Research Article - Research on Chronic Diseases (2018) Volume 2, Issue 1
Tonsillectomy combined with steroid pulse therapy for patients with immunoglobulin a nephropathy: A meta-analysis
- *Corresponding Author:
- Xiong L
Department of Health Services Management
Second Military Medical University
Shanghai, P.R. China
E-mail: xionglinping@aliyun.com
Abstract
Tonsillectomy combined with steroid pulse therapy has been reported to be effective in decreasing urinary abnormalities and preventing the progressive deterioration of renal function in patients with Immunoglobulin a nephropathy. However, the efficacy of the therapy remains controversial. We conducted a meta-analysis to assess the efficacy of tonsillectomy combined with steroid pulse therapy in achieving clinical remission. Studies from PubMed and Cochrane were searched up to the 20th of February 2017. Retrospective cohort studies, which reported tonsillectomy combined with steroid pulse therapy in patients with Immunoglobulin A nephropathy, were included. The fixed effect model was used to calculate the pooled hazard ratio. Finally, 7 retrospective cohort studies were included for analysis, with a total of 1474 participants. The pooled hazard ratio was 2.87 (95% confidence interval: 2.19- 3.77), comparing those who underwent tonsillectomy combined with steroid pulse therapy and those who did not. The subgroup analysis, sensitivity analysis and publication bias test suggested the overall result of the analysis was robust. The meta-analysis concludes that tonsillectomy combined with steroid pulse therapy has a significant effect on increasing the probability of clinical remission.
Keywords
tonsillectomy, steroid pulse therapy, immunoglobulin a nephropathy, clinical remission, meta-analysis
Abbreviations
TSP: Tonsillectomy Combined with Steroid Pulse Therapy;
IgA: Immunoglobulin A
Introduction
In 1968, Berger and Hinglais reported on Immunoglobulin A (IgA) nephropathy for the first time [1]. Studies performed in the last few decades have revealed that primary IgA nephropathy is an immune complex mediated glomerulonephritis that is immune histologically defined by the presence of glomerular deposits of IgA. (Emancipator, 1994) Now, IgA nephropathy is among the most common forms of glomerulonephritis in the world [2]. The clinical course of IgA nephropathy varies from asymptomatic microscopic hematuria to rapidly progressive kidney failure. It has been reported that, as a result of the progressive nature of IgA nephropathy, 30 to 40% of diagnosed patients reach end-stage renal disease within 10 to 25 years [3]. Many approaches to treatment have been proposed and reported because of the uncertainty of the pathogenesis of IgA nephropathy [4]. Additionally, the decreased immune regulation in the mucosa– bone marrow axis might be related [5,6]. Both steroid pulse therapy and tonsillectomy have demonstrated effectiveness for IgA nephropathy [7,8]. Additionally, the results of the current studies indicated that the total clinical remission rate for IgA nephropathy patients is significantly higher when other treatments are accompanied by tonsillectomy. For example, the clinical remission rate of tonsillectomy combined with steroid pulse therapy was better than that of simple tonsillectomy, steroid pulse therapy or general treatment, and the clinical remission rate of tonsillectomy plus steroid pulse was higher than that of normal-dose steroids alone [9]. Recently, tonsillectomy combined with steroid pulse therapy (TSP) has been reported to be effective in decreasing urinary abnormalities and preventing the progressive deterioration of renal function [10]. However, the efficacy of the therapy remains controversial. The purpose of the article is to evaluate the efficacy of TSP for patients with IgA nephropathy.
Methods
▪ Literature search
We used the medical subject headings “glomerulonephritis, iga”, “steroids”, and “tonsillectomy”, combined with free words, such as “tonsillectomy”, “pulse therapy”, “iga”, “immunoglobulin A nephropathy” or “Berger’s Disease”, in the title or abstract via a PubMed search. We used “iga” or “immunoglobulin A nephropathy” or “Berger’s Disease” or “glomerulonephritis” or “iga glomerulonephritis” in the title, abstract or keywords in Cochrane. We selected studies published in English-language journals by searching PubMed and Cochrane up to February 20th, 2017. Additionally, we tried to search for additional studies from literature references and review articles.
