Short Communication - Interventional Cardiology (2023)
Tissue reperfusion after ST-Elevation Myocardial Infarction (STEMI): Autoimmunity could explain the unexplainable
- Corresponding Author:
- Francesco Tona
Department of Cardiac, Thoracic, Vascular Sciences and Public Health, University of Padua, Padua, Italy,
E-mail: francesco.tona@unipd.it
Received date: 20-Oct-2023, Manuscript No. FMIC-23-117580;Editor assigned: 24-Oct-2023, PreQC No. FMIC-23-117580 (PQ); Reviewed date: 09-Nov-2023, QC No. FMIC-23-117580;Revised date: 16-Nov-2023, Manuscript No. FMIC-23-117580 (R);Published date: 23-Nov-2023, DOI: 10.37532/1755-5310.2023.15(S19).488
Abstract
ST-Elevation Myocardial Infarction (STEMI) is a major cause of premature death and morbidity. Although percutaneous coronary interventions are lifesaving, in up to 40% of patients reopening of the epicardial coronary artery doesn’t guarantee successful reperfusion of the myocardium. This phenomenon is called no-reflow and is a neglected problem in modern medicine. It has been recently demonstrated that autoantibodies targeting endothelin-1 type A receptor, by exerting their detrimental effects through vasoconstriction, fibrosis and inflammation, are associated with no-reflow. This finding provided, for the first time, evidence of an autoimmune predisposition to a worse outcome after myocardial infarction, creating opportunities to future therapeutic implications.
Keywords
No-reflow • Myocardial infarction • Endothelin • Autoantibodies • Autoimmunity • ETAR
Description
Every cardiologist has experienced this distressing revelation in his professional life: ST-Elevation Myocardial Infarction (STEMI) with short time from pain onset, optimal antithrombotic therapy before Percutaneous Coronary Intervention (PCI), accurate choice of the very-last-generation drug eluting stent, but, at post-PCI coronary angiography, very unsatisfactory coronary flow, and every cardiologist asked himself the very same question: why?
No-Reflow (NR), where restoration of myocardial perfusion is not obtained despite good epicardial coronary artery flow, is common after STEMI. Despite most cardiologists try to neglect the problem and swipe NR under the “we don’t diagnose what we can’t cure” carpet, around 40% of STEMI patients face NR after successful PCI, with tremendous implications in term of subsequent tissue necrosis, infarct expansion, congestive heart failure, and death [1]. Moreover, while acute treatment of patients presenting with STEMI has evolved enormously over the past several years, no change in infarct severity at the myocardial level has been observed [2]. Given the above, it’s clear how some important pathways linking STEMI to myocardial tissue damage are still unknown and, among these, the biggest question mark is probably that of NR.
Several mechanisms could contribute to the pathogenesis of NR, including extravascular compression, microvascular vasoconstriction, platelet–leukocyte capillary plugging 1 and reperfusion injury [3]. For sure, the coronary endothelium is the most important actor of the NR phenomenon and poor endothelial function increase the risk of NR [4].
Endothelin-1 is a product of the vascular endothelium and its release can be triggered by endothelial damage and hypoxia [5]. It has been proven that endothelin-1 levels rise in the early hours after acute myocardial infarction, [6] and its levels predict NR after successful PCI [7]. Indeed, in revascularized patients, endothelin-1 plays a detrimental role by inducing NR through the vasoconstrictor effects elicited by binding to Endothelin-1 Type A Receptor (ETAR).
Endothelin-1 receptor type A (ETAR) is a G-Protein-Coupled Receptor (GPCR) expressed on the surface of a great variety of cells, such as endothelial cells, vascular smooth muscle cells, immune cells and fibroblasts [8]. Interestingly, ETAR can be activated not only by endothelin-1 but also by autoantibodies specific for ETAR (ETAR-AAs), which can bind ETAR and regulate its function. The function of ETAR-AAs, which mirrors (and amplify) that of the natural ligand endothelin-1, involves not only vasoconstriction but also activation of pro-inflammatory and pro-fibrotic pathways [9]. Interestingly, these features are also described in NR. Additionally, histopathological features of tissue after ETAR-AAs mediated acute rejection resemble those typical of NR: Capillarity’s with diffuse blood extravasation, thrombosis inside small arteries and lymphomonocyte infiltration around arterioles [10-14].
