Mini Review - Interventional Cardiology (2023)
Transcatheter pulmonary valve-in-valve implantation
- Corresponding Author:
- Shabana Shahanavaz
Department of Pediatrics, University of Cincinnati College of Medicine, Ohio, USA,
E-mail: shabana.shahanavaz@cchmc.org
Received date: 31-Jul-2023, Manuscript No. FMIC-23-108527; Editor assigned: 02-Aug-2023, PreQC No. FMIC-23-108527 (PQ); Reviewed date: 18-Aug-2023, QC No. FMIC-23-108527; Revised date: 25-Aug-2023, Manuscript No. FMIC-23-108527 (R); Published date: 04-Sep-2023, DOI: 10.37532/1755-5310.2023.15(S19).468
Abstract
Surgically placed Bioprosthetic Valves (BPV) are widely used in the pulmonary position for dysfunctional Right Ventricular Outflow Tract (RVOT) in patients with Tetralogy of Fallot (TOF) as well as other conotruncal anomalies. The longevity of BPV in the pulmonary position is variable with inevitable dysfunction secondary to calcification, leaflet thickening, and subclinical thrombosis. Surgical replacement of dysfunctional BPV has been largely replaced by Transcatheter Pulmonary Valve (TPV) implantation, using the BPV frame as a landing zone, i.e., Valve-in-Valve (VIV) with good outcomes that are comparable to surgical valve replacement. In this mini review, we summarize outcomes, limitations, and technical considerations for transcatheter VIV implantation in the pulmonary position.
Keywords
Bioprosthetic valve; Heart disease; Melody valve; Pulmonary valves
Introduction
Surgical implantation of a Bioprosthetic Valve (BPV) in the pulmonary position is one of the options for the treatment of postoperative Right Ventricular Outflow Tract (RVOT) dysfunction in patients with repaired congenital heart disease [1,2]. With the evolution of transcatheter technology initially with the balloon-expandable Melody valve (Medtronic Inc, Minneapolis, MN) over two decades ago and subsequently with the Sapien XT (Edwards Lifesciences LLC, Irvine, California) and Sapien S3 valves, Transcatheter Pulmonary Valve (TPV) implantation is now considered first-line therapy to manage RVOT dysfunction in patients with favorable anatomy [3]. Both transcatheter and surgically placed bioprosthetic valves are prone to dysfunction due to various mechanisms, including calcification, subclinical leaflet thrombosis, and leaflet thickening [1,2,4]. TPV implantation provides an effective, and less invasive way of treating dysfunctional pulmonary valves, aiming to improve hemodynamics and reduce the total number of sternotomies for patients with RVOT dysfunction. While originally approved for implantation in dysfunctional RV-PA conduit, TPV has been implanted within surgical BPV in the pulmonary position, as well as dysfunctional TPV [4,5].
Literature Review
Deterioration of BPV and TPV
Outcomes of surgical BPV valve placement have been variable and reported 5 and 10-year freedom from dysfunction is 53% to 92% and 20% to 73% [2,6,7]. Both porcine and bovine pericardial BPV have similar rates of valve dysfunction [6,8]. As for the Melody TPV, freedom from dysfunction at 5 and 10 years was 73% and 53%, respectively, and was higher in patients older than 21 years of age [9]. This was similar to another report, where freedom from valve replacement for Melody valve at 5 and 10 years was 78.15% and 50.4%, respectively [10]. The data for Sapien Valves is shorter rates of surgical valve replacement at 3 and 5 years were 97.6 ± 2.3% (CI; 93.2-100.0) and 94.3 ± 4.0% (CI; 87.7-100.0) with Sapien-XT and 97.8 ± 2.1% (CI; 95.3-100) and 97.8 ± 2.0% CI; 95.3-100) with S3, respectively [10]. The cumulative incidence of re-intervention for TPV from a large international registry was 25% at 8 years, which is comparable to surgical BPV re-intervention.