Study selection
The inclusion criteria were as follows:
▪ the study design was a controlled clinical trial or cohort study,
▪ the study population included adults with biopsy-proven IgA nephropathy,
▪ the study compared the clinical outcomes of IgA nephropathy patients with or without TSP,
▪ the latest study was included if there were duplicates or data originating from the same study population,
▪ the study reported outcomes include achievement of clinical remission (CR), and
▪ studies of secondary IgA nephropathy (e.g., systemic lupus erythematosus and liver disease) or recurrent IgA nephropathy after kidney transplantation were excluded. Reviews or studies that failed to obtain data were excluded. Additionally, we tried to contact the communication author through e-mails for raw data of those studies that did not report available information if necessary.
▪ Data extraction and analysis
We used a standardized data collection form to extract the following information: name of the first author, publication year, details of the treatment protocol, definition of clinical remission, follow up years, samples, age, sex, effect measures with the corresponding 95% confidence interval (CI) and adjusted variables. The most adjusted data were selected if adjusted effect estimates were given in different levels of adjustment. Two authors independently performed the literature search, study selection, and data extraction. Disagreements during this process were resolved by discussion or consulting with another author. The hazard ratio (HR) was used as a common effect measure. In some cases, the HR was broadly equivalent to the RR or OR [11]. Therefore, we used the RR (or) OR as a replacement for some studies that did not report the HR. We used the I2 statistic (significance level at P<0.10) to assess the heterogeneity across studies [12,13]. If P>0.10 and I2 ≤ 50%, the heterogeneity was considered statistically insignificant.
If there was a relatively high heterogeneity in the results, we would use the random effect model as an alternative to calculate the pooled HR among studies. Alternatively, the fixed effected model was used. Subgroup analysis and meta regression were conducted to explore the heterogeneity. Additionally, we conducted subgroup analysis according to confounding factors that were adjusted to examine the causes of potential heterogeneity, including hypertension, disease period, body mass index and so on. A meta regression was conducted to explore the heterogeneity from the follow up years, samples and quality assessment scores. Sensitivity analysis was performed to detect the effects of an individual study on the pooled result by omitting one study per turn. Begg’s and Egger’s tests were used to detect the potential publication bias [14,15]. Data analysis was conducted using Stata, version 11.0
▪ Quality assessment
Another two authors performed the quality assessment using the Newcastle–Ottawa Scale (NOS) for cohort studies. The scale allocates a maximum of 9 points for the quality of selection, comparability and outcome of study par-ticipants. Disagreement between the two authors was solved by discussion or consulting with another author’s suggestions.
Results
▪ Description of selected studies
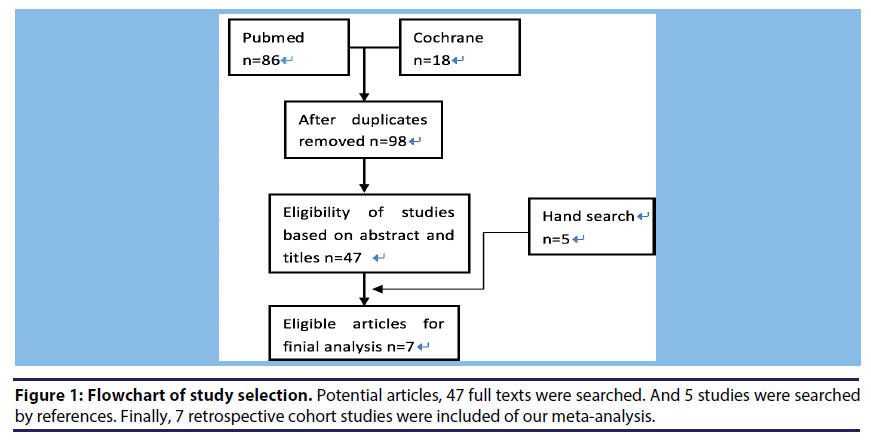
Figure 1 shows the process of searching studies in our meta-analysis. We initially identified 98 articles from PubMed and Cochrane. The majority were excluded after the first screening of abstract and titles, which was mainly because they were reviews, case report studies, or not relevant to our analysis. After assessing the abstracts of the flowchart of study selection.
