprisingly also the platinum refractory groups gained the highest survival estimates. In the end, the presence of platinum resistance or partial platinum sensitivity can be considered a risk factor for death in patients receiving weekly taxol regardless of histotype, the number of weekly taxol cycles, and progression to the fourth month after starting weekly taxol therapy. In the case of three or fewer previous chemotherapies before using the weekly taxol as rescue therapy, the woman had a lower OS. Furthermore, the toxicity profile of weekly taxol appeared good among the population under study. Even if not significant, there was a trend of higher PFI among BRCA 1/2 mutated than wild-type women.
Acknowledgement
This research was supported by the University of Bari and promoted by the Precision Medicine project of the Apulian Region.
Author contributions
All authors contributed to the study’s conception and design. The study was conceived and designed by Ettore Cicinelli, Gennaro Cormio, Vera Loizzi, and Rosalba De Nola. Material preparation and data collection were performed by Michele Ligorio and Rosalba De Nola. A review of the literature was performed by Michele Ligorio and Rosalba De Nola. Analysis was performed by Rosalba De Nola. The first draft of the manuscript was written by Rosalba De Nola and all authors commented on previous versions of the manuscript. Rosalba De Nola and Michele Ligorio equally contributed to this paper. All authors read and approved the final manuscript.
Compliance with ethical standards
This is an observational study. The Research Ethics Committee (Comitato Etico Interregionale, Policlinico di Bari, Bari, Italy) has confirmed that no ethical approval is required.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Wei T, Simko V, Levy M, et al. Package ‘corrplot’. Statistician 56:e24 (2017).
Google Scholar Crossref - Gastwirth JL, Gel YR, Hui WW, et al. Package ‘lawstat’. (2020).
Google Scholar Crossref - Therneau TM, Grambsch PM. The cox model. In modelling survival data: Extending the Cox model. Springer. 1st Edi:39-77 (2000).
Google scholar Crossref - Warnes GR, Bolker B, Lumley T, et al. Package ‘gmodels’. Vienna: R Foundation for Statistical Computing. (2018).
Google scholar Crossref - Fox J, Weisberg S, Price B. carData: Companion to applied regression data sets. CRAN. (2020).
Google scholar Crossref - Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. (2013).
Google scholar Crossref - Duska LR, Kohn EC. The new classifications of ovarian, fallopian tube, and primary peritoneal cancer and their clinical implications. Ann Oncol. 28:viii8-viii12 (2017).
Google scholar Crossref - Maheshwari A, Gupta S, Prat J. A proposal for updating the staging of endometrial cancer. Int J Gynecol Obs. 145:245-252 (2019).
Google scholar Crossref - Matsuo K, Machida H, Mandelbaum RS, et al. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 152:87-93 (2019).
Google scholar Crossref - Rustin GJ, Vergote I, Eisenhauer E, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer. 21:419-423 (2011).
Google scholar Crossref - Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45:228-247 (2009).
Google scholar Crossref - Gronlund B, Høgdall C, Hilden J, et al. Should CA-125 response criteria be preferred to response evaluation criteria in solid tumors (RECIST) for prognostication during second-line chemotherapy of ovarian carcinoma? J Clin Oncol. 22:4051-4058 (2004).
Google scholar Crossref - Davidson BA, Foote J, Clark LH, et al. Tumor grade and chemotherapy response in endometrioid endometrial cancer. Gynecol Oncol Rep. 17:3-6 (2016).
Google scholar Crossref - Friedlander M, Trimble E, Tinker A, et al. Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer. 21:771-775 (2011).
Google scholar Crossref - Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 30:672-705 (2019).
Google scholar Crossref - Boruta DM 2nd, Fowler WC Jr, Gehrig PA, et al. Weekly paclitaxel infusion as salvage therapy in ovarian cancer. Cancer Invest. 21:675-681 (2003).
Google scholar Crossref - Osman MA, Elkady MS, Nasr KE. Weekly Paclitaxel Versus Three-Weekly Paclitaxel in Recurrent Platinum-Resistant Epithelial Ovarian and Peritoneal Cancers: A Phase III Study. Clin Med Insights Oncol. 10:35-41 (2016).
Google scholar Crossref - Kogan L, Laskov I, Amajoud Z, et al. Dose dense carboplatin paclitaxel improves progression free survival in patients with endometrial cancer. Gynecol Oncol. 147:30-35 (2017).
Google scholar Crossref - Machida H, Moeini A, Ciccone MA, et al. Efficacy of Modified Dose-dense Paclitaxel in Recurrent Cervical Cancer. Am J Clin Oncol. 41:851-860 (2018).
Google scholar Crossref - Chen WC, Huang HJ, Chang TC, et al. Dose-dense chemotherapy with weekly paclitaxel and 3-weekly carboplatin for recurrent ovarian cancer. Taiwan J Obstet Gynecol. 59:21-27 (2020).
Google scholar Crossref - Markman M, Blessing J, Rubin SC, et al. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: A Gynecologic Oncology Group study. Gynecol Oncol. 101:436-440 (2006).
Google scholar Crossref - Peres LC, Cushing-Haugen KL, Köbel M, et al. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J Natl Cancer Inst. 111:60-68 (2019).
Google scholar Crossref - Ballester M, Bendifallah S, Daraï E. European guidelines (ESMO-ESGO-ESTRO consensus conference) for the management of endometrial cancer. Bull Cancer. 104:1032-1038 (2017).
Google scholar Crossref - Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 4:253-265 (2004).
Google scholar Crossref - Jordan MA, Wendell K, Gardiner S, et al. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 56:816-825 (1996).
Google scholar Crossref - Colevas A, Setser A. The NCI Common Terminology Criteria for Adverse Events (CTCAE) v 3.0 is the new standard for oncology clinical trials. J Clin Oncol. 22:6098-6098 (2004).
Google scholar Crossref