Outcomes of VIV for the pulmonary position
Transcatheter VIV represents an alternative to surgery and may reduce the total number of surgical interventions for patients with congenital heart disease. A recent meta-analysis indicated that the number of thoracotomies is a risk factor for VT in patients with tetralogy of Fallot [11]. Reports of transcatheter VIV in a dysfunctional BPV have shown high rates of immediate and short- term success [5,12]. Melody stent fracture has been described as one of the etiologies behind TPV dysfunction and stenosis [4,13]. Such a phenomenon is less likely to happen when the Melody valve is deployed within the stented BPV or with present placement [14]. The same is true when it comes to transcatheter VIV in a dysfunctional TPV. A recent study showed transcatheter VIV is more sustainable than mere balloon dilation of a dysfunctional Melody valve [4], especially when Melody stent fracture is present [4]. Freedom from re-intervention after transcatheter VIV (the second TPV) is reported to be higher when compared with the initial TPV [13].
Technical considerations
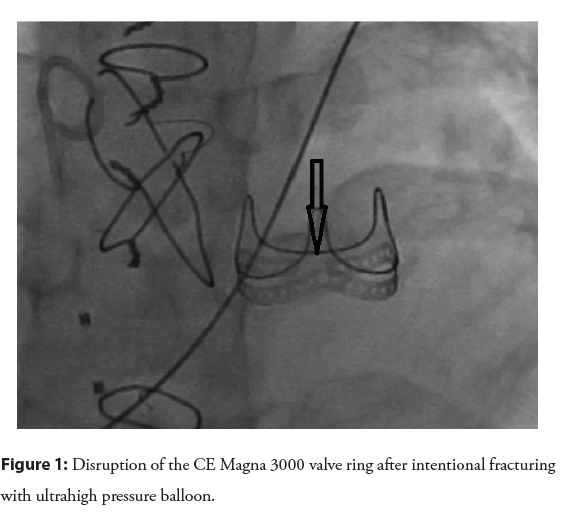
Intentional fracture: Unlike surgical BPV, homografts and conduits can be expanded beyond their nominal diameter [15]. BPV is composed of a rigid sewing ring that supports leaflets and commissural posts [16], which limits the maximum diameter that can be achieved with VIV. The true Inner Diameter (ID) is usually 2 mm-3 mm smaller than the labeled diameter, which refers to the outer diameter of the BPV supporting frame [16,17]. Implanting VIV within a limited diameter leads to a further decrease in ID and effective orifice, creating patient prosthesis mismatch (PPM) further impacting the ultimate effective orifice size and not relieving valvar gradient. Intentional fracture of the BPV ring (Edwards Perimount bioprosthesis) was first described less than a decade ago [18], using an ultra-high-pressure balloon (Atlas Gold balloon), which then allowed implantation of VIV at a larger diameter than the original BPV true ID. An important finding from bench testing is that the fabric covering the ring remains intact, protecting the adjacent structure from the edges of the broken ring [18]. Several other studies corroborated the safety and efficacy of intentional fracture before or after VIV implantation in the aortic and pulmonary position to achieve a larger effective orifice and better hemodynamics [17,19,20]. From a technical standpoint, an ultra-high-pressure balloon has been used, with a balloon size usually 1 mm larger than the nominal BPV diameter, which typically translates into 3 mm-5 mm larger than the true ID [17,19,21,22]. In some instances, the ring is fractured with a balloon diameter of 1 mm less than the nominal diameter [17]. BPV fracture is usually associated with a sudden drop in balloon pressure, occasionally audible crack, and fluoroscopic evidence of ring disruption [21,22] (Figure 1). The use of a larger balloon diameter relative to the stenosis may exert the same force with lower inflation pressure [23]. However, caution should be exercised when using a larger balloon, especially when the main pulmonary artery is relatively small. It was demonstrated before that the fabric covering of the ring remains intact despite the fracture of the ring itself. It is unclear whether this remains true when using larger balloons.
