Review Article - Interventional Cardiology (2013) Volume 5, Issue 4
Transcatheter pulmonary valve replacement: valves, techniques of implantation and outcomes
- Corresponding Author:
- Ziyad M Hijazi
Rush Center for Congenital & Structural Heart Disease
Rush University Medical Center, 1653 West
Congress Parkway, Jones 770, Chicago, IL 60612, USA
Tel: +1 312 9426800
Fax: +1 312 9428979
E-mail: zhijazi@rush.edu
Abstract
Transcatheter pulmonary valve replacement (tPVR) has revolutionized the world of interventional congenital and structural cardiology. Congenital heart disease affects up to one in every 100 live births in the USA and the tPVR procedure has allowed many of these patients to undergo a less invasive procedure instead of additional cardiac surgery. tPVR is performed primarily in patients with a right ventricle (RV) to pulmonary artery (PA; RV-PA) conduit and/or a bioprosthetic valve; however, it can also be performed in patients whose native right ventricular outflow tract (RVOT) was repaired surgically if there is significant stenosis. The RVOT can be stented, creating a ‘conduit’ between the RV and the PA.
Keywords
congenital heart disease, pulmonary regurgitation, pulmonary stenosis, pulmonary valve, right ventricular failure, transcatheter pulmonary valve replacement
Transcatheter pulmonary valve replacement (tPVR) has revolutionized the world of interventional congenital and structural cardiology. Congenital heart disease affects up to one in every 100 live births in the USA [1] and the tPVR procedure has allowed many of these patients to undergo a less invasive procedure instead of additional cardiac surgery. tPVR is performed primarily in patients with a right ventricle (RV) to pulmonary artery (PA; RV-PA) conduit and/or a bioprosthetic valve; however, it can also be performed in patients whose native right ventricular outflow tract (RVOT) was repaired surgically if there is significant stenosis. The RVOT can be stented, creating a ‘conduit’ between the RV and the PA.
In patients with a RVOT obstruction, implantation of a RV-PA conduit allows for the treatment and palliation of complex congenital heart disease that had previously been untreatable, and has contributed to the current survival rate of over 85% for congenital heart disease patients into adulthood [2–4]. Many patients with cardiac anomalies afflicting the RVOT, including pulmonary atresia with ventricular septal defect, Tetralogy of Fallot and truncus arteriosus, may require surgical correction with a conduit in the early neonatal period to improve blood flow to the lungs. Conduits are also used in patients with congenital aortic valve abnormalities when undergoing the Ross procedure (autotransplantation of the native pulmonary valve in the aortic position and placement of a conduit between the RV and the PA instead of the pulmonary valve being used for the aortic position). Owing to the inevitable development of pulmonary regurgitation and/or stenosis owing to calcification and thrombosis, these conduits have a limited lifespan [5,6]. On average, conduit replacement is necessary between 10 and 15 years postsurgical implantation in adults [7]. However, in children, this time interval may be considerably shorter. This means that patients who had their first conduit placed during infancy would require four or more operations over their lifespan. There is significant morbidity and mortality involved in reoperations in the setting of RV failure in these patients, making a less invasive option highly desirable.
History
In 2000, Bonhoeffer et al. published the first case report of a tPVR in an ovine model [8]. In this initial report, a fresh bovine jugular vein containing a native biological valve was sewn inside a platinum iridium stent. After being hand crimped onto a balloon catheter, device insertion was attempted via the internal jugular approach in 11 lambs. The device was successfully deployed in seven of the animal models and was in the desired location (native pulmonary valve) in five. The stents were explanted and examined 2 months after implantation. Four out of the five revealed the valves to be mobile and competent, with one valve displaying slight stenosis and macroscopically visible calcification.
Only 2 months after the initial animal trial, the first report of implantation of the valve in a human was published by the same group [9]. The patient was a 12-year-old boy with pulmonary atresia and a ventricular septal defect who had an 18-mm Carpentier-Edwards™ valve (Edwards Lifesciences, CA, USA) conduit from the RV to the PA placed at the age of 4 years. He was in the New York Heart Association (NYHA) functional class II, and echocardiography demonstrated significant stenosis and insufficiency of the conduit, leading to moderate dilatation of the RV. The above-mentioned bovine jugular venous valve was sewn onto a platinum stent and was successfully delivered into the degenerated valve of the conduit via the right femoral vein. There were no procedural complications and postimplantation hemodynamic study demonstrated a reduction in RV pressure. Echocardiography confirmed a competent pulmonary valve with no residual insufficiency.
Available valves
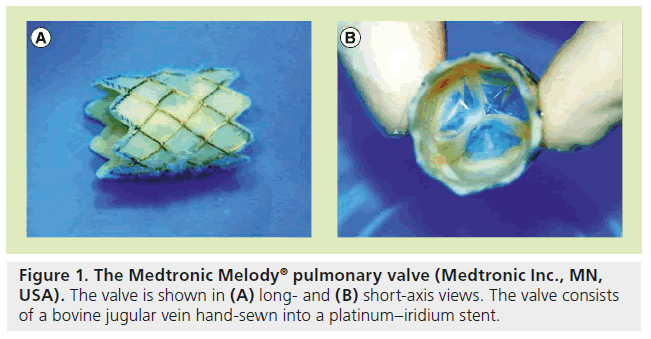
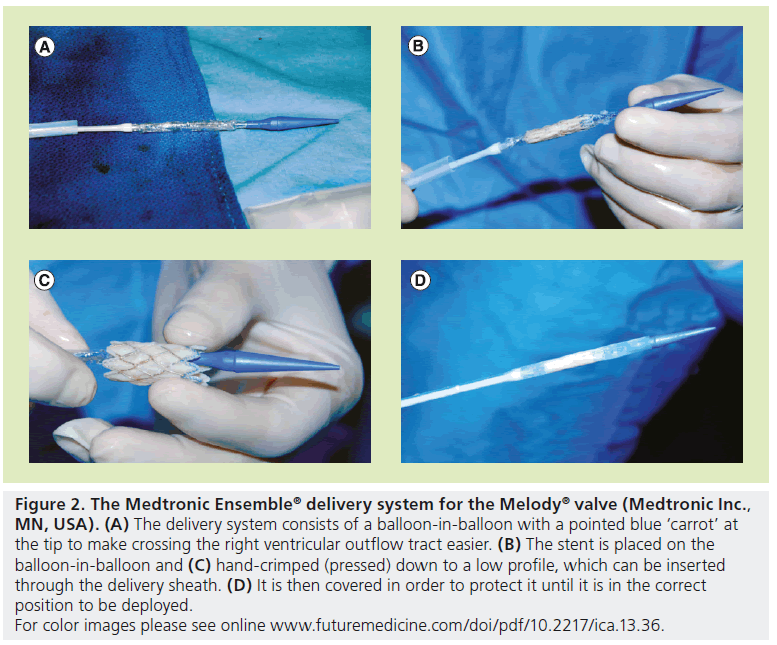
There are currently two available valves for percutaneous replacement in the pulmonary position. Bonhoeffer’s valve design was acquired by Medtronic and renamed the Melody® valve (Medtronic Inc., MN, USA), and was the first US FDA-approved percutaneous heart valve. It received approval in January 2010 under the Humanitarian Device Exemption program, which supports the development of medical devices intended for use in fewer than 4000 patients per year. Therefore, the Melody valve can only be used at medical institutions with an overseeing institutional review board [101]. The Melody valve continues to use a bovine internal jugular vein and the valve is sewn into a platinum iridium stent (Figure 1) [8,9]. The valve can be expanded up to 22 mm and requires a 22-Fr sheath for delivery. It is delivered using the Ensemble delivery® catheter system (Medtronic Inc.), which consists of a balloon-in-balloon (BiB; NuMED, NY, USA) at its distal end, onto which the valve is front loaded and crimped (Figure 2). The valve is covered by a retractable sheath, until it is at the target lesion, where the retractable sheath is withdrawn for valve deployment.