(A) After duplication, 98 articles from Pubmed (86) and Cochrane (18) were searched.
(B) Of these, the majority was excluded after the first screening of abstract and titles, only 47 studies were left.
(C) Finally, 7 retrospective cohort studies met the predetermined criteria of our meta-analysis.
The studies’ follow-up years ranged from 1 to 6.9 years. In brief, 45 studies are excluded for the following reasons: included children and young people (n=4), insufficient data (n=2), data were originated from the same study population (n=2), clinical outcomes were unexpected (n=3), similar studies were far from sufficient (n=3) (One is a random controlled trail [16] and another two are prospective studies [17,18], and not related (n=31). Finally, 7 retrospective cohort studies Hotta et al., [19-23] were included.
Table 1 shows the definitions of clinical remission and protocols of TSP. They were essentially similar among the included studies. And Table 2 summarizes the main characteristics of the selected studies for analysis. The 7 studies were all conducted in Japan. The population size per study ranged from 40 to 388, with a total of 1474 participants. Not all adjusted HR, RR or OR were reported in all studies, but we can extract related data and calculate them. Adjustment for potential confounding factors differed across studies, and the main adjusted factors were hypertension, disease period, body mass index, uric acid, triglycerides, serum immunoglobulin A, urinary protein, urinary red blood cells, angiotensin converting enzyme inhibitor or angiotensin II receptor blocker, serum cholesterol, estimated glomerular filtration rate, levels of hematuria, severity of diseases, diabetes and cyclophosphamide.
| Study | The Steroid Pulse Therapy Protocols | Clinical Remission |
|---|---|---|
| Miyamoto [19] | Oral prednisolone was approximately 0.5 mg/kg per day. And the prednisolone was gradually tapered. The median administration period was 411 (188–970) days. | Remission of both proteinuria (negative or trace proteinuria on the urine dipstick test or urine protein excretion <0.3 g/g creatinine) and hematuria (the absence of blood on the dipstick test or urine red blood cells (U-RBC) <5/high power field) at least two consecutive times. |
| Hoshino [20] | Intravenous methylprednisolone pulses of 500 mg/day for 3 consecutive days and two additional pulses within 6 months | <0.3 g/day proteinuria and <5 urinary red blood cells per high-powered field. |
| Ohya (Ohya et al., 2013) | 0.5 g of methylprednisolone on 3 consecutive days followed by oral prednisolone (30 mg/day) on 4 consecutive days, with the course repeated 3 times | Dipstick test and an erythrocyte count <1-4 cells per high- power field on 3 consecutive visits |
| Ochi [21] | Intravenous methylprednisolone pulses of 0.5 g/day for 3 consecutive days, and a further two pulses within 6 months after the initial pulse. Patients were given the oral prednisone at a dose of 0.5 mg/kg body weight on every alternate day for 6 months. After the third steroid pulse, the oral prednisone was gradually withdrawn over a period of 1 month. | Urinary protein excretion <0.3 g/g creatinine and urinary red blood cell count <5/high-power field. |