There is no consistent balloon size and/or inflation pressure that predicts BPV ring fracture due to variability between different BPV and factors related to valve-tissue interaction and geometry [17]. While most of the BPV can be fractured, several studies reported a failure to intentionally fracture the Trifecta and Hancock II valves [17,21,22].
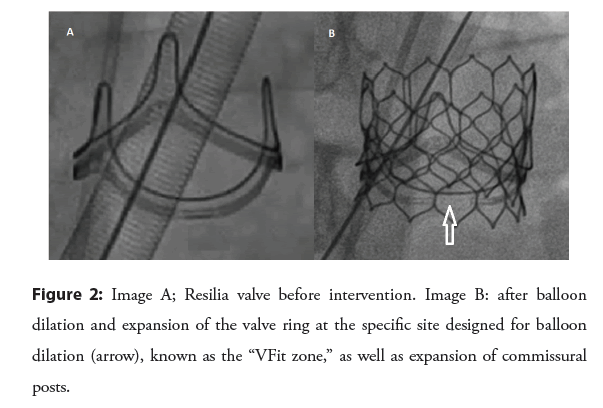
A newer iteration of BPV is now available with a ring that is designed to be balloon-dilated predictably [24]. Inspiris Resilia (Edwards Lifesciences) valve ring as well as the commisural posts are designed with overlapping segments at nominal diameter that allow expansion in a precise fashion during valvuloplasty [24] (Figure 2).
Pre-stenting: It is not clear whether pre-stenting is necessary after fracturing BPV and before VIV implantation. Pre-stenting seems appropriate when significant recoil of the valve ring is noted after fracture [17], and if using the Melody valve, which has a platinum- iridium stent frame with less radial strength than the cobalt- chromium frame of the Sapien Valve [25]. Pre-stenting may reduce the effective orifice by occupying space within the BPV ring.
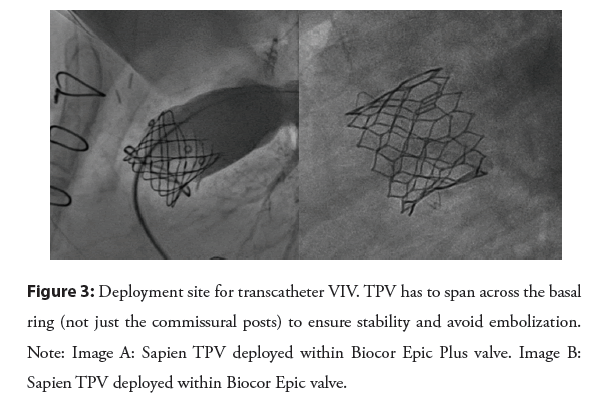
New valve deployment site: As mentioned before, the limiting factor for BPV is the basal ring and not the commissural post. This is important when trying to achieve a larger diameter by balloon dilation and/or intentional fracture. This is also important when deploying a pre-stent and/or TPV. The TPV stent has to span across the basal BPV ring for stability to avoid embolization of the stent/valve [17,26] (Figure 3). The length of the Melody valve is longer than most BPV, which may be an advantage when trying to correct the angulation between BPV and RVOT by deploying the Melody valve in a proximal position leading to stenting part of the RVOT [5].
Coronary compression: Coronary compression is less of a concern with VIV and has been reported rarely [5,12]. However, coronary proximity should be evaluated especially when intentional valve fracture is planned or the stent spans the RVOT or MPA beyond the BPV frame. Another reported mechanism of coronary compression without BPV fracture is the geometric change in the BPV with VIV [5], which can be predicted with balloon testing before VIV.
