Review Article - Interventional Cardiology (2012) Volume 4, Issue 5
Transcatheter valve interventions: mitral valve is the next quest
- Corresponding Author:
- Francesco Maisano
Cardiothoracic and Vascular Department
San Raffaele University Hospital
Via Olgettina 60, 20132 Milano, Italy
Tel: 09008740965
Fax: 416-603-6919
E-mail: Arina.Bingeliene@uhn.ca
Abstract
Keywords
mitral regurgitation, mitral repair, mitral replacement, percutaneous annuloplasty, transcatheter interventions
In the last 5 years, transcatheter aortic valve implantation (TAVI) has evolved from an innovative experimental approach to valve disease to a valuable therapeutical option for high-risk and inoperable patients with aortic stenosis. As technology evolves and clinical outcomes improve, more patients are referred to TAVI. In the last year, in most countries in Europe, approximately one out of four patients requiring a tissue aortic valve replacement had it implanted with a transcatheter approach. The threshold for indicating TAVI is lowering each year and there is potential to expand transcatheter treatment into the intermediate risk population.
In the meantime, mitral valve interventions have lagged behind. The reasons for a slower evolution and penetration into clinical practice are multiple. Compared with the aortic valve, the mitral valve anatomy and pathology is more complex and variable. As a consequence, a single device is not going to be enough to treat all patients; furthermore, many patients could require more than one device to fix mitral disease more effectively. In addition, since the mitral valve apparatus is a complex 3D and dynamic structure, a conventional stent-based approach (such as the one used for the aortic valve) is not applicable to replace or to repair the valve. Finally, the future of mitral valve intervention is closely linked to the evolution of imaging, due to the dynamicity and the complexity of the structures, where an angiographic approach is grossly insufficient to guide most of the procedures. All these factors generate challenges both in the development and in the clinical acceptance of the therapies.
However, it is very probable that we will see multiple successful devices and procedures introduced into clinical practice in the next 5 years. With the introduction of novel therapies, more patients will become eligible for percutaneous treatment, in addition, more effective and durable treatment should become available. The current review focuses on the current status of mitral valve interventions and on the evolution of this treatment.
The unmet need for less invasive treatments of mitral valve regurgitation
Surgery has achieved very high-quality standards, with low mortality and excellent long-term results, both for mitral repair and replacement [1]. Currently, most patients with degenerative mitral valve regurgitation are treated with repair, at an early stage of the disease [2]. A variety of techniques are available to correct mitral regurgitation (MR), to accommodate the wide variability of the disease and to cover the spectrum of anatomical disorders causing degenerative mitral valve regurgitation. In high volume centers, minimally invasive procedures are available, including the closed-chest robotic approach, which minimizes the esthetic impact of treatment and reduces rehabilitation times. In asymptomatic patients, hospital mortality following repair is below 1% [1]. However, mortality increases with age, comorbidites and in cases of combined procedures. Hospital mortality is higher for functional MR (FMR), as well as durability of repair is suboptimal [3]. Consequently, prognostic impact is uncertain [4]. As a matter of fact, Euro Heart Survey revealed that 50% of patients with severe and symptomatic MR are not referred to surgery for presumed contraindications [5]. Transcatheter valve interventions bring a new hope for those patients who are currently denied surgery, particularly for those who are inoperable or at high risk. In this field a multidisciplinary team approach (heart team), including surgeons, interventionalists, anesthesiologists and clinical cardiologists, has been proven successful and demanded by recent recommendations.
Transcatheter mitral valve interventions are the natural evolution of surgery towards less-invasive solutions [6] to better treat high-risk patients as well as to enable early correction of valvular disease [7]. Some diseases traditionally approached with surgical treatment are already treated with an interventional approach. A good example is mitral stenosis. Currently, in western countries, closed and open mitral valve commissurotomy is rarely performed as it has been replaced by catheter-based balloon valvuloplasty. Valvuloplasty is reproducing surgical commissurotomy, with the advantage of real-time echocardiographic guidance to support decision-making. Image guidance brings a new dimension in the treatment of valve disease, allowing correction under physiologic conditions, and real-time verification of the effects of therapy.
As MitraClip™ (Abbott Vascular Inc., CA, USA) achieved the first 5000 procedures performed worldwide in March 2012, a similar evolution should be expected for the treatment of MR.
A variety of different technologies are at different stages of development. A classification is difficult and cannot be complete at this time. Most technologies are based upon surgical techniques, while a minority are based on novel concepts. The latter approach is an attempt to simplify, to make procedures more reproducible, although demonstration of effectiveness is more challenging in the absence of a surgical background. Generally, transcatheter mitral devices should be divided in repair and replacement technologies. In the repair group, another classification should be made between leaflet, annular and ventricular repair.