Autoantibodies targeting GPCRs (and thus also ETAR-AAs) are compelling components of our immune system [15-18]. Although their importance has been neglected for many decades, rheumatologists and immunologists recently started to discover their central role in the pathogenesis of many different diseases. Pivotal to the scope of understanding their potential role in the NR phenomenon, autoantibodies anti-GPCRs have recently been described as powerful markers of chronic GPCR expression, reflecting chronic individual exposure to different environmental factors (“Exposome”). This antibody network (“Antibodiom”) is therefore determined both by environmental exposure and by the individual’s immune system.
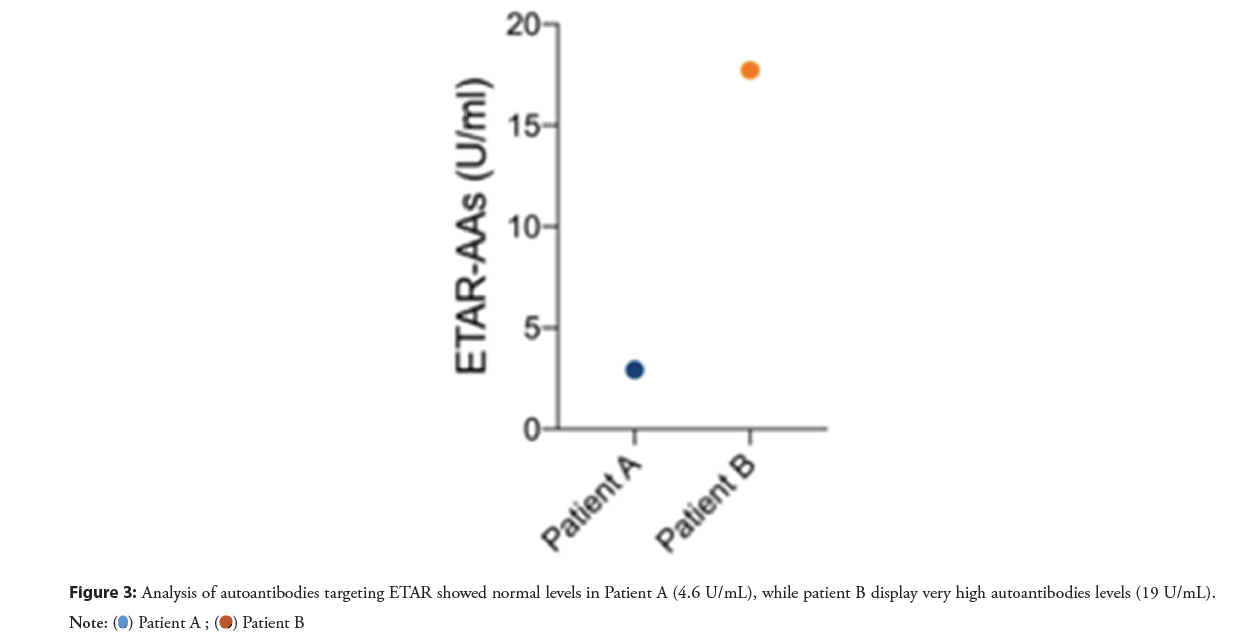
In our recently published paper “Association of autoantibodies targeting endothelin type-A receptors with no-reflow in ST-elevation myocardial infarction” we have showed, for the first time, how high ETAR-AAs levels are associated with higher risk of microvascular obstruction, which is the cardiac-magnetic-resonance equivalent of NR [19]. This association is independent from other know causes of NR (such as time before primary PCI and infarct size) and is presumably mediated by the deleterious effects of ETAR-AAs on coronary microcirculation (Figures 1-Figures 3).
Figure 1: Patient A, admitted for anterior STEMI (3-hours of chest pain). Coronary angiography revealed total proximal LAD occlusion A, that was effectively treated with drug eluting stent, obtaining TIMI 3. Cardiac magnetic resonance showed relevant infarct size, without signs of MVO at LGE sequences.