Figure 2: The Medtronic Ensemble® delivery system for the Melody® valve (Medtronic Inc., MN, USA). (A) The delivery system consists of a balloon-in-balloon with a pointed blue ‘carrot’ at the tip to make crossing the right ventricular outflow tract easier. (B) The stent is placed on the balloon-in-balloon and (C) hand-crimped (pressed) down to a low profile, which can be inserted through the delivery sheath. (D) It is then covered in order to protect it until it is in the correct position to be deployed.
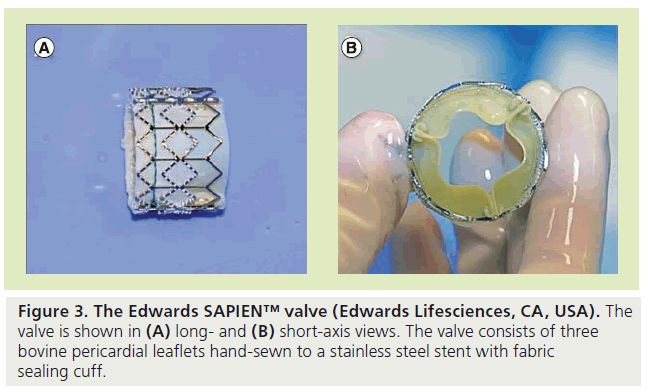
The second available valve is the Edwards SAPIEN™ valve (Edwards Lifesciences), which was FDA approved in November 2011 for use in patients with severe symptomatic aortic stenosis who are not surgical candidates (Figure 3) [102]. Although it was initially developed as a percutaneous substitute to surgical valve replacement in the aortic position [10,11], it has also emerged as a potential alternative to the Melody valve in the pulmonary position. It has been used successfully in RV-PA conduits since 2005 [12]. Since the SAPIEN valve is not currently FDA approved for use in the pulmonary position, it can only be implanted in centers participating in ongoing clinical trials for pulmonary indication or can be used off-label in the USA. In Europe, the SAPIEN and Melody valves have received CE mark and are widely used in the pulmonary position.
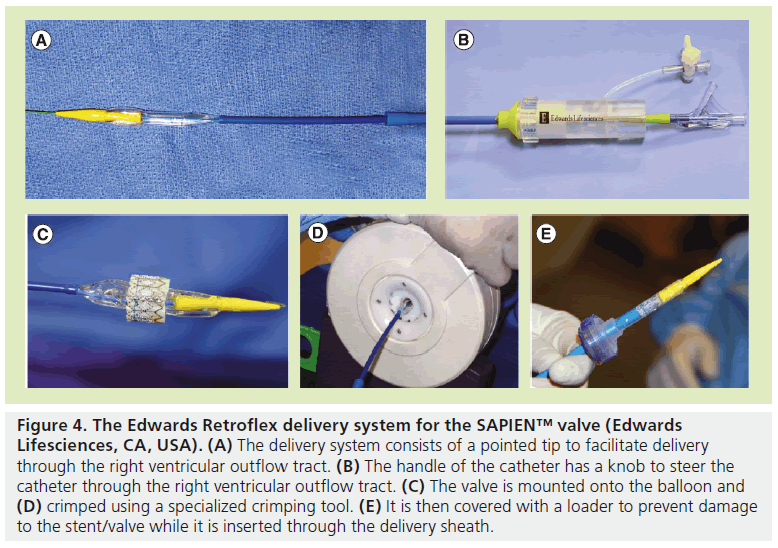
The SAPIEN valve is made up of three equalsized bovine pericardial leaflets that are handsewn to a stainless steel, balloon-expandable stent. There is a fabric cuff covering the lower portion of the stent to enable a seal with the calcified conduit and to prevent paravalvular leakage. The leaflet material has been designed to reduce leaflet stress and maximize coaptation. The pericardial tissue is processed with Thermafix™ (Edwards Lifesciences) anticalcification treatment, which is the same treatment utilized in the Carpentier-Edwards PERIMOUNT Magna surgical valve (Edwards Lifesciences). In the USA, it is currently available with 23 and 26 mm diameters with heights of 14.5 and 16 mm, respectively [13]. This valve can therefore be used in conduits up to 24 mm. The delivery system for the SAPIEN valve is the Retroflex III™ (Edwards Lifesciences), with a tapered-nose cone-shaped balloon catheter and a deflectable guiding catheter that requires either a 22 or 24 Fr hydrophilic sheath for the 23- and 26-mm valves, respectively (Figure 4) [13]; the sheath is 35 cm long. The Retroflex catheter has a handle with a control knob that can deflect the catheter through a passage into the RVOT.
Figure 4: The Edwards Retroflex delivery system for the SAPIEN™ valve (Edwards Lifesciences, CA, USA). (A) The delivery system consists of a pointed tip to facilitate delivery through the right ventricular outflow tract. (B) The handle of the catheter has a knob to steer the catheter through the right ventricular outflow tract. (C) The valve is mounted onto the balloon and (D) crimped using a specialized crimping tool. (E) It is then covered with a loader to prevent damage to the stent/valve while it is inserted through the delivery sheath.
Currently, the next generation of the SAPIEN valve, the SAPIEN™ XT (Edwards Lifesciences) is being evaluated for use in the aortic position in the PARTNER II trial [14]. In this newer version, the stent material has been changed to a cobalt–chromium alloy, which allows for a smaller delivery profile and sheath size. In addition, the valve is now available with a 29 mm diameter. There are case reports of this valve being used in the pulmonary position [15]; however, there are no available long-term data for this newer generation valve.