Repair technologies acting at the leaflet level
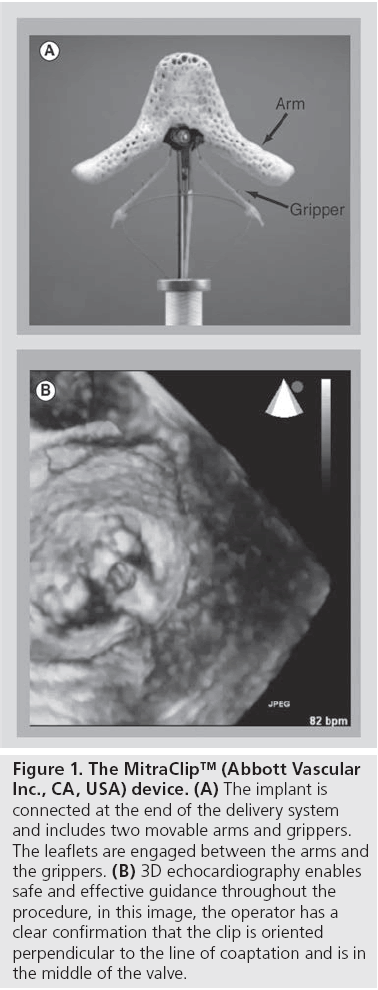
The MitraClip system is available in Europe as the only certified device to treat both degenerative mitral valve regurgitation and FMR (Figure 1). The device has been designed to reproduce the Alfieri technique [8], a surgical method to treat MR by suturing the free edge of the leaflets at the origin of the regurgitant jet. MitraClip therapy has been delivered to more than 5500 patients worldwide. The EVEREST was started in the USA after the first-in-man experience to evaluate safety and efficacy of the device [9]. Three cohorts of patients are included in the study: the EVEREST I feasibility cohort, the EVEREST II pivotal cohort [10] and the EVEREST II randomized trial. In addition, patients with a highrisk profile who had been excluded from the EVEREST trial (being nonsurgical candidates) have been enrolled in the high-risk registry [11]. Overall, the EVEREST trial demonstrated that MitraClip therapy is feasible and carries a lower risk than surgery in surgical candidates; however, surgery is more effective than the MitraClip in reducing the degree of MR. However, MitraClip reached noninferior clinical outcomes up to 3 years from the index procedure. Nevertheless, it must be mentioned that the EVEREST trial had several limitations and results are not applicable to the current patient population undergoing MitraClip procedures. For this reason, a number of registries and new studies are currently in progress.
Figure 1: The MitraClip™ (Abbott Vascular Inc., CA, USA) device. (A) The implant is connected at the end of the delivery system and includes two movable arms and grippers. The leaflets are engaged between the arms and the grippers. (B) 3D echocardiography enables safe and effective guidance throughout the procedure, in this image, the operator has a clear confirmation that the clip is oriented perpendicular to the line of coaptation and is in the middle of the valve.
With increasing experience, early and late outcomes are reproducible and predictable, and the procedure is performed in a more expeditious fashion. The procedure is performed under general anesthesia and is mostly guided by transesophageal echocardiography.
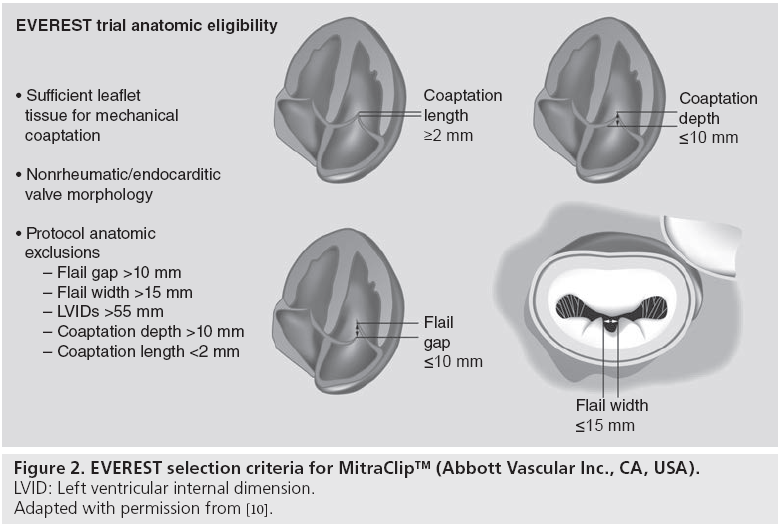
The main advantage of MitraClip is its versatility. It can be applied to both degenerative and FMR with good results. However, patient selection is crucial: the best candidates have a central MR jet, with limited intercommissural extension (Figure 2). The wider the jet, the greater the need for multiple clip implantation and the risk of residual MR or to induce mitral stenosis. In addition, commissural lesions are less ideal, although expert operators are now treating them. In cases involving prolapsing leaflets, the distance between the free edge of the prolapse and the opposing healthy leaflet (flail gap) should be limited to allow grasping. For FMR, coaptation depth and length have been used for patient selection in the EVEREST trial, but are rarely felt as an absolute contraindication in current practice [12].