| Nakagawa [22] | Intravenous methylprednisolone (500 mg/day) pulse therapy was administrated as three installations over course of 3 days, followed by 30 mg of prednisolone on alternate days for 8 weeks. | Negative proteinuria and urinary erythrocytes of less than 5/high-power field |
| Kawaguchi [23] | Methylprednisolone 0.5 g/day for 3 days for three courses, which was usually followed by oral prednisolone at an initial dose of 0.6 mg/kg per day. | Complete disappearance of urinary protein by dipstick and urinary erythrocytes less than 1/high-power field. |
| Hotta [24] | Methylprednisolone (0.5 g/d for 3 days for three courses) followed by oral prednisolone at an initial dose of 0.6 mg/kg on alternate days with a gradual decrease in dosage over 1 year | Negative proteinuria and hematuria by dipstick and urinary erythrocytes of 4/high-power field or less. |
Table 1. The steroid pulse therapy protocols and definitions of clinical remission in the included studies
| Study | Follow up Years | Samples(Intervention/Control) | Age | Male (%) | Treatment | Corresponding effect measures and 95% CI | Adjusted variables | |
|---|---|---|---|---|---|---|---|---|
| Intervention | control | |||||||
| Miyamoto [19] | 4.1 | 241(161/123) | 36 | 123(43.3%) | TSP | CT, CS | HR:2.08(1.33,3.33) (TSP vs. CT) | Hy, IgA |
| Hoshino [20] | 6 | 52(26/26) | 32.6 | 18(34.6%) | TSP | OS | HR:3.58(1.31,10.91) | Hy, Age, Dp, BMI, U-A, TG, IgA, U-P, U-RBC, ACEI/ARB, H-G TG, IgA, U-P, U-RBC, ACEI/ARB, H-G |
| Ohya(Ohya et al., 2013) | 2.5 | 62(41/21) | 34.6 | 20(33.3%) | TSP | SP | OR:1.55(0.54,4.47)# | Hy, Age, BMI, IgA, U- P, ACEI/ARB, Sex, Ch, eGFR, He, H-G, |
| Ochi [21] | 1 | 41(26/15) | 31.1 | 15(36.6%) | TSP | SP | OR:12.5(2.91,86.7) | Hy, Age, BMI, U-A, TG, IgA, U-P, U-RBC, ACEI/ARB, Sex, Ch, eGFR, H-G |
| Nakagawa [22] | 3 | 40(20/20) | 38.9 | 16(40%) | TSP | T | OR:3.67(0.96,14.03)# | Hy, Age, Dp, IgA, U-P, U-RBC, ACEI/ARB, eGFR, H-G, Dia |
| Kawaguchi [23] | 2 | 3888(240/148) | 34.3 | 177(45.6%) | TSP | N, T, SP | HR:3.10(2.02,4.77) (TSP vs N) | Hy, Age, BMI, IgA, U- P, U-RBC, ACEI/ARB, Cy |
| Hotta [24] | 6.9 | 329(153/176) | 36.1 | 178(54.1%) | TSP | SP, TCP, T, CS, N | OR:3.35(2.13,5.27)#(TSP | Cy Hy, Age, U-P, ACEI/ARB, Sex, He, |
aHere listed the average follow-up year of each study.
*As these studies did not report average follow-up years, so the minimums were listed in the table.
#The figures offered were calculated based on the original data in the corresponding studies.
Abbreviation: CS, conventional steroid therapy without tonsillectomy;
CT, conservative therapy with RAAS-I and/or antiplatelet agents or observation;
RAAS-I, renin–angiotensin–aldosterone system inhibitors;
OS, oral-corticosteroid without tonsillectomy;
SP, steroid pulse therapy without tonsillectomy;
T, tonsillectomy;
N, no particular therapy beyond supportive therapy, TCP, tonsillectomy combined with conventional steroid therapy, OP, other therapies including SP, TCP, T, CS and N;
Hy, hypertension, Dp, disease period, BMI, body mass index, U-A, uric acid, TG, triglycerides;
IgA, serum immunoglobulin A, U-P, urinary protein;
U-RBC, urinary red blood cells, ACEI/ARB, angiotensin converting enzyme inhibitor or angiotensin II receptor blocker, Ch, serum cholesterol, eGFR, estimated glomerular filtration rate, He, levels of hematuria;
H-G, severity of diseases, Dia, diabetes, Cy, cyclophosphamide.