Surgical replacement of dysfunctional BPV: Despite all the strides made in TPV, there are instances where surgical replacement becomes necessary. BPVs, especially those implanted with smaller nominal diameters, have a limited range of dilation due to the inherent characteristics of the valve itself. The angle of the BPV with the homograft/conduit might be prohibitive for VIV. Finally, coronary compression is rare but possible. Patients with conotruncal anomalies will eventually need multiple surgeries over their lifetime. Transcatheter VIV represents a reasonable way to reduce the total number of surgeries, which, to our knowledge, has not been studied systematically. There are instances where the decision between VIV within a dysfunctional BPV vs. surgical replacement of the BPV is equivocal. Collaboration between the surgical and cath teams is paramount to achieve the best outcomes.
Discussion
Intentional fracture of certain rigid-frame BPV devices in the pulmonary position is feasible, allows greater enlargement of the TPV compared with implant into an un-fractured BPV. With current valve technology there is concern for continued valve deterioration which can only be potentiated from stenosis due to patient prosthesis mismatch. With this in mind setting up the valve frame to decrease patient prosthesis mismatch with smaller BPV that is less than ideal for an adult, and placement of a TPV within the relatively small BPV which would result in further loss of orifice area would be potentiate early valve failure. Preliminary data published regarding intentional frame fracture technique both in the aortic and pulmonary position in encouraging but longer term data will be necessary to understand the applications and limitations of this adjunctive technique. The addition of stented trileaflet valve comprised of RESILIA bovine pericardial tissue that is mounted on a flexible frame gives the option of predictable expansion for valve in valve therapy.
Conclusion
In conclusion, transcatheter VIV in dysfunctional BPV is safe and effective. Intentional fracture of the BPV ring provides a larger effective valve orifice and better hemodynamics. Intentional fracture of the BPV ring is safe and achievable in most BPVs with an ultrahigh-pressure balloon. Specific technical aspects of the procedure should be considered to achieve optimal deployment of VIV. Newer stented BPV technology allow predictable expansion for VIV therapy.
References
- Baird CW, Chávez M, Sleeper LA, et al. Reintervention rates after bioprosthetic pulmonary valve replacement in patients younger than 30 years of age: A multicenter analysis. J Thorac Cardiovasc Surg. 161(2): 345-362 (2021).
- Egbe AC, Connolly HM, Miranda WR, et al. Outcomes of bioprosthetic valves in the pulmonary position in adults with congenital heart disease. Ann Thorac Surg. 108(5):1410-1415 (2019).
- Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease: The task force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Adult Congenital Heart Disease (ISACHD). Eur Heart J. 42(6):563-645 (2020).
- Shahanavaz S, Berger F, Jones TK, et al. Outcomes after transcatheter reintervention for dysfunction of a previously implanted transcatheter pulmonary valve. JACC Cardiovasc Interv. 13(13):1529-1540 (2020).
- Gillespie MJ, et al. Melody valve implant within failed bioprosthetic valves in the pulmonary position: A multicenter experience. Circ Cardiovasc Interv. 5(6):862-870 (2012).
- Patel PM, Zapata D, Qu W, et al. Bioprosthetic pulmonary valve dysfunction in congenital heart disease. Ann Thorac Surg. 115(3):641-648 (2023).
- Lee C, Park CS, Lee CH, et al. Durability of bioprosthetic valves in the pulmonary position: long-term follow-up of 181 implants in patients with congenital heart disease. J Thorac Cardiovasc Surg. 142(2): 351-358 (2011).
- Chen XJ, Smith PB, Jaggers J, et al. Bioprosthetic pulmonary valve replacement: Contemporary analysis of a large, single-center series of 170 cases. J Thorac Cardiovasc Surg. 146(6):1461-1466 (2013).
- Jones TK, McElhinney DB, Vincent JA, et al. Long-term outcomes after melody transcatheter pulmonary valve replacement in the US investigational device exemption trial. Circ Cardiovasc Interv. 15(1):e010852 (2022).
- Houeijeh A, Batteux C, Karsenty C, et al. Long-term outcomes of transcatheter pulmonary valve implantation with melody and SAPIEN valves. Int J Cardiol. 370: 156-166 (2023).