Both available valve systems have their unique benefits and drawbacks (Table 1). The SAPIEN valve is available in larger sizes than the current Melody system and, therefore, may be appropriate for placement in larger conduits, as are typically found in older patients. However, there have been reports of the Melody valve being used successfully in conduits up to 24 mm [16]. It is also important to remember that it is not necessarily the original conduit size, but the final size of the conduit after presenting that determines what size valve to use. The SAPIEN valve also has a shorter height, which is beneficial in certain anatomy; however, prestenting is necessary in order to give the short valve an adequate landing zone. The Melody delivery system, however, is less bulky and the retractable sheath protects the valve until it is deployed in the desired location. The bulkier delivery system of the SAPIEN valve makes it potentially more difficult to implant, especially in patients with a tortuous RVOT. Careful consideration must be given to the likelihood of procedural success before attempting valve implantation as the SAPIEN system does not use a covering sheath; therefore, once it exits its delivery sheath it may be difficult to retract. A single- center retrospective analysis of 33 patients who had undergone tPVR demonstrated comparable results in both types of valves [17].
| Characteristic | Melody valve (Medtronic Inc., MN, USA) [103] | SAPIEN valve (Edwards Lifesciences, CA, USA) [104] |
|---|---|---|
| Stent material | Iridium 10%, platinum 90% | Stainless steel |
| Valve material | Bovine jugular vein | Bovine pericardium treated with Thermafix™ |
| Available diameter size (mm) | 18, 20 and 22 | 23 and 26 |
| Stent height (mm) | 34 | 14.5 and 16 |
| Delivery sheath size (Fr) | 22 | 22 and 24 |
Table 1. Comparison of the Melody® and the SAPIEN™valves.
Indications
Since the procedure was first described 13 years ago, the field of tPVR has made great strides and the procedure has become much more widely practiced. The 2010 American Heart Association statement on the Indications for Cardiac Catheterization and Intervention in Pediatric Cardiac Disease was expanded to include a class IIa indication for tPVR. It recommends that: “It is reasonable to consider percutaneous pulmonary valve replacement in a patient with an RV-PA conduit with associated moderate-to-severe pulmonary regurgitation or stenosis, provided the patient meets inclusion/exclusion criteria for the available valve (Level of Evidence: B)” [18].
The inclusion and exclusion criteria for both the Melody and SAPIEN valve trials are summarized in (Table 2). However, the type of valve ultimately used is not decided by these inclusion and exclusion criteria, but is based on the patient’s anatomy and valve/conduit morphology. These criteria are based on surgical indications for RVOT revision. However, there is a lot of controversy surrounding the optimal timing of surgery to prevent irreversible RV damage. A study published in 2008 suggests that an aggressive surgical approach with an indexed RV end diastolic volume <150 ml/m2 at the time of surgery leads to improved RV function as well as normalization of RV volumes [19]. Another study suggested that an indexed RV end diastolic volume between 150 and 200 ml/m2 is an ideal window in which to perform pulmonary valve replacement in patients with Tetralogy of Fallot in order to promote RV remodeling and improve function [20]. However, the current American College of Cardiology/American Heart Association guidelines for repeat surgery in RV-PA conduits are fairly vague and are based primarily on symptoms and subjective assessments [4]. The typical criteria used if the patient is asymptomatic include a pulmonary regurgitant fraction of >40%, RV ejection fraction of <40% and an indexed RV end diastolic volume of >150 ml/m2, as determined by cardiac MRI. However, if the patient is symptomatic owing to severe pulmonary regurgitation or stenosis, then such criteria are not strictly enforced. Furthermore, attention to the QRS duration in patients with severe pulmonary regurgitation should be taken into account. A duration of >180 ms is associated with ventricular arrhythmias and sudden death [21].
| Criteria | Melody valve (Medtronic Inc., MN, USA) | SAPIEN valve (Edwards Lifesciences, CA, USA) |
|---|---|---|
| Inclusion | Age ≥5 years | Weight >35 kg |
| Original conduit diameter ≥16 mm | In situ conduit≥16 and≤24 mm | |
| Echocardiographic RVOT conduit dysfunction: | Dysfunctional RVOT conduit: | |
| – Patients classified as NYHA functional classes II, III and IV: | – ≥3 + PR by transthoracic echocardiography | |
| Doppler mean gradient ≥35 mmHg or at least moderate PR | – Pulmonary regurgitant fraction ≥40% | |
| – Patients classified as NYHA class I: Doppler mean gradient | – With or without pulmonary stenosis | |
| ≥40 mmHg or severe PR associated with TV annulus z-score | ||
| ≥2 or RVEF <40% | ||
| Exclusion | Active endocarditis | Active infection requiring antibiotics† |
| Major progressive noncardiac disease | History of, or active, endocarditis | |
| Central vein occlusion or significant obstruction | Intravenous drug abuse | |
| Pregnancy | Pre-existing prosthetic heart valve in any position | |
| Intravenous drug abuse | Pregnancy | |
| Contraindication to MRI | Severe chest wall deformity | |
| ECG evidence of intracardiac mass, thrombus or vegetation | ||
| Known intolerance to aspirin or heparin |
Table 2. Inclusion and exclusion criteria for US Melody® and SAPIEN™ valve trials.
Procedural technique
▪ Preprocedural assessment
Cardiac MRI has become a crucial component of patient selection for tPVR. MRI can help assess the severity of pulmonary valve dysfunction by calculating the regurgitant fraction, the RV ejection fraction and end-diastolic volumes. In addition, valuable information about the patient’s anatomy can be obtained by MRI, such as native RVOT dimensions, degree of conduit stenosis and the distance of the coronary arteries from the outflow tract or conduit, which is a critical step in the evaluation.
▪ Vascular access & procedure setup
tPVR is performed under general endotracheal anesthesia in the USA. However, in Europe, the procedure has been performed successfully under deep sedation. The femoral vein is the preferred route of delivery; however, the procedure can also be performed via the internal jugular vein. A small sheath (7 Fr) is used initially in the vein to perform right heart catheterization and angiography, and is later upsized to a larger sheath, depending on the size of the valve chosen. In addition, arterial access is obtained (5 or 6 Fr) for aortic root or selective coronary angiography. Once vascular access is obtained, the patient is given intravenous heparin for a goal activated clotting time of >200 s. The research protocols also include starting the patients on 81 mg of aspirin (for adult patients) the night prior to the procedure; however, this is not something routinely performed in our practice. All patients should be given antibiotic prophylaxis during the procedure and for the 24 h following the procedure.