Table 2. Characteristics of included studies
▪ Subgroup analysis and meta regression
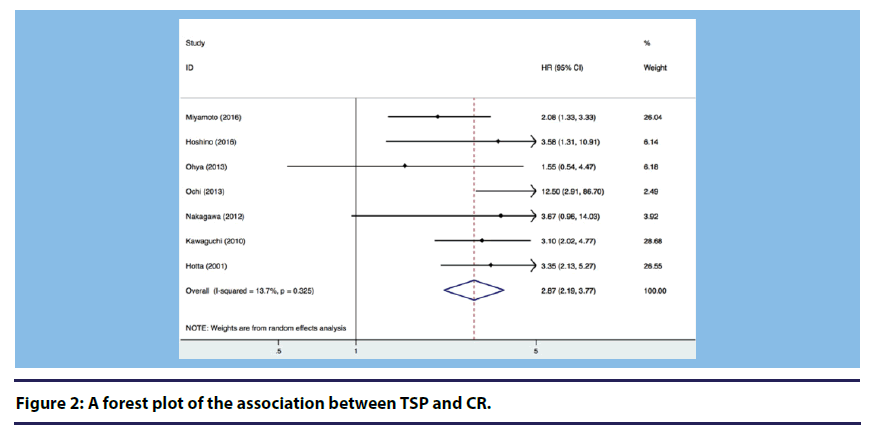
At first, we used the fixed effect model for analysis. If there was significant heterogeneity, we would use the random effect model. Figure 2 shows a forest plot presenting the association between conducting TSP in patients with IgA nephropathy and CR. Heterogeneity across studies was not statistically significant (I2=13.7%, P=0.325), and the fixed effect model suggested a significantly increased CR in patients with IgA nephropathy receiving TSP compared to those who were not (Overall HR=2.87, 95% CI: 2.19, 3.77).
(A) 7 studies were included in the meta-analysis.
(B) Heterogeneity across studies was not statistically significant (I2=38.3%, P=0.137).
(C) The fixed effect model suggested significant increased clinical remission in patients with IgA nephropathy receiving tonsillectomy combined with steroid pulse therapy compared to those who were not. (Overall HR=2.94, 95% CI: 2.00, 4.30).
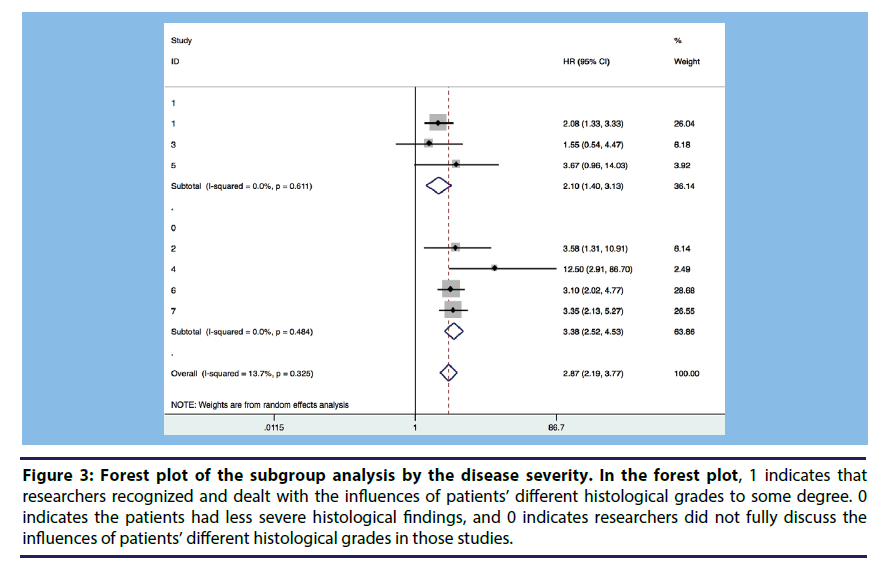
Subgroup analysis and meta regression were conducted to explore the heterogeneity between studies. We divided the studies into several subgroups according to adjusted variables. We conducted meta-regression focusing on the follow up years, samples and quality assessment scores. The subgroup analysis and the meta regression showed that the major factor influencing the association on TSP with CR was whether the researchers considered the histological grades of the selected patients. Figure 3 shows that if we divided the seven studies into two groups according to whether a study controlled for patients’ different histological grades in its analysis, the heterogeneity might be somewhat reduced.
Figure 3: Forest plot of the subgroup analysis by the disease severity. In the forest plot, 1 indicates that researchers recognized and dealt with the influences of patients’ different histological grades to some degree. 0 indicates the patients had less severe histological findings, and 0 indicates researchers did not fully discuss the influences of patients’ different histological grades in those studies.
▪ The seven studies were divided into three groups according to whether a study controlled histological grades in its analysis.