In our experience at San Raffaele Hospital (Milan, Italy), 77% of patients who underwent MitraClip procedure have anatomical or functional criteria beyond the limits described in the EVEREST trial [13]. The most typical criteria that are not met in the real world are coaptation depth and ejection fraction. Most patients treated have a very low ejection fraction and severely tethered leaflets. In our experience, the latter conditions are not associated with early and 1-year outcomes. Also, although most patients are at high risk, compared with the typical EVEREST II randomized controlled trial population, the safety profile of the procedure has remained excellent. Hospital mortality has been below 2%, with 1-year survival of 85%. At 1 year, approximately 80% of patients had a residual MR grade equal to or lower than 2+, and 70% of patients were in the New York Heart Association class I or II (as compared with preprocedural prevalence of 70% of patients being in class III or IV). Few anatomical contraindications remain in our experience: leaflet calcifications (in the target segments), not pliable leaflets (as for rheumatic disease), wide prolapse (more than 20 mm) in patients with small annulus or large flail gap (although this can be sometimes corrected with advanced techniques).
In patients with degenerative disease and unfavorable anatomy for MitraClip, a good alternative could be transcatheter neochordae implantation. Several devices are under development in this field, using a variety of approaches.
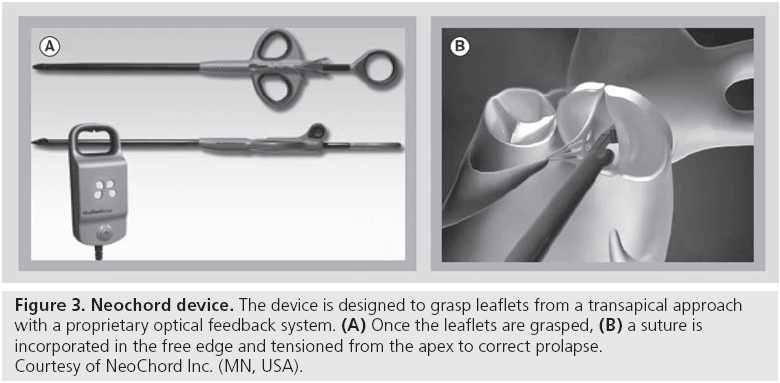
Neochordae implantation is a very common method to treat prolapsing lesions in open-heart surgery. Its adoption rate has increased significantly in surgery during the last 10 years, due to a potential advantage in coaptation surface (respecting rather than resecting coaptation tissue), and due to enhanced feasibility in minimally invasive approaches. Neochord Inc. (MN, USA) has developed a suture device to implant neochords on the beating heart with a transapical approach [14]. The device has a proprietary method to grasp leaflets and to confirm appropriate leaflet incorporation using a fiber optic feedback method (Figure 3). The procedure is guided by transesophageal echocardiography (similar to MitraClip), using both 2D and 3D views. Once the free edge of the prolapsing segment is grasped, a polytetrafluoroethylene suture is pierced into it and then carried externally, through the apex. The prolapse is corrected under echo guidance, by tethering the implanted suture to the appropriate length. Usually two or more sutures are used to treat prolapse and are secured to the apex with pledgets. The device is under evaluation in the TACT trial.
Figure 3: Neochord device. The device is designed to grasp leaflets from a transapical approach with a proprietary optical feedback system. (A) Once the leaflets are grasped, (B) a suture is incorporated in the free edge and tensioned from the apex to correct prolapse. Courtesy of NeoChord Inc. (MN, USA).
Other technologies are under development for treating leaflet lesions with neochordae. ValtechCardio (Or Yehuda, Israel) is working on a sutureless adjustable device, potentially adaptable for transcatheter procedures, to enable minimally invasive mitral valve repair with neochords: the v-chordal system [15]. This device has been designed to deliver adjustable length neochordae using a sutureless implant in the tip of the papillary muscle, delivering a couple of adjustable length sutures (polytetrafluoroethylene covered) that are attached to the free edge of the prolapsing leaflet. The device is currently under clinical study and open-heart implant followed by adjustment on the beating heart after discontinuation from cardiopulmonary by-pass. Off-pump adjustment is expected to improve surgical outcomes by enabling optimization of neochordae length under physiologic conditions as opposed to current techniques, where the surgeon has to guess neochordae length either by visual assessment or by preoperative evaluation. Further developments will enable total percutaneous implant.