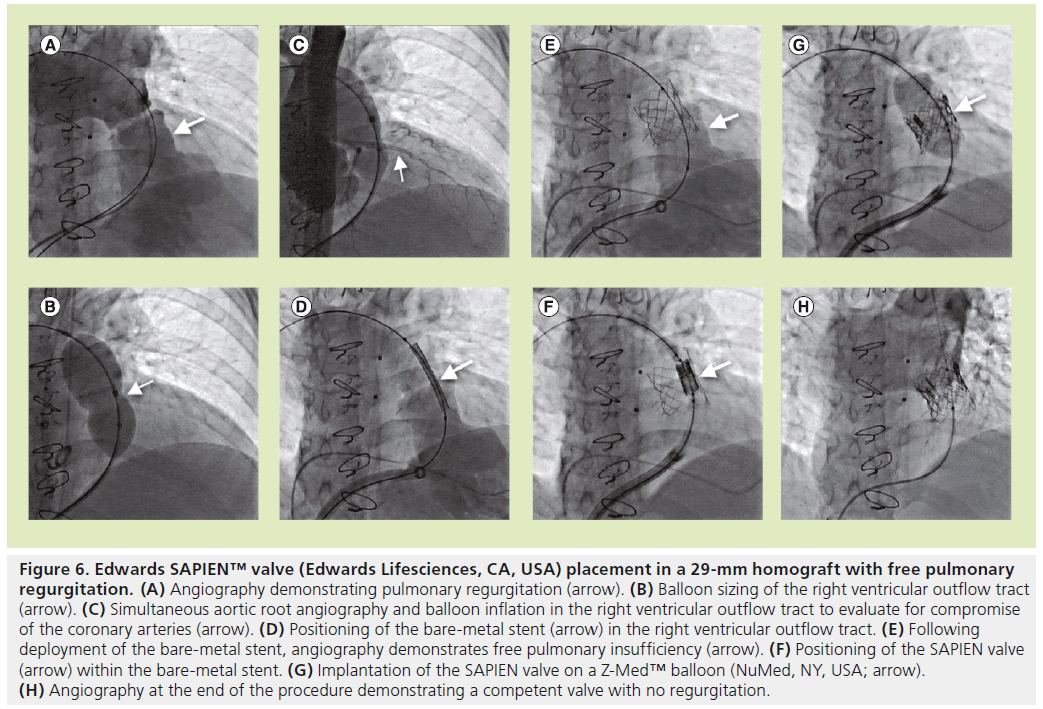
Standard right heart catheterization is performed to assess the preprocedural hemodynamics and the pressure gradient across the dysfunctional valve. Angiographic assessment of the RV-PA conduit is performed utilizing biplanar angiography to assess the degree of pulmonary regurgitation (Figures 5A & 6A) and the level of narrowing. Alternatively, 3D rotational angiography may be utilized to assess the RVOT in laboratories where it is available. The minimum diameter of the conduit can also be measured while inflating a sizing balloon across the pulmonary valve (Figure 6B). Aortic root angiography or selective coronary angiography is performed with simultaneous balloon inflation in the RVOT to assess for possible coronary artery compression (Figures 5B & 6C). The authors prefer to use a compliant sizing balloon for this purpose. This step is critical owing to the presence of coronary artery origin abnormalities in some patients with congenital heart disease. In the authors’ practice, if the distance between the RVOT and the origin of the left main coronary artery is more than 15 mm, balloon inflation in the RVOT is not often carried out; however, some operators advocate balloon testing in all cases, regardless of the distance of the RVOT from the coronary arteries. Furthermore, attention should also be given to the origin of the right coronary artery, not just the left; the operator has to make sure that a final conduit diameter will not impinge on the coronary flow.
Figure 5: Melody® valve (Medtronic Inc., MN, USA) placement in an 18-mm right ventricle to pulmonary artery homograft with severe pulmonary regurgitation. (A) Angiography demonstrating free pulmonary egurgitation (arrow). (B) Simultaneous aortic root angiography and balloon inflation in the right ventricular outflow tract to evaluate for compromise of the coronary arteries (arrow). (C) Positioning of the bare-metal stent (arrow) in the right ventricular outflow tract. (D) Following deployment of the bare-metal stent, angiography demonstrates free pulmonary insufficiency (arrow). (E) Positioning of the Melody valve (arrow) within the bare-metal stent. (F) Implantation of the Melody valve using a balloon-in-balloon. (G) Angiography at the end of the procedure demonstrating a competent valve with no regurgitation. (H) Short-axis view of the valve (large arrow) and stent showing uniform circular deployment (small arrow).
Figure 6: Edwards SAPIEN™ valve (Edwards Lifesciences, CA, USA) placement in a 29-mm homograft with free pulmonary regurgitation. (A) Angiography demonstrating pulmonary regurgitation (arrow). (B) Balloon sizing of the right ventricular outflow tract (arrow). (C) Simultaneous aortic root angiography and balloon inflation in the right ventricular outflow tract to evaluate for compromise of the coronary arteries (arrow). (D) Positioning of the bare-metal stent (arrow) in the right ventricular outflow tract. (E) Following deployment of the bare-metal stent, angiography demonstrates free pulmonary insufficiency (arrow). (F) Positioning of the SAPIEN valve (arrow) within the bare-metal stent. (G) Implantation of the SAPIEN valve on a Z-Med™ balloon (NuMed, NY, USA; arrow). (H) Angiography at the end of the procedure demonstrating a competent valve with no regurgitation.
▪ Prestenting
Prestenting of the RVOT with a bare-metal stent was not a part of the initial tPVR using the Melody valve protocol. However, owing to the high incidence of stent and valve fractures [22,23], this step has now become a routine part of the Melody valve implantation procedure. It is thought that more layers of metal around the valve would provide a grater resistance to fracture. This was validated in a bench model using a computerized finite element analysis [23,24]. Due to the short height of the SAPIEN transcatheter heart valve, we have incorporated stent implantation from the beginning in order to provide a landing zone for the valve. The baremetal stent is usually deployed on a BiB catheter over a stiff guidewire placed in either branch of the PA, but preferably the left (Figures 5C, 5D, 6D & 6E). General recommendations are to inflate the balloon to a diameter of up to 2 mm less than the original conduit size in stenotic conduits or slightly larger in conduits with no stenosis [25]. If there is significant recoil of the stent after BiB deflation, post dilation using a high-pressure balloon may be needed or, in some cases, multiple stents may be placed to create an adequate landing zone for the valve. In heavily calcified conduits, which are at risk for rupture after balloon inflation, covered stents may be used instead of bare-metal stents for prestenting in an effort to prevent rupture. Covered stents are not readily available in the USA, but may be fashioned by the operator in the laboratory by sewing Gore-Tex® (WL Gore & Associates, Inc., DE, USA) onto a bare-metal stent [26].
▪ Valve implantation
The final valve size is guided by the size of the bare-metal stent used for presenting of the RVOT. It is important to measure the fully expanded stent diameter in 2D (utilizing biplanar fluoroscopy) to ensure symmetrical stent expansion. In general, we implant the 23-mm SAPIEN valve to be no less than 21 mm in diameter and the 26-mm SAPIEN valve to be no less than 23 mm in diameter. The valve has been tested for durability and functionality for these diameters. Prior to valve insertion, the SAPIEN valve is crimped symmetrically using a specialized crimping device onto a 30-mm long presized balloon catheter. The Melody valve is hand crimped on an appropriately sized BiB catheter. The crimped valve is delivered across the pre stented outflow tract over a suprastiff guidewire (Meier wire [Boston Scientific, MA, USA] or Lunderquist wire [Cook Medical, IN, USA]). Multiple angiograms are performed prior to balloon inflation to ensure proper positioning of the stent (Figures 5E, 5F, 6F & 6G).