▪ Group 1 means, in those studies histological grades of the selected patients differed from each group. Group 2 means in these groups the patients were with less severe histological findings. While Group 0 means, in these studies patients with different histological grades in this study were well-distributed.
▪ The heterogeneity might be reduced to some degree in each group.
▪ Sensitivity and publication bias analyses
Sensitivity analysis showed that the changes were not significant when omitting any one of selected studies. Details for each study’s sensitivity analysis are presented in Table 3. It indicated that there was no significant trend of the overall result being influenced by any individual study. Additionally, no publication bias was found using asymmetry analysis in the funnel plot for both Begg’s test (P=0.548) and Egger’s test (P=0.436).
| Study omitted | Estimates | 95%CI |
|---|---|---|
| Miyamoto [19] | 3.21 | (2.43,4.24) |
| Hoshino [20] | 2.83 | (2.21,3.61) |
| Ohya (Ohya et al., | 2.95 | (2.32,3.77) |
| 2013) | ||
| Ochi [21] | 2.78 | (2.18,3.53) |
| Nakagawa [22] | 2.84 | (2.23,3.61) |
| Kawaguchi [23] | 2.76 | (2.08,3.67) |
| Hotta [24] | 2.69 | (2.04,3.56)) |
| Combined | 2.86 | (2.26,3.63) |
Table 3. Results of meta-analysis estimates by given name study is omitted
▪ Quality assessment
All 7 studies were determined to have moderate quality with a mean score of 7.6 (ranging from 7 to 8). Details for each study’s quality assessment are presented in Table 4.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Miyamoto [19] | 2016 | * | * | * | * | * | * | * | * | 8 |
| Hoshino [20] | 2016 | * | * | * | * | * | * | * | * | 8 |
| Ohya (Ohya et al., 2013) | 2013 | * | * | * | * | * | * | * | 7 | |
| Ochi [21] | 2013 | * | * | * | * | * | * | * | 7 | |
| Nakagawa [22] | * | * | * | * | * | * | * | * | 8 | |
| 2012 | ||||||||||
| Kawaguchi [23] | * | * | * | * | * | * | * | 7 | ||
| 2010 | ||||||||||
| Hotta [24] | 2001 | * | * | * | * | * | * | * | * | 8 |
Table 4. Results of quality assessment of the selected studies according to the NOS
Discussion
Our meta-analysis showed that TSP had a significant impact on increasing the probability of CR compared with other therapies, such as tonsillectomy alone, steroid pulses alone and so on, in IgA nephropathy patients. In 2001, Hotta [24] first reported the significant effect of tonsillectomy combined with steroid pulse therapy on the clinical remission of urinary abnormalities in IgA nephropathy. Since then, TSP has become one of the most widely used therapy protocols in adult patients in Japan. However, the efficacy of the therapy remains controversial.
According to our results, we speculated that conducting TSP in patients with IgA nephropathy might increase the rate of CR. A random controlled trail [16] and two prospective studies [17,18] were published to illustrate the issue. However, compared to retrospective cohort studies, the numbers of these studies were not sufficient to conduct a meta-analysis. Therefore, we conducted a meta-analysis of retrospective cohort studies. Our results provide useful information for conducting future random controlled trails and prospective studies. Treatment strategies leading to CR should be encouraged for patients with IgA nephropathy. Nonetheless, it is important to note that the quality and heterogeneity of selected studies might attenuate the strength of the results. We conducted meta-regression focusing on the follow up years, samples and quality assessment scores.
However, it seems to be invalid because of the limited numbers of studies and heterogeneities. Through subgroup analysis, we found that the most important factor influencing the relationship between the TSP and CR in patients with IgA nephropathy was whether the researchers considered the histological grades of the selected patients. Previous studies have also shown that TSP was more effective in those with less severe histological findings [18].