- Possner M, Tseng SY, Alahdab F, et al. Risk factors for mortality and ventricular tachycardia in patients with repaired tetralogy of fallot: A systematic review and meta-analysis. Can J Cardiol. 36(11):1815-1825 (2020).
- Cabalka AK, Asnes JD, Balzer DT, et al. Transcatheter pulmonary valve replacement using the melody valve for treatment of dysfunctional surgical bioprostheses: A multicenter study. J Thorac Cardiovasc Surg. 155(4):1712-1724 (2018).
- Nordmeyer J, Coats L, Lurz P, et al. Percutaneous pulmonary valve-in-valve implantation: A successful treatment concept for early device failure. Eur Heart J. 29(6):810-815 (2008).
- McElhinney DB, Cheatham JP, Jones TK, et al. Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation: Patient-related and procedural risk factors in the US melody valve Trial. Circ Cardiovasc Interv. 4(6):602-614 (2011).
- Cabalka AK, Hellenbrand WE, Eicken A, et al. Relationships among conduit type, pre-stenting, and outcomes in patients undergoing transcatheter pulmonary valve replacement in the prospective North American and European melody valve trials. JACC Cardiovasc Interv. 10(17):1746-1759 (2017).
- Bapat V, Mydin I, Chadalavada S, et al. A guide to fluoroscopic identification and design of bioprosthetic valves: A reference for valve-in-valve procedure. Catheter Cardiovasc Interv. 81(5):853-861 (2013).
- Shahanavaz S, Asnes JD, Grohmann J, et al. Intentional fracture of bioprosthetic valve frames in patients undergoing valve-in-valve transcatheter pulmonary valve replacement. Circ Cardiovasc Interv. 11(8):e006453 (2018).
- Tanase D, Grohmann J, Schubert S, et al. Cracking the ring of Edwards Perimount bioprosthesis with ultrahigh pressure balloons prior to transcatheter valve in valve implantation. Int J Cardiol. 176(3):1048-1049 (2014).
.[CrossRef][Google Scholar][PubMed]
- Chhatriwalla AK, Allen KB, Saxon JT, et al. Bioprosthetic valve fracture improves the hemodynamic results of valve-in-valve transcatheter aortic valve replacement. Circ Cardiovasc Interv. 10(7): e005216 (2017).
- Nielsen-Kudsk, JE, Andersen A, Therkelsen, CJ, et al. High-pressure balloon fracturing of small dysfunctional mitroflow bioprostheses facilitates transcatheter aortic valve-in-valve implantation. Euro Intervention: Journal of EuroPCR in collaboration with the working group on interventional cardiology of the European society of cardiology. EuroIntervention. 13(9): e1020-e1025 (2017).
- Johansen P, Engholt H, Tang M, et al. Fracturing mechanics before valve-in-valve therapy of small aortic bioprosthetic heart valves. EuroIntervention. 13(9):e1026-e1031 (2017).
- Allen KB, Chhatriwalla AK, Cohen DJ, et al. Bioprosthetic valve fracture to facilitate transcatheter valve-in-valve implantation. Ann Thorac Surg. 104(5):1501-1508 (2017).
- Capelli C, Nordmeyer J, Schievano S, et al. How do angioplasty balloons work: A computational study on balloon expansion forces? EuroIntervention. 6(5): 638-642 (2010).
- Batlivala SP, Hagel JA, Hirsch R, et al. Transcatheter pulmonary valve-in-valve implantation within the expandable Inspiris Resilia(R) bioprosthetic valve. Catheter Cardiovasc Interv. 99(4):1157-1160 (2022).
- Tanase D, Georgiev S, Eicken A, et al. The Sapien valve provides enough grip to be implanted in pulmonary position without a pre-stent. Cardiovasc Diagn Ther. 9:S264-S268 (2019).
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 121(16):1848-1857 (2010).