Edwards Lifesciences Corporation (CA, USA) developed a suture-based technology (currently on hold) named the Mobius that allowed leaflet capturing by suction and subsequent piercing with a polyproylene suture. The Mobius device has been tested in a clinical trial to enable double orifice technique, and in preclinical trials for transapical neochordae implantation. The technology has not been developed further due to durability issues in the clinical trial [16].
MitraFlex™ is a device which combines neochordae implantation with leaflet approximation according to the edge-to-edge procedure. The device is developed by Transcardiac Therapeutics (GA, USA) and is based on a transapical approach.
Treatment of prolapsing lesions includes plication devices to fold the prolapsing segment of a leaflet as opposed to resection. Leaflet folding is rarely used in surgery today; however, it was described in the past with satisfactory results. As an alternative, the redundant tissue associated with prolapsing lesions can be ablated with radiofrequency Thermocool Smarttouch® (Cordis Corporation, NJ, USA) in an attempt to remodel leaflet surface and enable better coaptation. The technology platform has been developed on a transfemoral retrograde approach to the mitral valve.
An innovative concept working at the leaflet level, with no surgical precedent, is the Percu-Pro™ (Cardiosolutions, MA, USA) device, a mitral valve spacer device consisting on an apical anchor and tether with a balloon inflated inside the mitral valve orifice with the intention of occupying the effective orifice area. This device should be delivered with a transfemoral transeptal approach.
Repair technologies acting at the annular level
Whatever leaflet repair technique is adopted in surgical practice, annuloplasty is routinely added to the repair to enhance short- and long-term outcomes. Annuloplasty is associated with longer durability and lack of annuloplasty is an incremental risk factor for reintervention and recurrent MR following repair, either for conventional techniques or for the edge-to-edge procedure.
Therefore, lack of viable annuloplasty devices is felt as an important unmet need in the field of percutaneous mitral valve interventions, and several devices are under development, using a wide variety of technologies and approaches.
Coronary sinus implants have been the first technologies developed and tested in clinical trials. The coronary sinus lies in close vicinity to the posterior mitral annulus, a device implanted herein has the potential to induce annular dimension changes. This approach is expected to be safer and simpler than others, although early experience has not been satisfactory in most cases. Three main devices have been assessed. The MONARC (Edwards Lifesciences, Corporation) is a Nitinol self-expanding device with two stents joined by a tension bridge segment designed to foreshorten over 3–4 weeks, due to progressive absorption of biocompatible beads embedded in the cells of the Nitinol bridge. The foreshortening of the bridge would approximate the two stents and such movement would be transferred to the coronary sinus and the nearby mitral annulus. MONARC has been tested in the safety and efficacy EVOLUTION trial. A total of 72 patients were enrolled at eight centers The MONARC device was implanted in 59 patients (82%). The primary safety end point (freedom from death, tamponade or myocardial infarction at 30 days) was met in 91% of patients at 30 days and in 82% at 1 year. Computed tomography documented crossing of the great cardiac vein over the circumflex artery in 55% of patients and was associated with coronary artery compression in 15 patients and myocardial infarction in two patients (3.4%). At 12 months, any degree of reduction in MR grade was observed only in 50% of patients [17]. The main limitation of the MONARC design has been the delayed foreshortening. Originally conceptualized to improve safety and efficacy by reducing the risk of device embolization, delayed bridge shortening impeded intraoperative recognition of device success, as well as risk of coronary occlusion. These objectives are met with the Carillon™ device (Cardiac Dimensions, WA, USA), a Nitinol wire with distal and proximal anchors connected by an intervening cable designed for acute coronary sinus foreshortening. The real-time effect on coronary sinus and mitral annulus allows intraprocedural guidance and identification of responders, as well as ruling out the risk of coronary compression. The device has been tested in the AMADEUS trial [18], which enrolled 48 patients with moderate- to-severe FMR, an ejection fraction <40% and a 6 min walking distance between 150 and 450 m. Eighteen patients did not receive a device because of access issues, insufficient acute FMR reduction or coronary artery compromise. In the remaining 30 patients, at 6 months, there was a demonstration of a slight improvement of MR degree but associated with functional improvement (6-min walking distance improvement). More recently, Siminiak et al. reported the outcomes of the TITAN trial, where patients in whom the device was placed then acutely recaptured for clinical reasons served as a comparator group [19]. In this trial, 36 patients received a permanent implant and 17 had the device recaptured. Compared with the control, the implanted cohort demonstrated significant reductions in FMR and reduction in left ventricular volumes, compared with progressive left ventricular dilation in the comparator. The device received the CE mark and it is currently available for clinical use in Europe. A third device, the PTMA™ (Viacor, MA, USA) has been implanted in clinical trials, but is not yet available for clinical use [20]. The device is implanted permanently in the coronary sinus, with a subcutaneous access port located in the jugular vein. Thin alloy rods can be inserted into the implant through this port to reshape the permanent implant and generate annular remodeling. Recently, some concerns about the safety of the device have been reported [21], although the device has been demonstrated to be efficacious in selected patients, with evidence of sustained reduction of the septalateral annular dimension [20].