▪ Poststent imaging
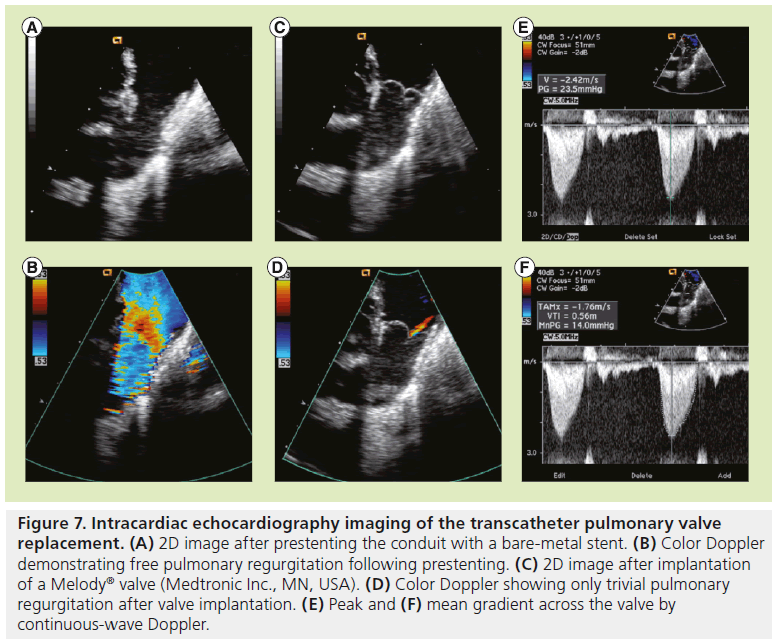
Valve performance is evaluated by angiography (Figures 5G & 6H) and/or intracardiac echocardiography. Continuous-wave Doppler as well as color Doppler across the valve are used to evaluate the gradient and assess for any perivalvular or valvular regurgitation (Figure 7).
Figure 7: Intracardiac echocardiography imaging of the transcatheter pulmonary valve replacement. (A) 2D image after prestenting the conduit with a bare-metal stent. (B) Color Doppler demonstrating free pulmonary regurgitation following prestenting. (C) 2D image after implantation of a Melody® valve (Medtronic Inc., MN, USA). (D) Color Doppler showing only trivial pulmonary regurgitation after valve implantation. (E) Peak and (F) mean gradient across the valve by continuous-wave Doppler.
▪ Hemostasis
It is recommended that venous hemostasis is achieved by utilization of a vascular closure device, such as two Perclose Proglide® sutures (Abbott Vascular, IL, USA), placed at the beginning of the procedure [23]. However, we have also utilized the ‘figure-of-eight’ suture effectively [27].
▪ Discharge & follow-up
Patients are usually kept for observation overnight and are discharged home the following day on 81-mg aspirin for 1 year. Follow-up examination and echocardiography are performed at 1, 6 and 12 months, and yearly thereafter. Chest radiographics were obtained at baseline and at 6 months to identify valve/stent position and any potential stent fracture.
Potential complications
There are multiple potential serious complications that are associated with tPVR. These can be divided into immediate complications and long-term complications. Immediate complications occur during or immediately following the procedure. These include: device embolization or migration; coronary artery compression or dissection; obstruction of either PA by the stent or device; damage to the tricuspid valve; guidewire injury of PA resulting in pulmonary hemorrhage; conduit rupture; femoral vein thrombosis or hematoma; arrhythmias; and hemodynamic instability. While these complications were initially reported to be as high as 12% in early smaller tPVR studies, more recent trials have shown a decrease of these adverse events to 5–6% [28,29]. This decrease in procedural complications is most probably owing to increased operator experience. In 2008, Bonhoeffer’s group published a study looking at the learning curve for tPVR since it was first used in 2001. They reported that after their initial 50 patients, the incidence of procedural complications fell to 2.9% [30].
Many of these potential complications can be avoided by taking some precautions. For example, using a vascular closure device, such as the Perclose (preclose with one or two sutures placed at the beginning of the case), may reduce access site complications. While there are no randomized trials to support the theory that vascular closure reduces vascular complications when compared with manual pressure, a recent study demonstrated that the technique was safe and effective in 243 patients undergoing structural interventions with no resulting vascular complications [31]. Some groups also advocate using a venous cutdown and repair of the vessel at the end of the procedure. The potential for coronary artery compression is not an uncommon problem; as many as 4.4% of the US Melody cohort had unsuitable anatomy and, therefore, did not undergo valve implantation [29]. This complication can be avoided by a computed tomography/magnetic resonance angiogram prior to the procedure to assess the distance between the conduit and the coronary artery. In addition, simultaneous nonselective aortic root angiography or selective coronary angiography with a balloon inflated in the RVOT in more than one view has become a routine part of the tPVR procedure and should prevent this catastrophic complication. In one case, which did not reveal coronary compression in the frontal view but was suggestive of compression in the lateral view, we used intravascular ultrasound in the left main coronary artery during balloon inflation to verify the suspicion. In this case, there was a greater than 50% reduction in the luminal area during balloon inflation, and tPVR was abandoned. Conduit rupture is a serious life-threatening complication, which may necessitate converting to open surgery. However, utilization of covered stents either prophylactically (when available) or as a bailout may be an effective way to avoid surgery in this situation. In situations where conduit rupture occurs distally to the stent and is caused by the distal end of the balloon catheter, covered stents may not be helpful as further stenting may propagate the tear. We believe that laboratories performing tPVR should have the appropriate-sized covered stents or stent grafts available either in the laboratory or easily and rapidly accessible nearby in the institution. Stent migration and embolization are typically successfully treated with percutaneous device retrieval and redeployment or surgery. Bailout periventricular pulmonary valve implantation following failed percutaneous attempts with stent migration has also been reported [32]. When these potential complications do occur, they can be catastrophic and require very quick actions. Therefore, it is imperative to have close collaboration between the interventional cardiologists and cardiac surgery colleagues. We perform all of our cases in a hybrid catheterization laboratory, which permits quick transition to a surgical procedure without delay, if needed.