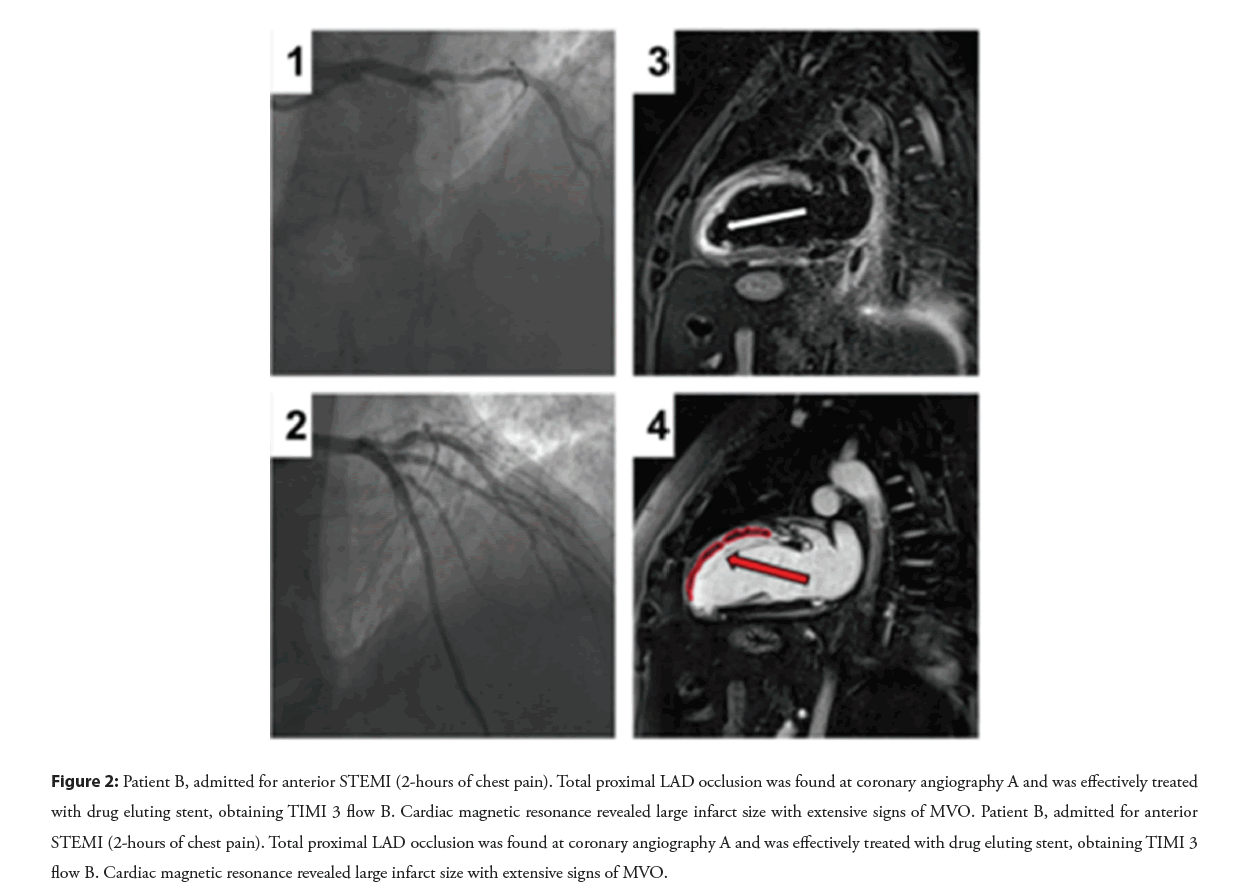
Figure 2: Patient B, admitted for anterior STEMI (2-hours of chest pain). Total proximal LAD occlusion was found at coronary angiography A and was effectively treated with drug eluting stent, obtaining TIMI 3 flow B. Cardiac magnetic resonance revealed large infarct size with extensive signs of MVO. Patient B, admitted for anterior STEMI (2-hours of chest pain). Total proximal LAD occlusion was found at coronary angiography A and was effectively treated with drug eluting stent, obtaining TIMI 3 flow B. Cardiac magnetic resonance revealed large infarct size with extensive signs of MVO.