As the EVOLUTION trial was discontinued due to suboptimal results, there has been discouragement around the coronary sinus approach. But the recent evidence accumulated with the TRITON trial bring some hope for this approach, which could be more easily applicable in selected heart failure patients due to its intrinsic simplicity as compared with more complex procedures including the MitraClip implant. However, coronary sinus anatomy could restrict applicability of this approach in up to 50% of patients with FMR, due to the risk of coronary compressions, and due to the unfavorable location of the coronary sinus (lack of anatomical continuity between coronary sinus and mitral annulus) [22,23]. In addition, the anterior aspect of the annulus cannot be addressed with a coronary sinus approach.
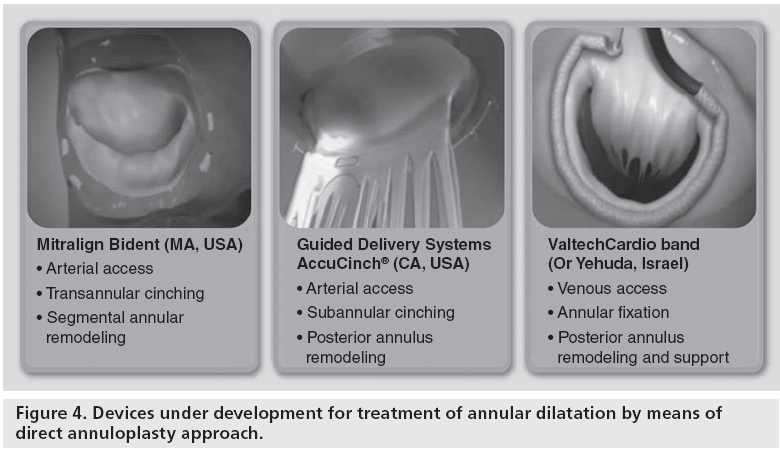
To overcome these limitations, great efforts have been made to develop direct annuloplasty methods, more closely reproducing surgical annular remodeling (Figure 4). Three technologies are being developed to achieve direct annuloplasty. Mitralign (MA, USA) has developed a system for selective annular plication with a retrograde transarterial approach. A delivery catheter is inserted into the left ventricle from an arterial approach. The tip of the catheter is positioned under the annulus, at the hinge of the posterior leaflet. The catheter design (Bident) is such that as it is positioned at the intended location, it transforms into a Y-shape, to deliver a pair of anchors. Using radiofrequency, the annulus is perforated from the ventricular to the atrial side, taking care to avoid any leaflet lesion. A pair of pledgets on both sides of the annulus are delivered a few millimeters apart using the Bident delivery system. Then the two pledgets are approximated using a cinching tether attached to each pledget on the ventricular side. Ideally, the procedure is carried out on two segments of the posterior annulus, on both sides of the medial scallop (P2) to reduce annular dimensions.
Guided Delivery Systems (CA, USA) is developing the AccuCinch® device, a cinching device implanted in the subvalvar area between the annulus and the base of the papillary muscles, with a retrograde approach. The procedure is guided mostly by fluoroscopy. A specially designed guiding catheter is introduced in the subannular space, and delivers a sequence of anchors, connected by a cable. At the end of the implant (ideally distributed from commissure to commissure), the anchors are cinched by pulling on the cable, under echo guidance. As the desired annular remodeling is achieved, the tethering cable is secured and cut to hold tension.
Both Mitralign and Guided Delivery Systems have limited clinical experience, and have undergone some technology iterations to improve performance of the device. New generation devices are currently under clinical trial.