Long-term complications are typically diagnosed following the procedure. The need for RVOT reintervention with either a second valve or balloon dilatation of the existing valve during the follow-up period was reported to be 7–14% in the Melody valve trials [29,30]. One cause for reintervention was in-stent restenosis secondary to the ‘hammock effect’, which was owing to the way the valve was sutured inside the stent. Since the recognition of this phenomenon, the way in which the sutures are placed has been changed and now the whole length of the bioprosthetic tissue supporting the valve is sutured to the stent [33]. Another problem, which can necessitate reintervention, is stent fracture. This was initially a major limitation of the Melody valve, with rates of stent fracture reported to be between 12 and 28% [28–30,34]. Prestenting of the RVOT with a bare-metal stent is thought to reduce the rate of stent fracture to less than 6% [22,23]. Another potential long-term complication using the Melody valve is endocarditis. In one Melody valve study, five patients (3.2%) were diagnosed with endocarditis over a mean follow-up of 5 months [30]. The updated American Heart Association guidelines recommend continuing lifelong endocarditis prophylaxis for patients who have a conduit [35]. In patients without a conduit, endocarditis prophylaxis is recommended for 6 months following tPVR. Although there have been fewer procedures performed compared with the Melody valve, no cases of endocarditis or stent fracture have been reported using the SAPIEN valve in the pulmonary position. However, there have been reports of endocarditis of the SAPIEN valve in the aortic position [36].
Current literature
The first reports of tPVR in a large number of patients were published in 2005. This initial paper, detailing the experience with Melody valve implantation in 59 patients in Europe, showed the procedure to be safe, with a significant reduction in pulmonary regurgitant fraction and indexed right ventricular volumes, as well as improvement in NYHA functional class during a mean follow-up of 10 months. There were no mortalities reported and, at 12 months, there was an 83.3% freedom from surgical explantation for valve dysfunction and a major complication rate of 6% [27]. A further report from this group in 2008 demonstrated superior outcomes with a freedom from reoperation of 95% at a follow-up of 10 months and a complication rate of 3% after the operators had become more experienced [30].
Meanwhile, the multicenter US Melody valve trial was underway and, in 2009, the report of the first 34 patients appeared to be promising at 6-months follow-up [34]. The short- and medium-term outcomes were published in 2010 and demonstrated an ongoing high rate of procedural success (the valve was successfully placed in 100% of patients attempted), improvement in NYHA functional class and a 95.4% survival free from RVOT reintervention at 1 year [29].
In 2011, a German report of two centers performing 102 consecutive Melody valve cases between 2006 and 2010 was published. The procedure was performed successfully in all patients. Complications included one death from coronary artery compression and one surgical explantation for bacterial endocarditis. Eight patients required reintervention at a median follow-up of approximately 1 year [37]. Most recently, the results of the Italian Melody valve registry were published [38]. They reported that between 2007 and 2010, 63 patients were included in the registry; there were six major complications and freedom from valve failure at the latest follow-up was 81.4.
Despite the plethora of data on the SAPIEN valve in the aortic position, there are not many large registries for this valve in the pulmonary position. In 2011, the COMPASSION trial demonstrated an effective reduction of RVOT gradient with a reduction in clinical symptoms and maintenance of pulmonary valve competence at 6-months follow-up [25]. The study included 36 patients recruited from four centers (three from the USA and one European center). Implantation was successful in 97.1% of patients.
The most recent data on the SAPIEN valve in the pulmonary position are the initial results in 22 European patients [39]. They reported a 95.5% procedural success rate (21 of 22 patients). There were three reported complications, including inability to pass the valve past the inferior vena cava owing to severe occlusion, one stent embolization and one brachial plexus injury owing to arm positioning.
There have been no randomized controlled trials to evaluate the relative effectiveness of the two available valves. Our group recently published a retrospective analysis of 33 patients who underwent tPVR at our institution (20 with the SAPIEN valve and 13 with the Melody valve). It was shown that the two valves were comparable in efficacy at a median of 6 months of follow-up. There was a greater residual RVOT gradient with the SAPIEN valve initially, which was reduced on follow-up and may represent a more conservative early prestenting approach with this valve [17].
Conclusion
Over the last decade, tPVR has emerged as a viable alternative to surgical pulmonary valve replacement in patients with complex congenital heart disease. With the two currently available valves; the Medtronic Melody valve and the Edwards SAPIEN valve, the procedure has been shown to be safe in both children and adults with favorable long-term outcomes. The procedure is usually performed via the femoral vein or, occasionally, via the internal jugular vein. Patients are usually discharged the following day and much of the morbidity associated with open heart surgery is eliminated. The future for the procedure is exciting and we hope that the formal indications for tPVR in the USA will be expanded to include surgically patched RVOTs and even native RVOTs in some instances. In addition, the prospect of performing ‘valve-in-valve’ procedures will allow the time between surgical procedures to be prolonged even further. Newer valve technology with much smaller delivery systems is on the horizon.
Future perspective
More than a decade has passed since the first report of tPVR and, in that time period, huge strides have been made in this emerging field in interventional cardiology. Patients, who in the past have required multiple open heart surgeries with cardiopulmonary bypass, can now prolong the lifespan of their conduits with a minimally invasive procedure and an overnight stay in the hospital. Long-term outcomes post-tPVR appear to be promising and, as operators become more comfortable with the procedure, the low rate of procedural complications will drop even further [30].
Currently, tPVR in a patient without a RV-PA conduit is considered to be off-label. In an effort to expand the patient population in which tPVR is indicated, there have been reports of successful implantation of the Melody valve in patients with Tetralogy of Fallot with RVOT patch repair and pulmonic regurgitation [40]. However, tPVR in native RVOTs is currently difficult owing to the available valve sizes (up to 22 mm for Melody and up to 26 mm for SAPIEN). In 2012, Boshoff et al. described a series of 23 Belgian patients considered to be off-label in whom tPVR was performed safely and effectively. These patients included those with a ‘conduit-free’ RVOT following transannular/infundibular patch, small conduits (<16 mm) and two elderly patients with pulmonary valve stenosis and severe RVOT calcification [41]. Hopefully, with more reports of tPVR being performed in patients who have anatomy other than the typical RV-PA conduits, the indications for use will be expanded.
Another off-label indication for tPVR is the placement of a valve inside a previously placed bioprosthetic valve. Recently, the Melody investigators published a series of 104 patients who underwent implantation of a Melody valve within a previously placed surgical bioprosthetic valve that had degenerated. The procedure was successful, showing a significant decrease in RVOT gradient. At a median follow-up of 12 months, no patients had more than mild regurgitation [42]. A similar but smaller series, limited to children, was published 2 years earlier showing good outcomes with Melody valve implantation in patients with bioprosthetic valves [43]. One of the most exciting prospects of tPVR is the possibility of performing more than one transcatheter procedure on the same valve. This would allow lengthening the time between surgeries for these patients to up to 20 years, something that was unimaginable just a few years ago. This has been performed successfully, even with a Melody valve implanted within a previously placed SAPIEN valve and vice versa [44]. As valve technology improves, the hope is that we can eliminate the need for reoperation in some of these patients altogether.