Although speculative, our hypothesis is that every patient has his “own” titers of ETAR-AAs, which are a signature of his “exposome” and which influence his response to an acute ischemic event and his likelihood to develop no reflow, irrespective of infarct extension. Endothelin-1, far from being a mere bystander, is produced by the endothelium after myocardial infarction, reflects infarct extension and has an add-on value to the effects of ETAR autoantibodies. Endothelin-1 and ETAR-AAs, however, are not the only players of the game. In rat’s models, ischemia and reperfusion increases endothelin-1 binding sites in cardiac membranes [20]. Infarct extension and NR can therefore be influenced both by the ligand (increased endothelin-1 production and elevated titers of ETAR-AAs) and by the receptor (increased ETAR expression).
The important question that arises at this point is: Can we do something to prevent the detrimental effects of ETAR-AAs? The short answer is “we don’t know”. But the long answer starts with a “maybe”. Indeed, previous studies about the role of ETAR-AAs in preeclampsia showed that treatment with ETAR antagonists can block and prevent the effects of ETAR-AAs [21]. Moreover, ETAR antagonists were also found to improve microvascular flow after myocardial infarction. The existing evidence is therefore promising and, if translational cardiology will start to focus on the possible role of autoimmunity in acute coronary syndromes, exciting results will come.
Conclusion
The immune system is a powerful mediator of disease. While in the last decades the role of autoimmunity in cardiovascular medicine has been limited to the niche of myocardial inflammation, we have recently provided evidence that ETAR-AAs are associated with the phenomenon of NR after STEMI, leading the way to future therapies against NR that could target this pathway. In the meantime, while looking at unsatisfactory post-PCI coronary angiographies and asking themselves “why?”, cardiologists should think to ETAR-AAs travelling in their patients’ blood and feel less frustrated about their powerlessness.
References
- Schwartz BG, Kloner RA. Coronary no reflow. J Mol Cell Cardiol. 52(4):873-882 (2012).
- Lechner I, Reindl M, Tiller C, et al. Temporal trends in infarct severity outcomes in st‐segment-elevation myocardial infarction: A cardiac magnetic resonance imaging study. JAHA. e028932 (2023).
- Maznyczka AM, McCartney PJ, Oldroyd KG, et al. Effects of intracoronary alteplase on microvascular function in acute myocardial infarction. JAHA.9(3):e014066 (2020).
- Kloner RA, King KS, Harrington MG, et al. No-reflow phenomenon in the heart and brain. Am J Physiol Heart Circ Physiol. 315(3):H550-H562 (2018).
- Dhaun N, Webb DJ. Endothelins in cardiovascular biology and therapeutics. Nat Rev. 16(8):491-502 (2019).
- Galiuto L, DeMaria AN, del Balzo U, et al. Ischemia-reperfusion injury at the microvascular level: Treatment by endothelin A-selective antagonist and evaluation by myocardial contrast echocardiography. Circulation.102(25):3111-3116 (2000).
- Niccoli G. Endothelin-1 and acute myocardial infarction: A no-reflow mediator after successful percutaneous myocardial revascularization. Eur Heart J. 27(15):1793-1798 (2006).
- Horinouchi T, Terada K, Higashi T, et al. Endothelin receptor signaling: New insight into its regulatory mechanisms. J Pharmacol Sci. 123(2):85-101 (2013).
- Philogene MC, Johnson T, Vaught AJ, et al. Antibodies against angiotensin II type 1 and endothelin A receptors: Relevance and pathogenicity. Hum Immunol. 80(8):561-567 (2019).
- Hiemann NE, Meyer R, Wellnhofer E, et al. Non-HLA antibodies targeting vascular receptors enhance alloimmune response and microvasculopathy after heart transplantation. Transplantation. 94(9):919-924 (2012).
- Cozzi E, Calabrese F, Schiavon M, et al. Immediate and catastrophic antibody-mediated rejection in a lung transplant recipient with anti-angiotensin II receptor type 1 and anti-endothelin-1 receptor type a antibodies. Am J Transplant.17(2):557-564 (2017).
- Kloner RA, Rude RE, Carlson N, et al. Ultrastructural evidence of microvascular damage and myocardial cell injury after coronary artery occlusion: Which comes first? Circulation. 62(5):945-952 (1980).
- Krug A, Rochemont DM, Korb G, et al. Blood supply of the myocardium after temporary coronary occlusion. Circ Res.19(1):57-62 (1966).
- Kloner RA, Ganote CE, Jennings RB, et al. The “No-Reflow” phenomenon after temporary coronary occlusion in the dog. J. Clin. Invest. 54(6):1496-1508 (1947).