There remains controversy about the pathogenesis of IgA nephropathy. Two main pathways, upstream and downstream, have been proposed to illustrate the pathogenic mechanisms. The upstream involves continuous antigenic stimulation of the innate immune system by the tonsillar mucosa via the mucosa–bone marrow axis. In the downstream pathway, the anomalous stimulated immune response in the bone marrow results in the production of aberrantly glycosylated IgA1 and its subsequent deposition within the meningeal area. [16,20,21]. The severity of glomerular IgA nephropathy lesions in situ is associated with such deposition [25]. From the mechanisms described above, tonsillectomy may have an effect on the upstream pathway by eliminating antigenic stimuli from the tonsillar mucosa. Steroid pulse therapy may influence the other pathway by suppressing the abnormal immune response in the bone marrow that leads to subsequent inflammation in renal glomeruli. As an intervention against both pathogenic and immunological mechanisms, TSP might have a more effective influence on IgA nephropathy than tonsillectomy or steroid pulses alone [23].
The result was similar to other studies that showed urinary remission or renal prognosis by TSP is linked with histological severity [16- 18]. Non-immunological mechanisms may have a more important effect on the progression of renal function in advanced IgA nephropathy. Therefore, steroid pulse and tonsillectomy, as immunological interventions, may not necessarily have a great benefit. To preserve the residual renal function in the long run, TSP might effectively lead to CR in an earlier stage of IgA nephropathy. Further studies are also needed to confirm the relationship between TSP and CR and exclude the effects of other confounding factors [23,26].
The results about whether TSP in patients with IgA nephropathy tended to result in CR are widely different between studies, and the link remains unclear in the literature. The inconsistent results can be explained by differences in the study designs, follow up times and samples, and questions/hypotheses being investigated. Additionally, there have been studies published of meta- analyses discussing the efficacy of tonsillectomy in patients with IgA nephropathy. However, the conclusions were different. Wang [27] reported tonsillectomy treatment alone failed to increase the clinical remission rate in IgA nephropathy patients. However, tonsillectomy combined with either normal steroid or steroid pulse treatment results in a higher remission rate with favorable long-term efficacy. Liu [27] concluded that, as adjunct or independent therapy, tonsillectomy may induce CR in patients with IgA nephropathy. Both studies have common features, but the results differed. Increasing numbers of researchers have focused on the issue. To the best of our knowledge, only one random controlled trial [16] has been conducted to study the efficacy of TSP in IgA nephropathy patients. The trial indicated that TSP has no beneficial effect on steroid pulses alone to attenuate hematuria and to increase the incidence of clinical remission. However, the conclusion is controversial because only 72 patients were evaluated with 1 year of follow up. More randomized controlled trails need to be conducted to better understand this issue.
We must note several limitations of our study. First, although the heterogeneity was not obvious in our analysis, we cannot ignore that all recruited studies were not equally designed. The population characteristics, follow-up duration and degree of adjustment were different across studies. The retrospective nature of the studies and limited number of included studies might affect the conclusions. Second, different studies used different indexes. Overall, we used the HR, but as some studies did not report the HR, we had to calculate and use the OR instead. The follow-up times were different among the included studies such that we cannot fully explain the event rates in the active and control groups equally. Third, only articles written in English were included, which may have introduced selection bias. Finally, all studies were conducted in Japan, which limits generalizability of the conclusions. According to our findings, we support that TSP is an effective therapy for achieving CR in patients with IgA nephropathy. Additionally, more attention needs to be paid on IgA nephropathy.
Funding
Our study is supported by Shanghai Three-year Planning on Public Health System Construction (SCREENING Study GWIV-18).
Competing and conflicting Interests
None.
References
- Berger J, Hinglais N. Less depots inter capillaries d’IgA–IgG. Urol. Nephrol. (Paris). 74, 694–695 (1968).
- Amico DG, Imbasciati E, Barbiano DBG et al. Idiopathic IgA meningeal nephropathy–Clinical and histological study of 374 patients. Medicine. 64, 49–60 (1985).
- ChauveauD, Droz D. Follow up evaluation of the first patients with IgA nephropathy described at Necker hospital contributions to nephrology. 104, 1–5 (1993).
- Barratt J, Feehally J. Treatment of IgA nephropathy. Kidney. International. 69, 1934–1938 (2006).
- Wyatt RJ, Julian BA. IgA nephropathy. N. Engl. J. Med. 368, 2402–2414 (2013).
- Floege J. The pathogenesis of IgA nephropathy what is new and how does it change therapeutic approaches. Am. J. Kidney. Dis. 58, 992–1004 (2011).