Finally, two important advances are already underway, including the hybrid approach in smaller patients who cannot accommodate the delivery system. This will certainly expand the pool of patients eligible for this technology to prolong the lifespan of their conduits. The other advancement is miniaturization of the delivery system. In preclinical laboratory testing, we have been working on a new valve that requires a 12–14 Fr delivery system for valves ranging from 20–30 mm in diameter. This valve, known as Colibri (Colibri Heart Valve, LLC, CO, USA), is still being developed and, over the next few years, the hope is that we will be able to apply it in humans.
Financial & competing interests disclosure
ZM Hijazi is a consultant, serves on the advisory board and holds stock options in the Colibri Heart Valve. He is also a consultant to Venus Medical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Congenital heart disease
▪ Patients with right ventricle (RV) to pulmonary artery (PA) conduit will ultimately develop conduit failure leading to RV dysfunction, arrhythmias and possibly death.
▪ These patients may ultimately require four or more open heart surgeries over the course of their lifetime to replace the failing conduits.
History of transcatheter pulmonary valve replacement
▪ The first transcatheter pulmonary valve replacement (tPVR) was reported in 2000 by Bonhoeffer et al. The valve was constructed from a bovine internal jugular valve sewn onto a platinum–iridium stent.
Available valves
▪ Bonhoeffer’s valve design was purchased by Medtronic and renamed as the as Melody® valve (Medtronic Inc., MN, USA).
▪ The Edwards SAPIEN™ valve (Edwards Lifesciences, CA, USA), which was originally designed for implantation in the aortic position, has been implanted in the pulmonary position since 2005.
Indications
▪ Currently, tPVR has a level IIa indication by the American Heart Association guidelines.
▪ Indications are controversial but may include a pulmonary regurgitant fraction of >40%, RV ejection fraction of <40% and an indexed RV end diastolic volume of >150 ml/m2 as determined by cardiac MRI in conjunction with the patient’s clinical picture and symptoms.
▪ tPVR is approved for implantation in RV-PA conduits; however, many procedures have been performed worldwide in both native right ventricular outflow tract and previously placed bioprosthetic valves.
Procedural technique
▪ tPVR is typically performed via the femoral vein, but may be performed via the internal jugular vein.
▪ The available valve systems require a 20–24 Fr sheath.
▪ The procedure includes: a complete right heart catheterization for hemodynamic assessment; angiography to fully evaluate the failing valve or conduit, including coronary compression assessment; prestenting with a bare-metal or covered stent; and valve implantation over a stiff guidewire.
▪ Hemostasis is achieved using either two Perclose Proglide® suture devices (Abbott Vascular, IL, USA) or a ‘figure-of-eight’ suture.
Literature
▪ The US Melody valve trial and multiple large European registries have demonstrated a high success rate for the Melody valve (approaching 100%) with a low rate of complications. Freedom from reoperation was 95% at 10 months.
▪ The SAPIEN valve COMPASSION trial demonstrated an effective reduction of right ventricular outflow tract gradient with a reduction in clinical symptoms and maintenance of pulmonary valve competence at 6-months follow-up with a procedural success rate of 97%.
Future directions
▪ tPVR is being performed in patients without a RV-PA conduit as ‘off-label’.
▪ Other off-label indications include patients with previously placed bioprosthetic valves in the pulmonary position and ‘valve-in-valve’ in patients with previous tPVR.
▪ As valve technology is advancing, there will be even more possibilities for tPVR in the future.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am. Heart J. 147, 425–439 (2004).
- Bove EL, Lupinetti FM, Pridjian AK et al. Results of a policy of primary repair of truncusarteriosus in the neonate. J. Thorac. Cardiovasc. Surg. 105, 1057–1065 (1993).
- Rastelli GC, Wallace RB, Ongley PA. Complete repair of transposition of the great arteries with pulmonary stenosis. A review and report of a cases corrected by using a new surgical technique. Circulation 39, 83–95 (1969).
- Warnes CA, Williams RB, Bashore TM et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 118, 714–833 (2008).
- Abd El Rahman MY, Abdul-Khaliq H, Vogel M et al. Relation between right ventricular enlargement, QRS duration, and right ventricular function in patients with tetralogy of Fallot and pulmonary regurgitation after surgical repair. Heart 84, 416–420 (2000).
- de Ruijter FT, Wernicnk I, Hirchcock FJ et al. Right ventricular dysfunction and pulmonary valve replacement after correction of tetralogy of Fallot. Ann. Thorac. Surg. 73, 1794–1800 (2002).
- Therrien J, Siu SC, McLaughlin PR et al. Pulmonary valve replacement in adults late after repair of tetralogy of Fallot: are we operating too late? J. Am. Coll Cardiol. 36, 1670–1675 (2000).
- Bonhoeffer P, Boudjemline Y, Zakhia S et al. Transcatheter implantation of a bovine valve in pulmonary position: a lamb study. Circulation 102, 813–816 (2000).
- Bonhoeffer P, Boudjemline Y, Saliba Z et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 356, 1403–1405 (2000).
- Webb JB, Chandavimol M, Thompson CR et al. Percutaneous aortic valve implantationretrograde from the femoral artery. Circulation 113, 842–850 (2006).
- Leon MD, Smith CR, Mack M et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363, 1597–1607 (2010).
- Garay F, Webb J, Hijazi ZM. Percutaneous replacement of pulmonary valve using the Edwards-Cribier percutaneous heart valve: first report in a human patient. Catheter. Cardiovasc. Inter. 67, 659–662 (2006).
- Boone RH, Webb JG, Horlick E et al. Transcatheter pulmonary valve implantation using the Edwards SAPIEN™ transcatheter heart valve. Catheter. Cardiovasc. Interv. 75, 286–294 (2010).
- Webb JG, Altwegg L, Masson J et al. A new transcatheter aortic valve and percutaneous valve delivery system. J. Am. Coll. Cardiol. 53, 1855–1858 (2009).
- Lauten A, Hoyme M, Figulla HR. Severe pulmonary regurgitation after tetralogy-of-Fallot repair: transcatheter treatment with the Edwards SAPIEN XT heart valve. Heart 98, 623–624 (2012).
- Cheatham SL, Holzer RJ, Chisolm JL et al. The Metronic Melody® transcatheter pulmonary valve implanted at 24 mm diameter… IT works. Catheter. Cardiovasc. Interv. doi:10.1002/ccd.24821 (2013) (Epubahead of print).
- Faza NN, Kenny D, Kavinsky C et al. Single center comparative outcomes of the edwards SAPIEN and medtronic melody transcatheter heart valves in the pulmonary position. Catheter. Cardiovasc. Interv. doi:10.1002/ccd.24680 (2012) (Epub ahead of print).
- Feltes TF, Bacha E, Beekman RH 3rd et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation 123, 2607–2652 (2011).
- Frigiola A, Tsang V, Bull C et al. Biventricular response after pulmonary valve replacement for right ventricular outflow tract dysfunction: is age a predictor of outcome? Circulation 118(14 Suppl.), S182–S190 (2008).