ValtechCardio is developing the Cardioband™ transfemoral and direct access systems. Cardioband is the closest percutaneous replica of a surgical anuloplasty device. With a sutureless approach, a Dacron band is implanted from commissure to commissure either with a direct approach or with a transfemoral, antigrade approach (involving transseptal punture). Following implantation, the Dacron band is cinched under echo-guidance to obtain up to 40% linear reduction of the annulus. The direct atrial approach device is currently under clinical trial [24], while the percutaneous version has completed the preclinical studies and is expected to start clinical trials in the last quarter of 2012. The main advantage of Cardioband compared with any other transcatheter annuloplasty device is that it most closely reproduces the shape and function of surgical annuloplasty rings, therefore, efficacy is predicted to be similar to what is achievable with surgical annuloplasty. Also, a surgical version of the transcatheter device is expected to be developed to enable faster and more reproducible minimally invasive surgical implantation owing to the sutureless technology method of annular fixation.
Other annular remodeling approaches include the use of energy (radiofrequency and ultrasounds) to induce shrinkage of annular collagen [25,26]. Although preclinical experience has been promising, initial clinical experience seems to be less satisfactory.
Overall, the development of effective solutions for annular remodeling will be an important adjunct to MitraClip in the armamentarium for treating patients with a percutaneous approach. The addition of annuloplasty is expected to expand the indications, to improve early outcomes, as well as to expand durability. Potentially, when annuloplasty will become available, percutaneous mitral repair could become available to the majority of patients, and it could become a real alternative to open heart surgery.
Mitral valve transcatheter implantation
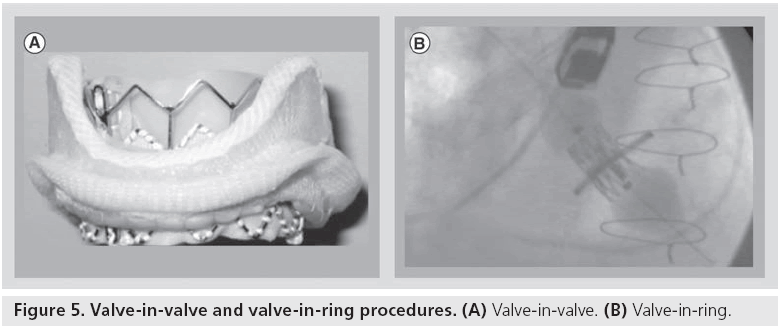
Currently, mitral valve replacement is an option for those patients with a failing surgical prostehsis undergoing valve-in-valve or valve-in-ring procedures using a transcatheter aortic valve prosthesis (Figure 5) [27–29].
To complete the portfolio of treatment options, mitral valve implantation in the native anatomy is also developing, although not yet performed in humans. There are several challenges to bring this therapy into clinical practice. Mitral valve anatomy and physiology is very different from the aortic valve, making technologies used for TAVI scarcely useful in the mitral space. Compared with the aortic valve, the mitral valve is anatomically larger. This implies the use of larger devices, which will require bulkier delivery systems. However, more importantly, anchoring of such devices seems to be a major challenge. Radial force fixation and anchoring is probably not feasible for several reasons. Firstly, due to the size of the native annulus, very large devices should be required to obtain enough radial force. In addition, a device using radial force to anchor itself to the mitral annulus could induce aortic valve dysfunction, left ventricular outflow tract obstruction as well as potential for annular erosions. Another challenge is the asymmetric and dynamic features of the mitral valve apparatus, which could require asymmetric devices, requiring orientation at the time of implant. Finally, if perivalvular leakage is relatively well tolerated following aortic valve implantation, in the case of mitral valve implantation, this could be associated with greater hemodynamic consequences, as well as with a higher risk of hemolysis.
Despite these challenges, several devices are under development and will become available in the future. Extensive preclinical work has demonstrated feasibility of mitral valve implantation with different approaches [30–32].
Conclusion and future perspective
The next 5 years will bring a number of innovative solutions for transcatheter mitral valve interventions ranging from mitral valve repair to mitral replacement. Current indications for transcatheter valve interventions will be revised, and more treatment options will become available for patients that are not currently being considered for surgical therapy. Advanced imaging will be instrumental for the development of these technologies, both for screening and selection of appropriate device, and for effective guidance. Evolution of transcatheter procedures will also influence further improvements in surgical outcomes, thanks to crossfertilization and incorporation of interventional features and technologies into surgical tools. However, for the time being, while we wait for more reliable data supporting these therapies, surgery should be still considered the gold standard for the treatment of mitral disease whenever feasible.