- Pozzi C, Andrulli S, Del VL et al. Corticosteroid effectiveness in IgA nephropathy long term results of a randomized controlled trial. JASN. 15, 157–163 (2004).
- Chen Y, Tang Z, Wang Q et al. Long–term efficacy of tonsillectomy in Chinese patients with IgA nephropathy. Am. J. Nephrol. 27, 170–175 (2007).
- Wang, Y, Chen J, Chen Y et al. A meta–analysis of the clinical remission rate and long–term efficacy of tonsillectomy in patients with IgA nephropathy (Provisional abstract). Nephrol. Dial. Transplant. 1923–1931(2011).
- Hotta O, Miyazaki M, Furuta T et al. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am. J. Kidney. Dis. 38, 736–743 (2001).
- Spruance S, Reid J, Grace et al. Hazard ratio in clinical trials. Antimicrob. Agents. Chemother. 48, 2787–2792 (2004).
- Higgins J, Thompson S, Deeks J et al. Measuring inconsistency in meta–analyses. BMJ Clinical. Research. Ed. 327, 557–560 (2003).
- Higgins J, Thompson S. Quantifying heterogeneity in a meta–analysis. Stat. Med. 21, 1539–1558 (2002).
- Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 50, 1088–1101 (1994).
- Egger M, Smith G D, Schneider M et al. Bias in meta–analysis detected by a simple graphical test. BMJ. 315, 629–634 (1997).
- Kawamura T, Yoshimura M, Miyazaki Y et al. A multicentre randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin nephropathy. European. Renal. Association. 29, 1546–1553 (2014).
- Komatsu H, Fujimoto S, Hara S et al. Effect of tonsillectomy plus steroid pulse therapy on clinical remission of IgA nephropathy a controlled study. CJASN. 3, 1301–1307 (2008).
- Miyazaki M, Hotta O, Komatsuda A et al. A multicentre prospective cohort study of tonsillectomy and steroid therapy in Japanese patients with IgA nephropathy a 5–year report. IgA. Nephropathy. Today. 157, 94–98 (2007).
- Miyamoto T, Nishino T, Nakata et al. Impact of tonsillectomy combined with steroid pulse therapy on immunoglobulin A nephropathy depending on histological classification: a multicentre study. J. Clin. Exp. Nephrol. 20, 50–57 (2016).
- Hoshino Y, Moriyama T, Uchida K et al. Comparison of oral steroids with tonsillectomy plus steroid pulse therapy in patients with IgA nephropathy. J. Clin. Exp. Nephrol. (2016).
- Ochi A, Moriyama, T, Takei T et al. Comparison between steroid pulse therapy alone and in combination with tonsillectomy for IgA nephropathy. Inter. Urol. Nephrol. 45, 469–476 (2013).
- Nakagawa N, Kabara M. Retrospective comparison of the efficacy of tonsillectomy with and without steroid–pulse therapy in IgA nephropathy patients. Inter. Med. 51, 1323–1328 (2012).
- Kawaguchi T, Ieiri N, Yamazaki S et al. Clinical effectiveness of steroid pulse therapy combined with tonsillectomy in patients with immunoglobulin a nephropathy presenting glomerular haematuria and minimal proteinuria. Nephrology. 15, 116–123 (2004).
- Hotta O. Use of corticosteroids, other immunosuppressive therapies, and tonsillectomy in the treatment of IgA nephropathy. Semin. Nephrol. 24, 244–255 (2004).
- Giannakakis K, Feriozzi S, Perez M et al. Aberrantly glycosylated IgA1 in glomerular immune deposits of IgA nephropathy. J. Am. Soc. Nephrol. 18, 3139–3146 (2007).
- Sato M, Hotta O, Tomioka S et al. Cohort study of advanced IgA nephropathy: efficacy and limitations of corticosteroids with tonsillectomy. Nephron. Clinical. Practice. 93, 137–145 (2003).
- Liu LL, Wang LN, Jiang Y et al. Tonsillectomy for IgA nephropathy a meta–analysis. American journal of kidney diseases the official journal of the National Kidney Foundation. 65, 80–87 (2015).