- Buechel ER, Dave HH, Kellenberger CJ et al. Remodelling of the right ventricle after early pulmonary valve replacement in children with repaired tetralogy of Fallot: assessment by cardiovascular magnetic resonance. Eur. Heart J. 26(24), 2721–2727 (2005).
- Gatzoulis MA, Till JA, Somerville J et al. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation 92, 231–237 (1995).
- Demkow M, Biernacka EK, Spiewak M et al. Percutaneous pulmonary valve implantation preceded by routine presenting with a bare metal stent. Catheter. Cardiovasc. Interv. 77, 381–389 (2011).
- Nordmeyer J, Lurz P, Khambadkone S et al. Pre-stenting with a bare metal stent before percutaneous pulmonary valve implantation: acute and 1-year outcomes. Heart 97, 118–123 (2011).
- Schievano M, Petrini L, Migliavacca F et al. Finite element analysis of stent deployment: understanding stent fracture in percutaneous pulmonary valve implantation. J. Interven. Cardiol. 20, 546–554 (2007).
- Kenny D, Hijazi ZM, Kar S et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early Phase 1 results from an international multicenter clinical trial. J. Am. Coll. Cardiol. 58, 2248–2256 (2011).
- Kenny D, Cao QL, Kavinsky C, Hijazi ZM. Innovative resource utilization to fashion individualized coveredstents in the setting of aortic coarctation.Catheter. Cardiovasc. Interv. 78(3), 413–418 (2011).
- Morgan GJ, Waragai T, Eastaugh L et al. The fellows stitch: large caliber venous hemostasis in pediatric practice. Catheter. Cardiovasc. Interv. 80(1), 79–82 (2012).
- Khambadkone S, Coats L, Tahlor A et al. Percutaneous pulmonary valve implantation in humans results in 59 consecutive patients. Circulation 112, 1189–1197(2005).
- McElhinney DB, Hellenbrand WE, Zahn EM et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation 122, 507–516 (2010).
- Lurz P, Coats L, Khambadkone S et al. Percutaneous pulmonary valve implantation impact of evolving technology and learning curve on clinical outcomes. Circulation 117(15), 1964–1972 (2008).
- Hamid T, Rajagopal R, Pius C, Clarke B, Mahadevan VS. Perclose of large-sized venous access sites in adults undergoing transcatheter structural interventions. Catheter. Cardiovasc. Interv. 81, 586–590 (2013).
- Cubeddu R, Hijazi ZM. Bailout perventricular pulmonary valve implantation following failed percutaneous attempt using the Edwards SAPIEN transcatheter heart valve. Catheter. Cardiovasc. Interv. 77, 276–280 (2011).
- Schievano S, Petrini L, Migliavacca F et al. Finite element analysis of stent deployment: understanding stent fracture in percutaneous pulmonary valve implantation. J. Interven. Cardiol. 20, 546–554 (2007).
- Zahn EM, Hellenbrand WE, Lock JE et al. Implantation of the Melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit. J. Am. Coll. Cardiol. 54, 1722–1729 (2009).
- Wilson W, Taubert KA, Gewitz M et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 116, 1736–1754 (2007).
- Zytowski M, Erb M, Albes JM, Hartrumpf M. Infective endocarditis 4 months after transapical aortic valve implantation with Edwards SAPIEN™ XT. Eur. J. Cardiothorac. Surg. doi:10.1093/ejcts/ezt215 (2013) (Epubahead of print).
- Eicken A, Ewert P, Hager A et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. Eur. Heart J. 32(10), 1260–1265 (2011).
- Butera G, Milanesi O, Spadoni I et al. Melody transcatheter pulmonary valve implantation. Results from the registry of the Italian society of pediatric cardiology. Catheter. Cardiovasc. Interv. 81, 31–316 (2013).
- Haas NA, Moysich A, Neudorf U et al. Percutaneous implantation of the Edwards SAPIEN pulmonic valve: initial results in the first 22 patients. Clin. Res. Cardiol. 102(2), 119–128 (2013).
- Momenah T, El Oakley R, Al Najashi K et al. Extended application of percutaneous pulmonary valve implantation. J. Am. Coll. Cardiol. 53, 1859–1863 (2009).
- Boshoff DE, Cools BM, Heying R et al. Off-label use of percutaneous pulmonary valved stents in the right ventricular outflow tract: time to rewrite the label? Catheter. Cardiovasc. Interv. 81(6), 987–995 (2013).
- Gillespie M, Rome J, Levi D et al. Melody valve implant within failed bioprosthetic valves in the pulmonary position: a multicenter experience. Circ Cardiovasc. Interv. 5, 862–870 (2012).
- Asoh K, Walsh M, Hickey E et al. Percutaneous pulmonary valve implantation within bioprosthetic valves. Eur. Heart J. 31, 1404–1409 (2010).
- Nordmeyer J, Coats L, Lurz P et al. Percutaneous pulmonary valve-in-valve implantation: a successful treatment concept for early device failure. Eur. Heart J. 29, 810–815 (2008).
- Medtronic Melody® transcatheter pulmonary valve – H080002. New humanitarian device approval. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm199258.htm (Accessed 7 June 2013)
- Edwards SAPIEN™ Transcatheter Heart Valve (THV) – P100041. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm280840.htm (Accessed 7 June 2013)
- Medtronic. The Melody® System. www.medtronic.com/melody/melody-system. html (Accessed 3 April 2013)
- Edwards. Transcatheter heart valve. www.edwards.com/products/ transcathetervalve/Pages/THVcategory.aspx (Accessed 3 April 2013)
- COMPASSION: COngenital Multicenter Trial of Pulmonic VAlve Regurgitation Studying the SAPIEN InterventIONal THV. http://clinicaltrials.gov/show/NCT00676689 (Accessed 7 July 2013)
- Melody Transcatheter Pulmonary Valve Study: Post Approval Study of the Original IDE Cohort (Melody IDE). http://clinicaltrials.gov/ct2/show/ NCT00740870 (Accessed 7 July 2013)
▪ Only available report comparing the outcomes of Melody® (Medtronic Inc., MN, USA) valve and Edwards SAPIEN™ (Edwards Lifesciences, CA, USA) valve implantation in a single-center trial.
▪ Demonstrates better outcomes of transcatheter pulmonary valve replacement (tPVR) with prestenting, which has now become a routine part of the procedure.
▪▪ Reviews the data of tPVR using the Edwards SAPIEN valve.
▪▪ First series of patients treated with the Melody valve.
▪▪ First large series of Melody valve implantation in the USA.
▪ Demonstrates the importance of the learning curve and patient volume on improving procedural success and reducing complications.
▪ Reviews the use of tPVR in an expanded patient population.
▪ Reviews the use of tPVR in pre-existing percutaneous valves that have failed.
▪ Websites