Financial and competing interests disclosure
F Maisano is a consultant for Abbott Vascular Inc., ValtechCardio, Medtronic Inc., St Jude Medical Inc., 4Tech and AFFix. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Mitral valve interventions: unmet needs & challenges
▪ Mitral valve anatomy and physiology is very complex. Mitral valve disease is a dynamic entity, with large variability. Consequently, mitral valve interventions require multiple solutions to accomplish for individual variability.
▪ Imaging is key in this field, where a combination of fluoro ad echo based guidance is needed.
▪ The Euro Heart Survey revealed that 50% of patients with severe and symptomatic mitral regurgitation are not referred to surgery. Transcatheter valve interventions bring a new hope for those patients who are currently denied surgery.
MitraClip™ (Abbott Vascular Inc., CA, USA): the current solution for high risk & inoperable patients with mitral regurgitation
▪ MitraClip™ is the first therapy clinically available in Europe. With increasing experience, early and late outcomes are reproducible and predictable, and the procedure is performed in a more expeditious fashion. The procedure is performed under general anesthesia and is mostly guided by transesophageal echocardiography. Results appear to be durable over time, with more than three out of four patients experiencing a sustained clinical benefit.
Future developments of mitral valve interventions
▪ Annuloplasty devices include both indirect (mainly through the coronary sinus) and direct approaches, simulating the surgical procedure of annular remodeling and reshaping.
▪ Mitral valve replacement represents a major challenge. Delivery, anchoring and sealing are more difficult than for the aortic valve, due to the size differences and to the more dynamic and asymmetric anatomy of the mitral valve.
▪ Despite the challenges, mitral interventions will develop in the next 5–10 years. Evolution of transcatheter procedures will also influence further improvements in surgical outcomes, thanks to crossfertilization and incorporation of interventional features and technologies into surgical tools.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Gammie JS, Sheng S, Griffith BP et al. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann. Thorac. Surg. 87(5), 1431–1437; discussion 1437–1439 (2009).
- Tribouilloy C, Grigioni F, Avierinos JF et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J. Am. Coll. Cardiol. 54(21), 1961–1968 (2009).
- Tahta SA, Oury JH, Maxwell JM, Hiro SP, Duran CM. Outcome after mitral valve repair for functional ischemic mitral regurgitation. J. Heart Valve Dis. 11(1), 11–18; discussion 18–19 (2002).
- Bolling SF, Pagani FD, Deeb GM, Bach DS. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J. Thorac. Cardiovasc. Surg. 115(2), 381–386; discussion 387–388 (1998).
- Mirabel M, Iung B, Baron G et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur. Heart J. 28(11), 1358–1365 (2007).
- Mack MJ. Minimally invasive and robotic surgery. JAMA 285(5), 568–572 (2001).
- David TE, Ivanov J, Armstrong S, Rakowski H. Late outcomes of mitral valve repair for floppy valves: implications for asymptomatic patients. J. Thorac. Cardiovasc. Surg. 125(5), 1143–1152 (2003).
- Maisano F, LA Canna G, Colombo A, Alfieri O. The evolution from surgery to percutaneous mitral valve interventions: the role of the edge-to-edge technique. J. Am. Coll. Cardiol. 58(21), 2174–2182 (2011).
- Feldman T, Foster E, Glower DD et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 364(15), 1395–1406 (2011).
- Feldman T, Kar S, Rinaldi M et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J. Am. Coll. Cardiol. 54(8), 686–694 (2009).
- Whitlow PL, Feldman T, Pedersen WR et al. Acute and 12‑month results with catheterbased mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to- Edge Repair) High Risk Study. J. Am. Coll. Cardiol. 59(2), 130–139 (2012).
- Maisano F, Godino C, Giacomini A et al. Clinical trial experience with the MitraClip catheter based mitral valve repair system. Int. J. Cardiovasc. Imaging 27(8), 1155–1164 (2011).
- Tamburino C, Ussia GP, Maisano F et al. Percutaneous mitral valve repair with the MitraClip system: acute results from a real world setting. Eur. Heart J. 31(11), 1382–1389 (2010).
- Seeburger J, Leontjev S, Neumuth M et al. Trans-apical beating-heart implantation of neo-chordae to mitral valve leaflets: results of an acute animal study. Eur. J. Cardiothorac. Surg. 41(1), 173–176; discussion 176 (2012).
- Maisano F, Cioni M, Seeburger J et al. Beating-heart implantation of adjustable length mitral valve chordae: acute and chronic experience in an animal model. Eur. J. Cardiothorac. Surg. 40(4), 840–847 (2011).
- Webb JG, Maisano F, Vahanian A et al. Percutaneous suture edge-to-edge repair of the mitral valve. EuroIntervention 5(1), 86–89 (2009).
- Harnek J, Webb JG, Kuck KH et al. Transcatheter implantation of the MONARC coronary sinus device for mitral regurgitation: 1‑year results from the EVOLUTION phase I study (clinical evaluation of the edwards lifesciences percutaneous mitral annuloplasty system for the treatment of mitral regurgitation). JACC Cardiovasc. Interv. 4(1), 115–122 (2011).
- Schofer J, Siminiak T, Haude M et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON mitral annuloplasty device European Union study. Circulation 120(4), 326–333 (2009).
- Siminiak T, Wu JC, Haude M et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN Trial. Eur. J. Heart Fail. 14(8), 931–938 (2012).
- Sack S, Kahlert P, Bilodeau L et al. Percutaneous transvenous mitral annuloplasty: initial human experience with a novel coronary sinus implant device. Circ. Cardiovasc. Interv. 2(4), 277–284 (2009).
- Machaalany J, St-Pierre A, Senechal M et al. Fatal late migration of viacor percutaneous transvenous mitral annuloplasty device resulting in distal coronary venous perforation. Can. J. Cardiol. doi: 10.1016/j. cjca.2012.03.014 (2012) (Epub ahead of print).
- Lansac E, Di Centa I, Al Attar N et al. Percutaneous mitral annuloplasty through the coronary sinus: an anatomic point of view. J. Thorac. Cardiovasc. Surg. 135(2), 376–381 (2008).
- Choure AJ, Garcia MJ, Hesse B et al. In vivo analysis of the anatomical relationship of coronary sinus to mitral annulus and left circumflex coronary artery using cardiac multidetector computed tomography: implications for percutaneous coronary sinus mitral annuloplasty. J. Am. Coll. Cardiol. 48(10), 1938–1945 (2006).
- Maisano F, Vanermen H, Seeburger J et al. Direct access transcatheter mitral annuloplasty with a sutureless and adjustable device: preclinical experience. Eur. J. Cardiothorac. Surg. 42(3), 524–529 (2012).
- Jilaihawi H, Virmani R, Nakagawa H et al. Mitral annular reduction with subablative therapeutic ultrasound: pre-clinical evaluation of the ReCor device. EuroIntervention 6(1), 54–62 (2010).
- Goel R, Witzel T, Dickens D, Takeda PA, Heuser RR. The QuantumCor device for treating mitral regurgitation: an animal study. Catheter. Cardiovasc. Interv. 74(1), 43–48 (2009).
- Nunez-Gil IJ, Goncalves A, Rodriguez E et al. Transapical mitral valve-in-valve implantation: a novel approach guided by three-dimensional transoesophageal echocardiography. Eur. J. Echocardiogr. 12(4), 335–337 (2011).
- Cheung AW, Gurvitch R, Ye J et al. Transcatheter transapical mitral valve-in-valve implantations for a failed bioprosthesis: a case series. J. Thorac. Cardiovasc. Surg. 141(3), 711–715 (2011).
- De Weger A, Ewe SH, Delgado V, Bax JJ. First-in-man implantation of a trans-catheter aortic valve in a mitral annuloplasty ring: novel treatment modality for failed mitral valve repair. Eur. J. Cardiothorac. Surg. 39(6), 1054–1056 (2011).
- Ma L, Tozzi P, Huber CH, Taub S, Gerelle G, Von Segesser LK. Double-crowned valved stents for off-pump mitral valve replacement. Eur. J. Cardiothorac. Surg. 28(2), 194–198; discussion 198–199 (2005).
- Iino K, Boldt J, Lozonschi L et al. Off-pump transapical mitral valve replacement: evaluation after one month. Eur. J. Cardiothorac. Surg. 41(3), 512–517 (2012).
- Lutter G, Quaden R, Osaki S et al. Off-pump transapical mitral valve replacement. Eur. J. Cardiothorac. Surg. 36(1), 124–128; discussion 128 (2009).
▪▪ Describes the current status of mitral valve surgery in the USA. It reports data on almost 60,000 patients undergoing mitral valve surgeries in the Society of Thoracic Surgeons database.
▪ The EuroHeart survey describes the current practice in Europe regarding referral practice in patients with mitral regurgitation and was the first to report how many patients and why patients are not referred to surgery.
▪▪ Useful overview of the surgical background of the Alfieri repair and of its percutaneous evolution into the MitraClip™ (Abbott Vascular Inc., CA, USA) device.
▪▪ The EVEREST trial, although debated, represents the first trial ever designed to test efficacy of interventional treatment strategies in the field of mitral valve regurgitation.